Evaluation of Water Ammonium Ion Test Kit and Its Feasibility for the Analysis of Protein in Food
Abstract
:1. Introduction
2. Results and Discussion
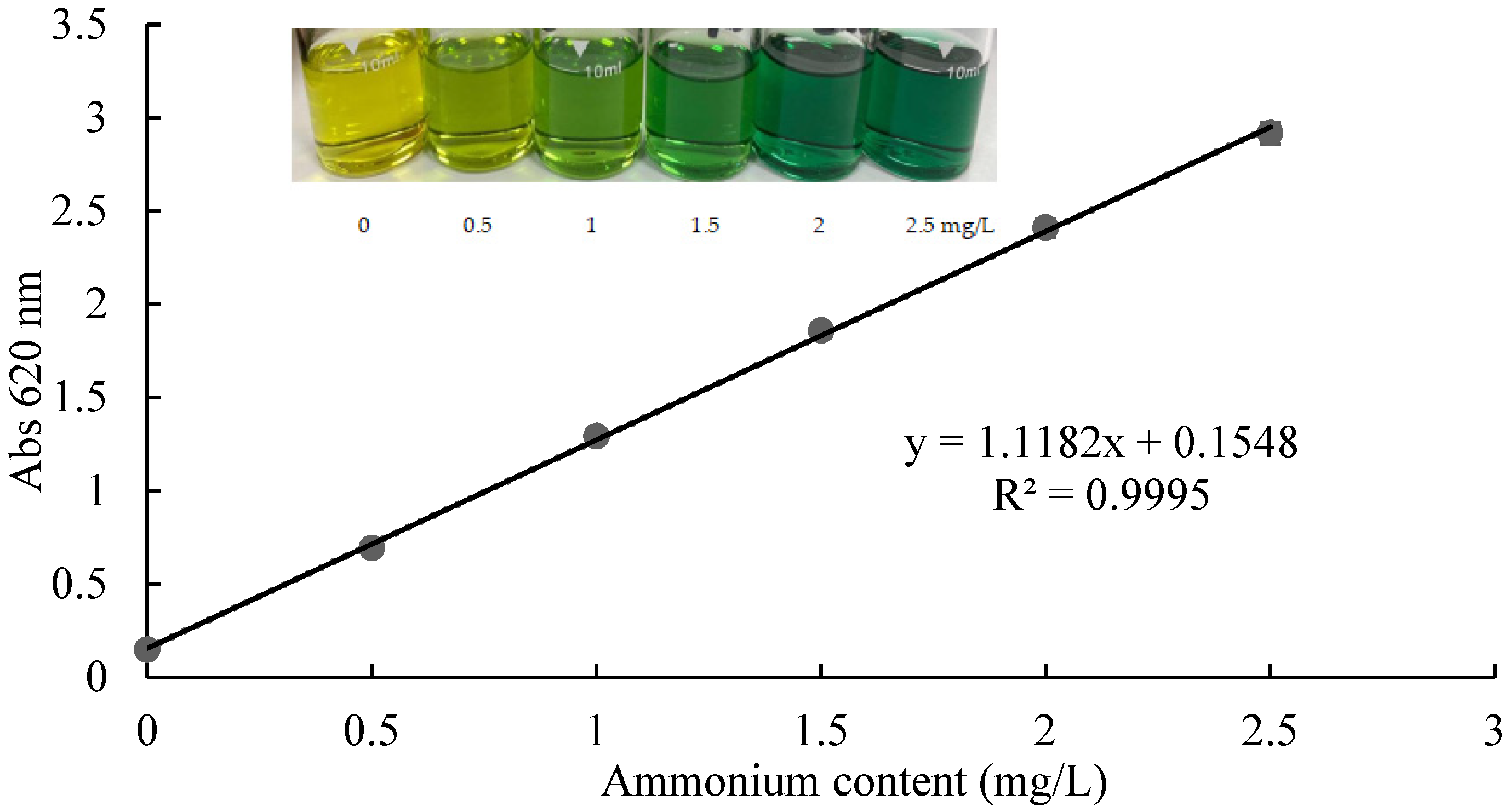
2.1. The Ammonium Standard Curve for the Test Kit Method
