Abstract
In the current study, the anti-inflammatory and analgesic potential of Alnus nitida (leaves and fruits) was evaluated in the Sprague-Dawley rat. Traditionally, A. nitida was used for the treatment of inflammatory ailments. However, A. nitida leaves and fruits have not been yet reported regarding any potential medicinal effects. Leaves/fruits of A. nitida were extracted with methanol and fractionated to attain n-hexane, chloroform, ethyl acetate and aqueous fractions. These extracts were then evaluated for in vivo analgesic and anti-inflammatory potential. For in vivo anti-inflammatory activity, carrageenan-induced paw edema assay, Freunds’ complete adjuvant-induced edema, xylene-induced ear edema and histamine-induced paw edema models were used in rats, which showed significant (p < 0.01) reduction (70–80%) in edema in comparison of inflammatory controls. On other hand, for the analgesic assessment, hot plate assay and acetic acid-induced writhing tests were used, which showed a significant (p < 0.01) rise in latency time (40–60%) as compared with pain-induced controls. These results were comparable with standard drugs in a concentration-dependent manner and no mortality or toxicity was observed during all experiments. Then, for the identification of chemical constituents gas chromatography–mass spectrometry (GC-MS) analysis was performed, which indicated the presence of neophytadiene, 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, phytol and vitamin E, justifying the use of A. nitida to treat inflammatory disorders.
1. Introduction
Although inflammation is a protective response of the body, it can also cause various degrees of injury, including allergic reactions, edema, effusion and scarring. Currently major global health concerns include inflammatory diseases, such as arthritis, cardiovascular disease, diabetes mellitus and others [1,2]. The inflammatory process involves a series of events that can be elicited by numerous stimuli, for example, infection agents, ischemia, antigen–antibody interactions and chemical, thermal or mechanical injury [3]. Several animal models were employed in this study to assess the anti-inflammatory effects and the rationale behind utilizing different model is that each model represents distinct mechanistic approach. The carrageenan-induced acute inflammatory process in rats comprises three phases: the first phase releases histamine and serotonin; the second phase is mediated by kinins; and the final phase is mediated by prostaglandins [4]. Therefore, it appears to cause inflammation by a different mechanism than that of substances like xylene, which causes an allergic response to dextran. When xylene is administered topically, a neurogenic inflammation is induced. The molecular mechanisms governing the production of pro-inflammatory chemicals from sensory neurons are mainly highlighted by this inflammation model [5]. Likewise, histamine induces the activation of microglia in vivo and prompts proinflammatory cytokine release, which suggests that histamine plays an important role in microglia activation and neuroinflammation-related diseases [6]. On the other hand, adjuvant-induced arthritis is developed after induction of complete Freund’s adjuvant into rats [7]. It is a systemic disease with articular and visceral manifestation that resembles rheumatoid arthritis. It is characterized by chronic evolution with recurrent inflammatory bouts resulting in periarticular, articular and bone lesion [4].
Pain is the most common symptom associated with minor ailments to life-threatening conditions. Analgesic agents are drugs that release pain without disturbing its cause, including non-steroidal anti-inflammatory drugs (NSAIDs) and antidepressants [8]. NSAIDs are among the most broadly used of all therapeutic classes of drugs because they are both anti-inflammatory and analgesic. Analgesic opioids are generally comprised of sulfentanil, codeine, fentanyl, morphine, methadone and meperidine. However, all these come with side effects like gastrointestinal and renal injuries [9,10,11]. Few NSAIDs have been associated with augmented blood pressure, the increased danger of congestive heart failure and the existence of thrombosis [12]. Two different analgesic testing methods were used in the current investigation to identify possible peripheral and central analgesic effects of extracts. To evaluate for a possible central anti-nociceptive effect, the hot plate test was used while for peripheral anti-nociceptive effects, the acetic acid-induced writhing test was employed.
Natural anti-inflammatory and analgesic agents based on plants are growing in popularity because of their minimal or non-existent side effects. The expansion of pharmacological mediators has been a chief focus of analgesic- and inflammatory-associated study regarding the outlook of their mass from a biomedicinal perspective. Therefore, the search for complementary and alternative sources has become increasingly vital to develop innovative types of natural ethnomedicinal drugs. Medicinal plants have been a chief reservoir of variety of biochemical compounds for several centuries and have been consumed broadly in raw or purified form to cure different ailments, including inflammation [13]. The use of plants and their parts and extracts as anti-inflammatory agents is widespread in many geographical areas. Atriplex vesicaria is used to treat inflammatory conditions and painful disorders [14]. The anti-inflammatory action of Salvia sagittata ethanolic extracts, and Zanthoxylum myriacanthum var. pubescens bark extracts has been documented [15,16]. Similarly, in Pakistan, various plants, including Aristolochia indica, Melilotus indicus, Tribulus terrestris and Cuscuta pedicellata, exhibit anti-inflammatory properties [17].