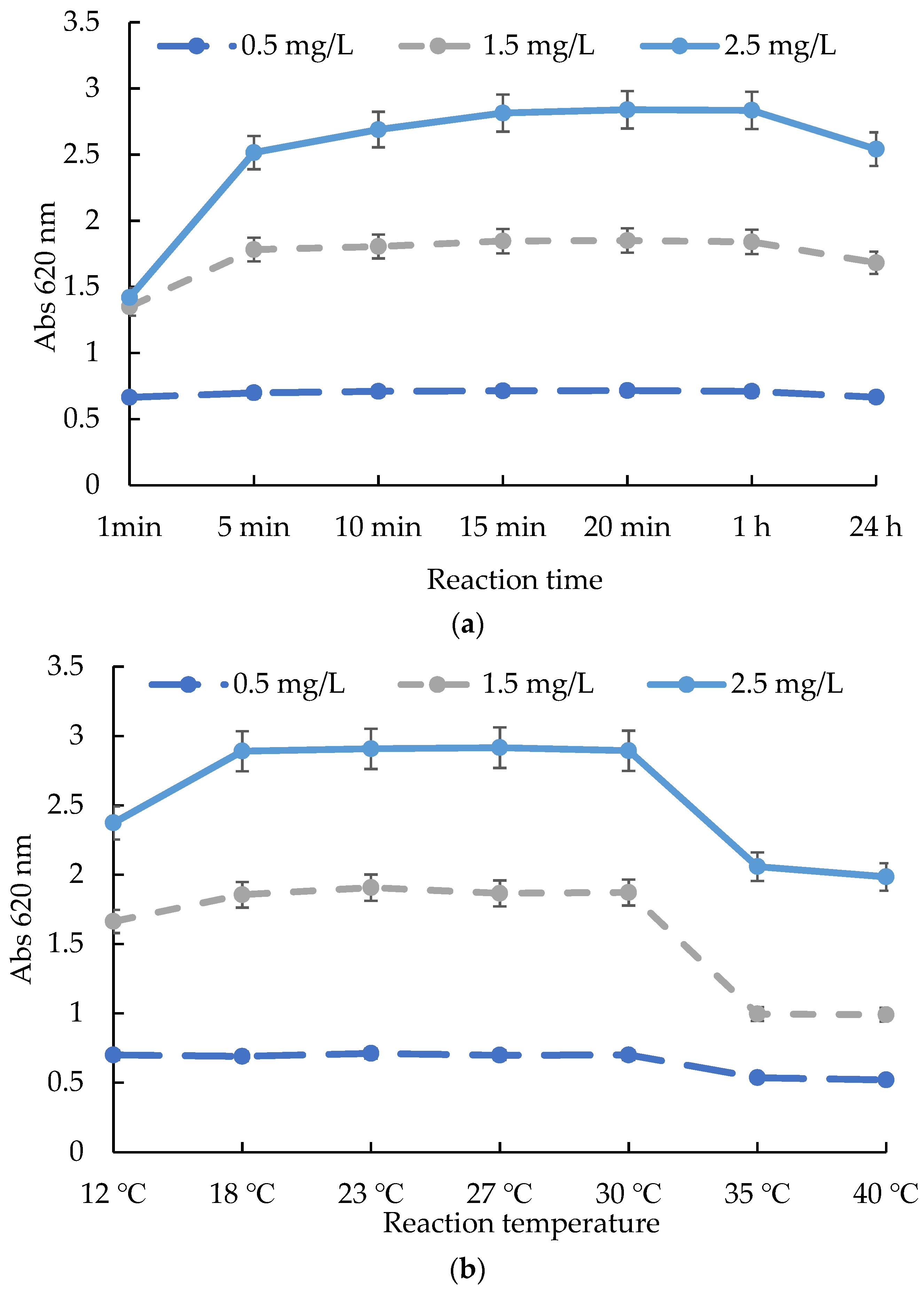
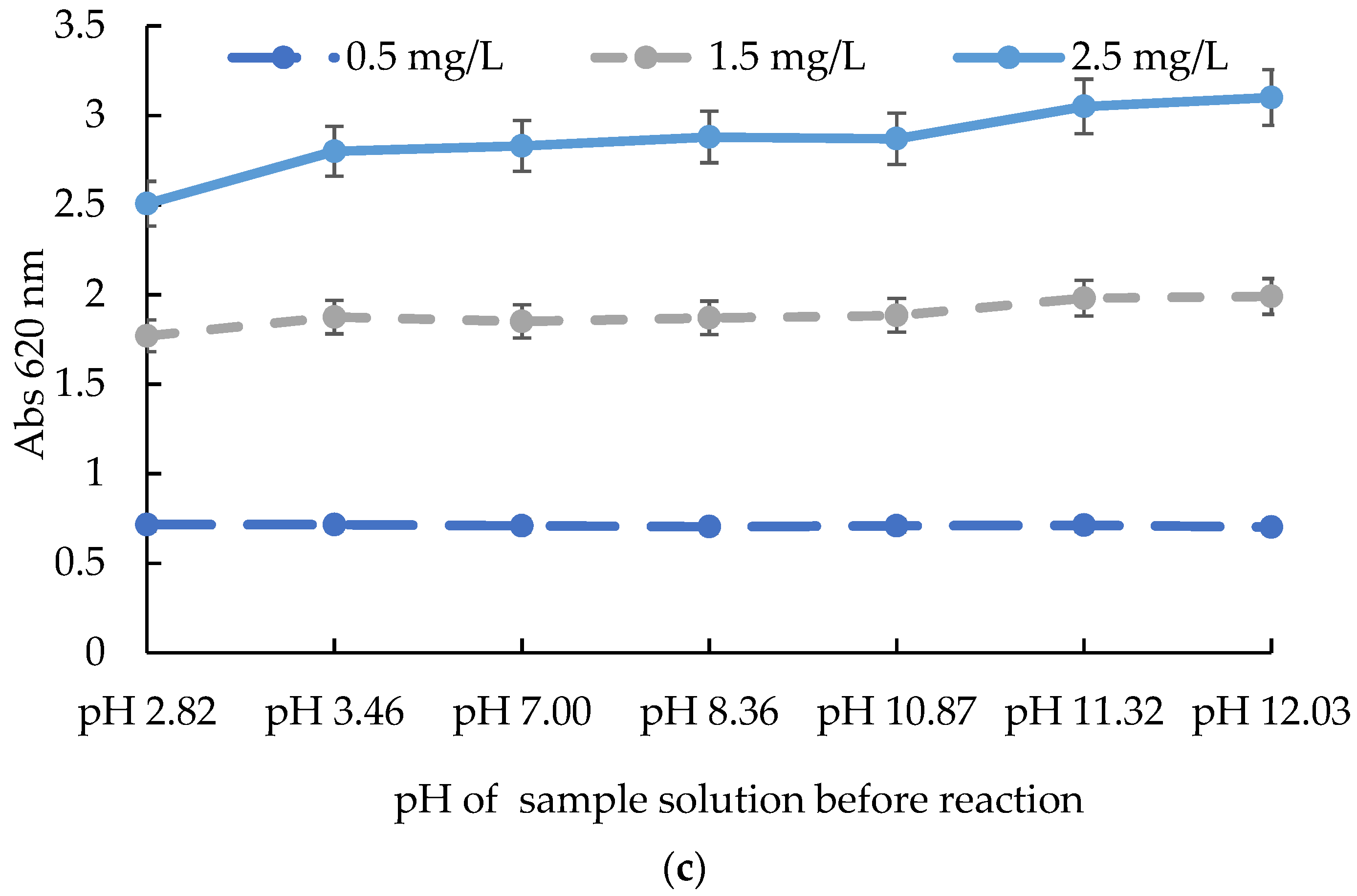
2.2. The Influences of Time, Temperature, and pH on the Test Kit Method
2.3. Precision and Repeatability of the Test Kit Method
2.4. The Recovery of the Test Kit Method