A. nitida (spach)-Endl. of the family Betulaceae is found in the Western Himalayas. In Punjab, its local name is Sharoliin, whereas in Kashmir it is known as Seril [18,19]. Alnus species contain diaryl heptanoids of 1,7-diphenylheptane skeleton and are reported to have different healing effects, such as antioxidant, anti-inflammatory and antitumor. About four hundred diarylheptanoids [20] have been identified from various species of Alnus depicting numerous pharmacological actions: anti-inflammatory [21,22], anti-influenza [23], hepatoprotective [24], etc. From A. nitida, two diarylheptanoids nitidone A and nitidone B have been isolated [25].
The leaves of A. nitida (spach)-Endl. are usually used as anti-inflammatory agents by local practitioners. They usually warm the leaves to cure boils and swellings [26]. Previously, we reported on the bark of A. nitida as a potential candidate for injuries and swelling [27]. However, A. nitida leaves have not been yet reported for any potential medicinal effects. Therefore, in the current study, we evaluated the anti-inflammatory and analgesic perspective of A. nitida leaves and fruit. The GC-MS analysis of A. nitida leaves and fruits extracts was carried out to identify the biochemical compounds responsible for these therapeutic effects.
2. Results
2.1. Acute Toxicity Studies
The animals did not show any kind of abnormal behaviors, such as water and food intake, attention, locomotion, appetite, analgesia, writhing, nasal release, aggressiveness, posture and urination and neither exhibited any change in the death rate.
2.2. Assessment of Anti-Inflammatory Activity
2.2.1. Carrageenan-Induced Paw Edema
The extracts/fractions of A. nitida leaves and fruits showed a dose- and time-dependent decline in edema (Table S1 in the Supplementary Materials). The percentage activity is presented in Figure 1. The activity became significant (p < 0.01) at the lowest dose after 2 h of carrageenan injection while edema was strongly inhibited after 4 h of injection. The A. nitida leaves chloroform fraction (ANLC) and A. nitida leaves methanolic extract (ANLM) at the highest dose decreased the edema to 91.23 ± 2.80% and 87.23 ± 1.52%, whereas diclofenac potassium and fluoxetine showed 86.63 ± 3.42% and 84.71 ± 2.46% inhibition after 4 h of injection (Figure 1A and Table S1). Likewise, in fruit ANFC after 4 h showed 87.86 ± 2.78% inhibition of edema (Figure 1B and Table S2).
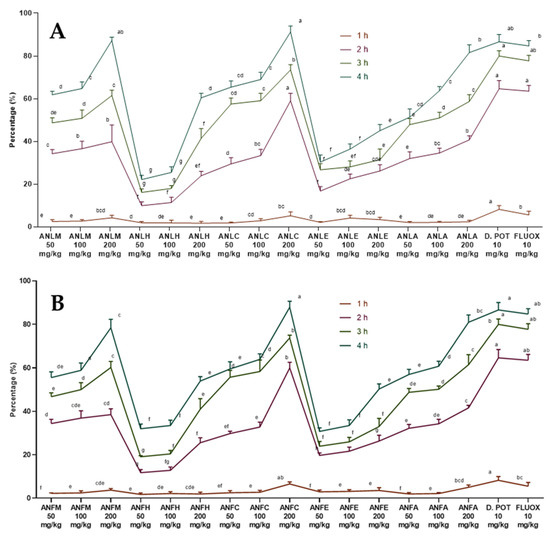
Figure 1.
Percentage inhibition of A. nitida on carrageenan-induced paw edema in rats (A) A. nitida leaves methanolic extract (ANLM), A. nitida leaves n-hexane fraction (ANLH), A. nitida leaves chloroform fraction (ANLC), A. nitida leaves ethyl acetate fraction (ANLE), A. nitida leaves aqueous fraction (ANLA), and (B) A. nitida fruit methanolic extract (ANFM), A. nitida fruit n-hexane fraction (ANFH), A. nitida fruit chloroform fraction (ANFC), A. nitida fruit ethyl acetate fraction (ANFE), A. nitida fruit aqueous fraction (ANFA). D.POT: Diclofenac potassium; FLUOX: Fluoxetine. Data were analyzed using seven replicates as mean ± SD by a two-way ANOVA followed by Bonferroni’s test. Different letters represent the significance level of p < 0.05 with respect to all other samples “a, b” with reference to positive controls, “c” with reference to methanolic extract and “d, e, f, g” with reference to n-hexane, chloroform, ethyl acetate and aqueous fractions, respectively.
2.2.2. Freunds’ Complete Adjuvant Induced Arthritis
The observations obtained from this test are reported in Figure 2 and Tables S3 and S4. After edema induction (14 days), ANLC and A. nitida fruit chloroform fraction (ANFC) at all doses (50, 100 and 200 mg/kg) showed markedly (p < 0.01) decreased edema. The edema was further significantly (p < 0.01) repressed after 21 days by chloroform, methanol and aqueous fractions of A. nitida leaves and fruit. However, multiple comparisons of many treatments showed that ANLC and ANFC at 200 mg/kg almost equally decreased in edema, as shown by diclofenac potassium and fluoxetine. However, the decrease in edema of these dosages was considerably higher (p < 0.01) relative to other samples.
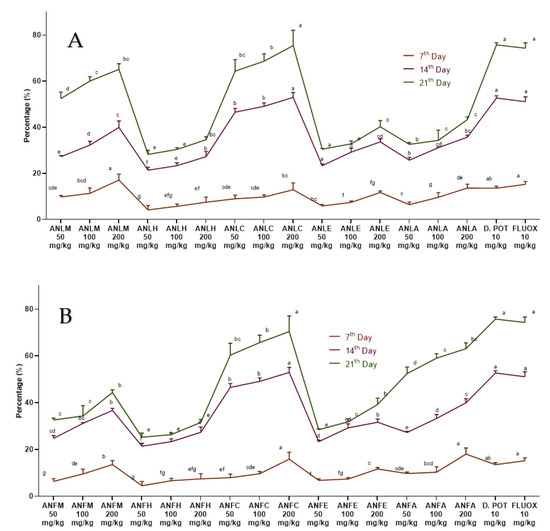
Figure 2.
Percentage inhibition of A. nitida on Freunds’ complete adjuvant induced arthritis. (A) A. nitida leaves methanolic extract (ANLM), A. nitida leaves n-hexane fraction (ANLH), A. nitida leaves chloroform fraction (ANLC), A. nitida leaves ethyl acetate fraction (ANLE), A. nitida leaves aqueous fraction (ANLA), and (B) A. nitida fruit methanolic extract (ANFM), A. nitida fruit n-hexane fraction (ANFH), A. nitida fruit chloroform fraction (ANFC), A. nitida fruit ethyl acetate fraction (ANFE), A. nitida fruit aqueous fraction (ANFA). D.POT: Diclofenac potassium; FLUOX: Fluoxetine. Data were analyzed using seven replicates as mean ± SD by two-way ANOVA followed by Bonferroni’s test. Different letters represent the significance level of p < 0.05 with respect to all other samples “a, b” with reference to positive controls, “c” with reference to methanolic extract and “d, e, f, g” with reference to n-hexane, chloroform, ethyl acetate and aqueous fraction, respectively.
2.2.3. Histamine Induced Paw Edema Method
A moderate antihistaminic effect was detected for ANLC, ANFC (200 mg/kg) after 2 h of histamine administration and edema volume was remarkably (p < 0.01) repressed after 4 h with percent inhibition of 73.91 ± 7.09% and 64.56 ± 3.09% as compared to the standard drug chlorpheniramine maleate (78.26 ± 3.54%). The methanol extract and aqueous fractions of both organs also exhibited good antihistaminic activity (Figure 3 and Tables S5 and S6).