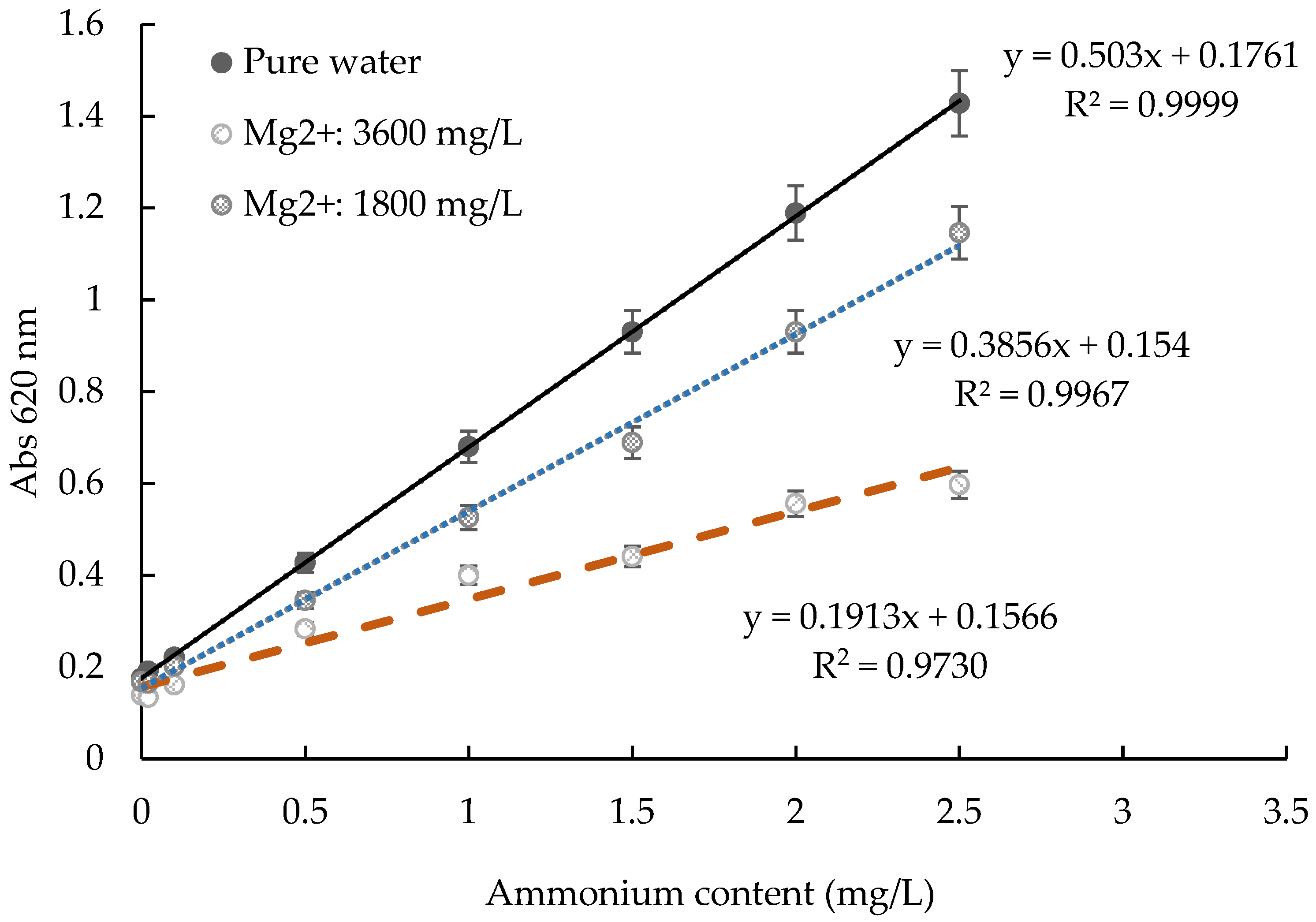
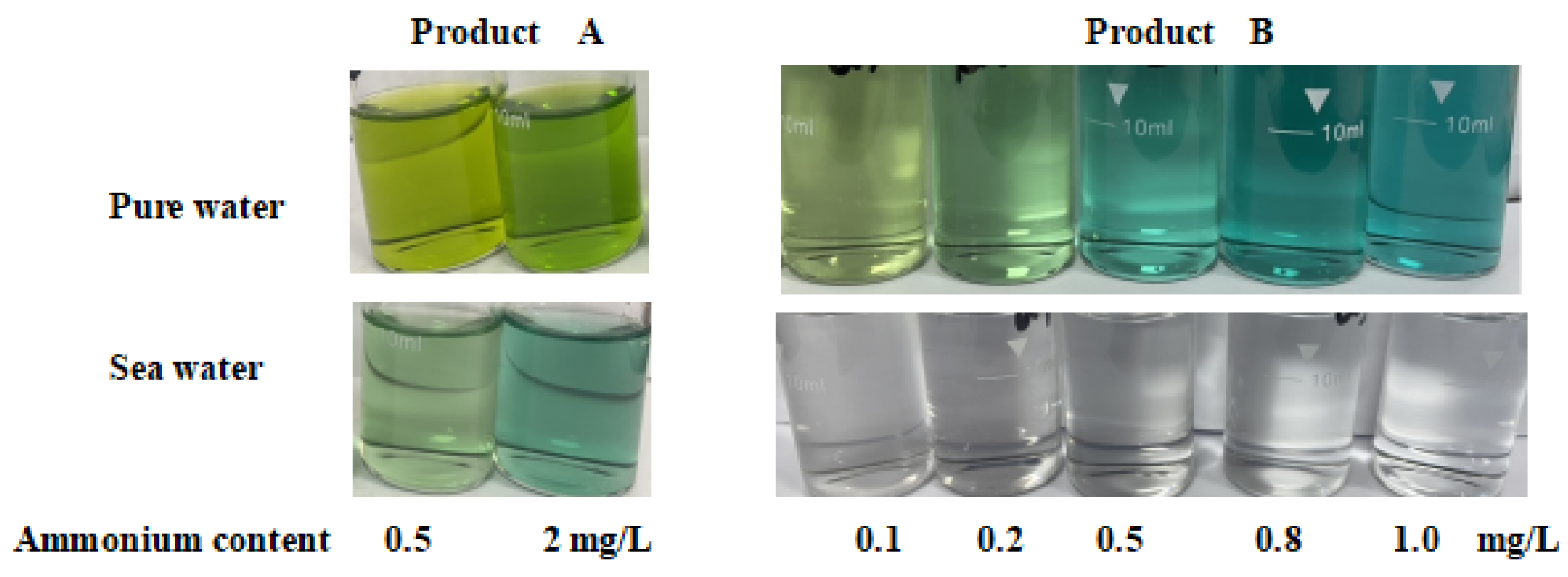
2.5. The Influence of Different Ions in Water for Test Kit Method
2.6. Comparison of National Method and Test Kit Method for Analysis of Food Protein
3. Materials and Methods
3.1. Materials
3.2. Ammonium Standard Curve for the ATK
3.3. Evaluation of the Influences of Time, Temperature, and pH on the ATK
3.4. Precision and Repeatability Evaluation of the ATK
3.5. Evaluation of the Recovery of and the Influence of Ions on the ATK
3.6. Protein Analysis
- —protein content (g/100 g);
- —protein conversion coefficient (refer to GB 5009.5-2016 [28]);
- —nitrogen content in sample solution to be measured (mg/L);
- —dilution ratio.