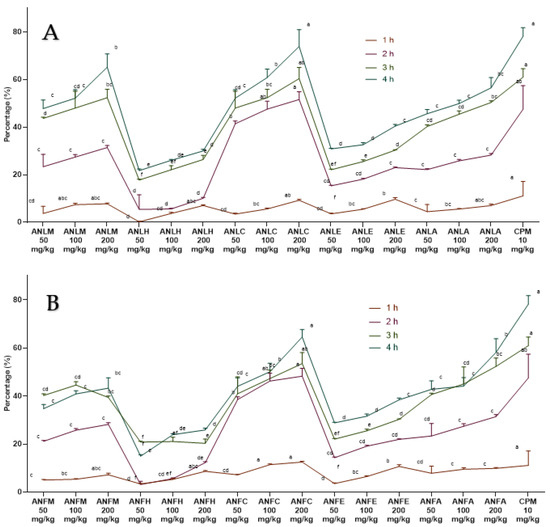
Figure 3.
Percentage inhibition of A. nitida on histamine induced paw edema in rats (A) A. nitida leaves methanolic extract (ANLM), A. nitida leaves n-hexane fraction (ANLH), A. nitida leaves chloroform fraction (ANLC), A. nitida leaves ethyl acetate fraction (ANLE), A. nitida leaves aqueous fraction (ANLA), and (B) A. nitida fruit methanolic extract (ANFM), A. nitida fruit n-hexane fraction (ANFH), A. nitida fruit chloroform fraction (ANFC), A. nitida fruit ethyl acetate fraction (ANFE), A. nitida fruit aqueous fraction (ANFA). CPM: chlorpheniramine maleate. Data were analyzed using seven replicates as mean ± SD by two-way ANOVA followed by Bonferroni’s test. Different letters represent the significance level of p < 0.05 with respect to all other samples “a” with reference to positive control, “b” with reference to methanolic extract and “c, d, e, f” with reference to n-hexane, chloroform, ethyl acetate and aqueous fraction, respectively.
2.2.4. Xylene-Induced Ear Edema
The results of leaves and fruits showing a dose- and time-dependent decline in edema are shown in Figure 4 and Tables S7 and S8. Saline rats exhibited an increase in ear weight (13.97 ± 0.01 mg), while the ANLC administration inhibited the gain in weight gain by 69.87 ± 0.19% (4.21 ± 0.02 mg) and 79.32 ± 0.58% (2.89 ± 0.08 mg) for 100 and 200 mg/kg, respectively. Likewise, ANFC remarkably (p < 0.01) inhibited a gain in weight by 75.42 ± 3.84% at 200 mg/kg dosage to the rats. The positive control diclofenac potassium and fluoxetine depicted 81.32 ± 0.15% (2.61 ± 0.02 mg) and 85.31 ± 0.04% (2.35 ± 0.04 mg) decrease in weight gain at 10 mg/kg, which was comparable to the doses of different fractions of A. nitida.
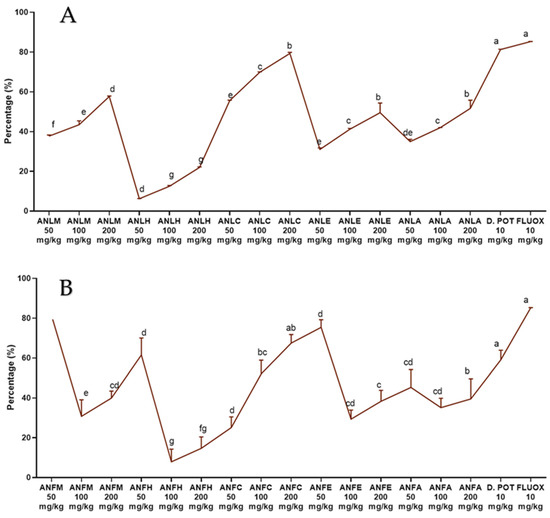
Figure 4.
Percentage inhibition of A. nitida on xylene-induced ear edema in rats (A) A. nitida leaves methanolic extract (ANLM), A. nitida leaves n-hexane fraction (ANLH), A. nitida leaves chloroform fraction (ANLC), A. nitida leaves ethyl acetate fraction (ANLE), A. nitida leaves aqueous fraction (ANLA) and (B) A. nitida fruit methanolic extract (ANFM), A. nitida fruit n-hexane fraction (ANFH), A. nitida fruit chloroform fraction (ANFC), A. nitida fruit ethyl acetate fraction (ANFE), A. nitida fruit aqueous fraction (ANFA). D.POT: Diclofenac potassium; FLUOX: Fluoxetine. Data were analyzed using seven replicates as mean ± SD by one way ANOVA followed by Tukey’s test. Different letters represent the significance level of p < 0.05 with respect to all other samples “a, b” with reference to positive controls, “c” with reference to methanolic extract and “d, e, f, g” with reference to n-hexane, chloroform, ethyl acetate and aqueous fraction, respectively.