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Chen, J.; Yuan, D.; Yang, Z.; Ran, L. Development of analytical methods for ammonium determination in seawater over the last two decades. TrAC Trends Anal. Chem. 2019, 119, 115627. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, Y. Research progress in field determination method of ammonia nitrogen. Chem. Bioeng. 2020, 37, 11–16. (In Chinese) [Google Scholar]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- Cogan, D.; Cleary, J.; Fay, C.; Rickard, A.; Jankowski, K.; Phelan, T.; Bowkett, M.; Diamond, D. The development of an autonomous sensing platform for the monitoring of ammonia in water using a simplified Berthelot method. Anal. Methods 2014, 6, 7606–7614. [Google Scholar] [CrossRef] [Green Version]
- Ruppersberg, H.S.; Goebel, M.R.; Kleinert, S.I.; Wünsch, D.; Trautwein, K.; Rabus, R. Photometric determination of ammonium and phosphate in seawater medium using a microplate reader. J. Mol. Microbiol. Biotechnol. 2017, 27, 73–80. [Google Scholar] [CrossRef]
- Kaewwonglom, N.; Jakmunee, J. Sequential injection system with multi-parameter analysis capability for water quality measurement. Talanta 2015, 144, 755–762. [Google Scholar] [CrossRef]
- Khongpet, W.; Pencharee, S.; Puangpila, C.; Hartwell, S.K.; Lapanant-noppakhun, S.; Jakmunee, J. A compact hydrodynamic sequential injection system for consecutive on-line determination of phosphate and ammonium. Microchem. J. 2019, 147, 403–410. [Google Scholar] [CrossRef]
- Vieira, G.P.; Crispino, C.C.; Perdigao, S.R.W.; Reis, B.F. An environmentally friendly photometric procedure for ammonium determination in rainwater employing a multicommutation approach. Anal. Methods 2013, 5, 489–495. [Google Scholar] [CrossRef]
- Muraki, H.; Higuchi, K.; Sasaki, M.; Korenaga, T.; Tôei, K. Fully automated system for the continuous monitoring of ammonium ion in fish farming plant sea water by flow-injection analysis. Anal. Chim. Acta 1992, 261, 345–349. [Google Scholar] [CrossRef]
- Infante, C.M.C.; Masini, J.C.; dos Santos, A.C.V. Development of a spectrophotometric sequential injection analysis (SIA) procedure for determination of ammonium: A response surface methodology (RSM) approach. Microchem. J. 2011, 98, 97–102. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.H.; Chun, H.; Kim, Y.S. Selective colorimetric determination of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens. Actuators B Chem. 2018, 256, 167–175. [Google Scholar] [CrossRef]
- HJ 168-2020; Technical Guideline for the Development of Environmental Monitoring Analytical Method Standards. China National Standard. Ministry of Environmental Protection of China: Beijing, China, 2020.
- Wu, D.; Zhou, S.R.; Liu, Y. Study on rapid detection method of ammonia nitrogen in aquatic environment. Chin. J. Anal. Lab. 2017, 34, 429–432. [Google Scholar]
- Shoji, T.; Nakamura, E. Collection of indonaphthol blue on a membrane filter for the spectrophotometric determination of ammonia with 1-naphthol and dichloroisocyanurate. Anal. Sci. 2010, 26, 779–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Yang, P.; Dong, F.; Sun, M. Detection of ammonia nitrogen in seawater in aquaculture based on microfluidics and colorimetric spectroscopy. Trans. Chin. Soc. Agric. Mach. 2020, 51, 397–404. [Google Scholar]
- Ma, R.; Liu, J.; Liu, Y. The Improvement of salicylic acid method to determine ammonia nitrogen in water. Environ. Monit. China 2019, 35, 160–164. [Google Scholar]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer–Lambert law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- Zhu, L.; Zhan, T.; Yu, H.; Huang, Z.; Yin, F.; Tao, J. Comparison between the national standard detection method and rapid detection method of water quality. Acta Agric. Shanghai 2014, 30, 121–123. (In Chinese) [Google Scholar]
- ASTM D1141-1998 (2013); Standard Practice for the Preparation of Substitute Ocean Water. American Society for Materials and Testing: West Conshohocken, PA, USA, 2021; Volume 11.02. [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A guide for selecting the most appropriate method for ammonium determination in water analysis. TrAC-Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Jafari, M.T.; Khayamian, T. Direct determination of ammoniacal nitrogen in water samples using corona discharge ion mobility spectrometry. Talanta 2008, 76, 1189–1193. [Google Scholar] [CrossRef]
- Huangpu, M.M. Study on the Method of Field Detection of Ammonia Nitrogen in Seawater. Master’s Thesis, Tianjing University, Tianjing, China, 2016. (In Chinese). [Google Scholar]
- Pai, S.C.; Tsau, Y.J.; Yang, T. pH and buffering capacity problems involved in the determination of ammonia in saline water using the indophenol blue spectrophotometric method. Anal. Chim. Acta 2001, 434, 209–216. [Google Scholar] [CrossRef]
- SC/T 1001-2008; Parameters of Ecological Type of Lake Fishery. China National Standard. The Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2008.