2.3. Analgesic Activity of A. nitida
2.3.1. Hotplate Analgesic Test
The A. nitida leaves and fruits methanol extract and their fractions displayed a dose-dependent rise (Figure 5) in latency time and repressed pain perception in a pattern close to morphine and aspirin. The effect of ANLC was shown to be quite pronounced (p < 0.01) after 120 min at 200 mg/kg dosage relative to standard drug (Figure 5A and Table S9). In fruit, similar dose-dependent results were shown by ANFC with an inhibition of 62.31 ± 1.86% after 120 min of administration (Figure 5B and Table S10).
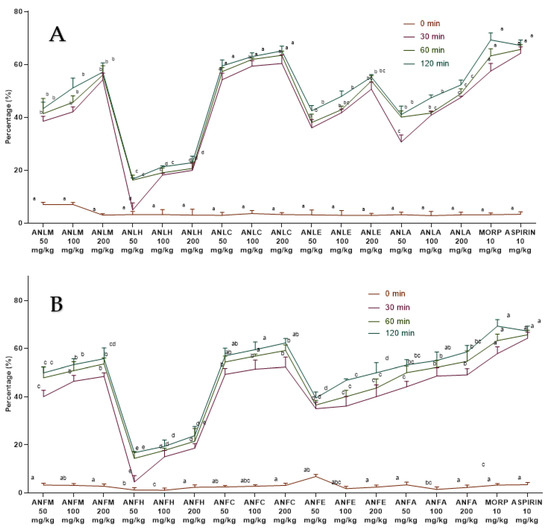
Figure 5.
Percentage inhibition of A. nitida on hot plate test in rats (A) A. nitida leaves methanolic extract (ANLM), A. nitida leaves n-hexane fraction (ANLH), A. nitida leaves chloroform fraction (ANLC), A. nitida leaves ethyl acetate fraction (ANLE), A. nitida leaves aqueous fraction (ANLA) and (B) A. nitida fruit methanolic extract (ANFM), A. nitida fruit n-hexane fraction (ANFH), A. nitida fruit chloroform fraction (ANFC), A. nitida fruit ethyl acetate fraction (ANFE), A. nitida fruit aqueous fraction (ANFA). MORP: morphine. Data were analyzed using seven replicates as mean ± SD by two-way ANOVA followed by Bonferroni’s test. Different letters represent the significance level of p < 0.05 with respect to all other samples “a, b” with reference to positive controls, “c” with reference to methanolic extract and “d, e, f, g” with reference to n-hexane, chloroform, ethyl acetate and aqueous fraction, respectively.
2.3.2. Acetic Acid-Induced Writhing Test
The ANLM and its resulting fractions ANLC and A. nitida leaves aqueous fraction (ANLA) considerably (p < 0.01) and dose-dependently (50, 100 and 200 mg/kg) decreased the number of abdominal contractions tempted after the acetic acid administration to the rats. The preventive effect of aspirin and morphine was non-considerably different from the defensive effect after exposure by ANLC and ANLM (50, 100 and 200 mg/kg) (Figure 6A and Table S11). Similar results were recorded for ANFC with 75.62 ± 2.06% inhibition of writhing (Figure 6B and Table S12). The writhing test in mice is regarded as being relatively reliable for the experimental evaluation of analgesic agents. However, using rats may have limitations as the challenge of the irritant can’t be repeated at short intervals and chronic challenges of irritants may cause injury to abdominal organs [28].
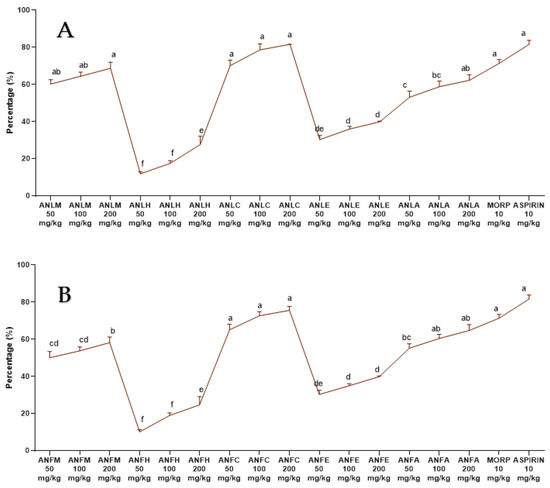
Figure 6.