- HJ 536-2009; Water Quality-Determination of Ammonia Nitrogen-Salicylic Acid Spectrophotometry. China National Standard. Ministry of Environmental Protection of China: Beijing, China, 2009.
- GB 17378.4-2007; The Specification for Marine Monitoring-Part 4: Seawater Analysis. China National Standard. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- GB 5009.5-2016; National Standard for Food Safety—Determination of Protein in Food—Kjeldahl Method. China National Standard. Ministry of Health of the People’s Republic of China: Beijing, China, 2016.
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. China National Standard. State Administration for Market Regulation (SAMR): Beijing, China, 2018.
- Jamal, S.; Jamil, D.; Khidhir, Z. Protein determination in some animal products from sulaymaniyah markets using kjeldahl procedure. J. Food Dairy Sci. 2020, 11, 343–346. [Google Scholar] [CrossRef]
- Chen, J.; Ren, X.; Shen, Q. Application of NIR transmission spectroscopy with effective wavelength selection in non-destructive determination of essential amino acid content of foxtail millet. Qual. Assur. Saf. Crops Foods 2017, 9, 237–248. [Google Scholar] [CrossRef]
- Jia, F.; Dai, F.; Wu, B.; Zhang, H.; Wang, P.; Yu, X. Determination of protein in solid drink by spectrophotometry. J. Food Saf. Qual. 2018, 9, 4725–4728. (In Chinese) [Google Scholar]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]

| Analytical Method | Manufacturer | Detection Range mg/L | Advantages and Disadvantages |
|---|---|---|---|
| IPB-salicylate | Macherey-Nagel GmbH& Co. KG (Düren, Germany) | 0.05–3.00 | Seawater needs 1 + 1 dilution; turbidity makes the measurements too high |
| 0.01–2.50 | Suitable for seawater; turbidity makes the measurements too high | ||
| Kyoritsu Chemical-Check Lab., Corp (Tokyo, Japan) | 0.5–20 | Apply to industrial wastewater, sea water | |
| 0.2–10 | Suitable for cleaner water, river water, groundwater, and drinking water; not suitable for wastewater | ||
| Hach Company (Loveland, CO, United States) | 0.02–2.5 | Iron ions interfere with the determination; monoammonium chloride, hydrazine, amino-acetic acid, turbidity, and chromaticity may cause high measurement results | |
| 0.4–50 | |||
| 0.01–0.50 | |||
| Zhejiang Lohand Environmental Technology Co. Ltd. (Jiubao Town, China) | 0.01–2.00 | Suitable for low-hardness water | |
| 0.02–2.50 | Suitable for high-hardness water, wastewater | ||
| Guangdong Huankai Microbial Technology Co., Ltd. ((Guangzhou, China) | 0.01–1.00 | Not suitable for sea water | |
| 0.4–50 | Suitable for clean seawater; detection limit is high | ||
| IPB-phenol | Macherey-Nagel GmbH& Co. KG (Germany) | 0.5–8.0 | Seawater needs to be diluted more than 10 times for determination |
| IPB-phenol | Macherey-Nagel GmbH& Co. KG (Germany) | 0.1–2.5 | Seawater needs to be diluted more than 10 times for determination |
| Concentration (mg/L) | Lab (A) Precision (n = 5) | Lab (B) Precision (n = 5) | Lab (C) Precision (n = 5) | Repeatability (n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean /(mg/L) | RSD % | Mean /(mg/L) | RSD % | Mean /(mg/L) | RSD % | Mean /(mg/L) | RSD % | |
| 0.5 | 0.48 | 0.94 | 0.48 | 2.28 | 0.50 | 0.90 | 0.49 | 1.71 |
| 1 | 1.02 | 1.08 | 0.93 | 2.37 | 0.99 | 1.52 | 0.98 | 3.86 |
| 1.5 | 1.52 | 1.35 | 1.51 | 3.33 | 1.54 | 1.34 | 1.52 | 2.52 |
| 2 | 2.02 | 1.15 | 1.91 | 1.19 | 1.97 | 1.01 | 1.96 | 4.86 |
| 2.5 | 2.47 | 1.74 | 2.43 | 2.07 | 2.53 | 2.29 | 2.48 | 4.71 |
| Sample | Background Concentration (mg/L) | Concentration after Addition (mg/L) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|
| 0.5 mg/L | 1 mg/L | 2 mg/L | 0.5 mg/L | 1 mg/L | 2 mg/L | ||
| Tap water | 0 | 0.490 ± 0.010 | 0.950 ± 0.080 | 2.060 ± 0.120 | 97.40 | 95.40 | 103.00 |
| River water | 0.05 | 0.480 ± 0.010 | 0.990 ± 0.060 | 1.940 ± 0.080 | 96.60 | 98.70 | 97.05 |
| Artificial seawater | 0 | 0.459 ± 0.010 | 0.997 ± 0.030 | 1.951 ± 0.100 | 91.80 | 99.70 | 97.55 |
| Artificial seawater diluted twice | 0 | 0.450 ± 0.010 | 0.973 ± 0.030 | 1.959 ± 0.090 | 90.00 | 97.30 | 97.95 |
| Artificial seawater diluted four times | 0 | 0.484 ± 0.010 | 0.974 ± 0.040 | 1.866 ± 0.110 | 96.80 | 97.40 | 93.30 |
| Ions | Coexisting Content mg/L | Ions | Coexisting Content mg/L | Ions | Coexisting Content mg/L |
|---|---|---|---|---|---|
| Ca2+ | 1400 | Cd2+ | 250 | As3+ | 25 |
| Mg2+ | 1800 | Cr6+ | 25 | SO42− | 10,000 |
| Al3+ | 250 | Mo6+ | 1250 | PO43− | 1250 |
| Mn2+ | 250 | Co2+ | 6.25 | NO3− | 1250 |
| Cu2+ | 31.25 | Ni2+ | 250 | NO2− | 250 |
| Pb2+ | 250 | V5+ | 250 | F− | 1250 |
| Zn2+ | 250 | Ag+ | 6.25 | Cl− | 23,778 |
| Item | Sample | National Method | Kit Method | t Test | |
|---|---|---|---|---|---|
| T | ρ | ||||
| Protein content (g/100 g) | Milk powder | 18.50 ± 0.50 | 18.57 ± 0.64 | 0.24 | 0.82 |
| Rice flour | 6.02 ± 0.19 | 5.96 ± 0.28 | 0.49 | 0.64 | |
| Wheat flour | 10.38 ± 0.22 | 10.36 ± 0.88 | 0.49 | 0.96 | |
| Soy | 32.05 ± 0.75 | 32.40 ± 0.97 | 0.70 | 0.50 | |
| Banana | 3.27 ± 0.14 | 3.32 ± 0.19 | 0.56 | 0.59 | |
| Milk | 3.47 ± 0.25 | 3.62 ± 0.25 | 1.09 | 0.30 | |
| Fish food | 9.90 ± 0.28 | 10.19 ± 0.20 | 2.09 | 0.06 | |
| Chicken food | 10.82 ± 0.07 | 10.87 ± 0.06 | 1.40 | 0.19 | |
| Dog food | 20.85 ± 3.06 | 20.07 ± 1.61 | 0.55 | 0.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Xia, Q.; Zhou, J.; Ye, X. Evaluation of Water Ammonium Ion Test Kit and Its Feasibility for the Analysis of Protein in Food. Molecules 2022, 27, 4689. https://doi.org/10.3390/molecules27154689
Wu D, Xia Q, Zhou J, Ye X. Evaluation of Water Ammonium Ion Test Kit and Its Feasibility for the Analysis of Protein in Food. Molecules. 2022; 27(15):4689. https://doi.org/10.3390/molecules27154689
Chicago/Turabian StyleWu, Dan, Qile Xia, Jianfeng Zhou, and Xingqian Ye. 2022. "Evaluation of Water Ammonium Ion Test Kit and Its Feasibility for the Analysis of Protein in Food" Molecules 27, no. 15: 4689. https://doi.org/10.3390/molecules27154689
APA StyleWu, D., Xia, Q., Zhou, J., & Ye, X. (2022). Evaluation of Water Ammonium Ion Test Kit and Its Feasibility for the Analysis of Protein in Food. Molecules, 27(15), 4689. https://doi.org/10.3390/molecules27154689