Percentage inhibition of A. nitida on acetic acid induced writhing in rats (A) A. nitida leaves methanolic extract (ANLM), A. nitida leaves n-hexane fraction (ANLH), A. nitida leaves chloroform fraction (ANLC), A. nitida leaves ethyl acetate fraction (ANLE), A. nitida leaves aqueous fraction (ANLA) and (B) A. nitida fruit methanolic extract (ANFM), A. nitida fruit n-hexane fraction (ANFH), A. nitida fruit chloroform fraction (ANFC), A. nitida fruit ethyl acetate fraction (ANFE), A. nitida fruit aqueous fraction (ANFA). MORP: morphine. Data were analyzed using seven replicates as mean ± SD by one way ANOVA followed by Tukey’s test. Different letters represent the significance level of p < 0.05 with respect to all other samples: “a, b” with reference to positive controls, “c” with reference to methanolic extract and “d, e, f, g” with reference to n-hexane, chloroform, ethyl acetate and aqueous fraction, respectively.
2.4. GC-MS Analysis
The methanolic extract of A. nitida leaves and fruit was subjected to GC-MS analysis to identify the bioactive compounds responsible for anti-inflammatory and analgesic effects. The graph of GC-MS exploration of ANLM (Figure 7A,B) and A. nitida fruit methanolic extract (ANFM) are illustrated (Figure 7C,D). Moreover, detail of 23 eluted components from ANLM and 24 from ANFM are presented in Table 1 and Table 2, respectively. The identification of bioactive compounds was based on a comparison of mass spectra and relative retention times with NST/NBS and Wiley spectral libraries (Table 1 and Table 2). In ANLC, total 23 compounds were identified, and their relative abundance is documented on the basis of peak area consisting of 1 alkenes (1.2%), 3 alcohols (7.25%), 1 amine (0.07%), 1 arene (0.33%), 1 terpene alcohols (2.59%), 2 ketones (2.05%), 5 esters (68.07%), 1 phosphol (15.23%), 1 acid amide (0.10%), 1 terpenoid (1.68%), 5 alkanes (0.67%) and 1 carotenoid (0.11%). In ANFC, there were 2 acid (0.12%), 2 alkenes (2.18%), 1 thioether (0.41%), 1 isothiazolinones (0.07%), 1 terpene (0.07%), 5 alcohols (8.62%), 1 terpene alcohols (2.61%), 5 esters (80.57%), 2 ketones (0.51%), 1 ether (0.33%), 1 benzopyridines (1.83%), 1 alkane (0.07) and 1 fatty acid (0.10%).
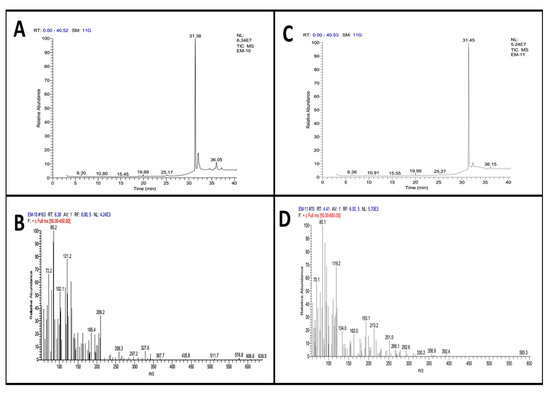
Figure 7.
GC-MS graph presenting retention time and m/z ration (A) leaves retention time and (C) fruits retention time, (B) leaves m/z ration and (D) fruit m/z ration.

Table 1.
GC-MS analysis of A. nitida leaves crude extract.

Table 2.
GC-MS analysis of A. nitida fruit crude extract.
3. Discussion
Plants have been a standard reservoir of drugs for centuries, especially in developing countries. In fact, many presently used drugs originated either directly or indirectly from plant sources [29]. In current study, firstly, we investigated the toxicity of A. nitida leaves and fruit extracts/fractions in mice. The administration dose of 2000 mg/kg did not produce any sign of toxicity nor morality. Next, we investigate the anti-inflammatory activity of A. nitida leaves and fruit extracts/fractions, as carrageenan is broadly utilized to prompt inflammation in rats to validate anti-inflammatory action of herbs. Edema development is a triphasic phenomenon involving the production of inflammatory markers. Early phase of edema (0–1 h) is endorsed by the discharge of bradykinin, histamine and 5-hydroxytryptamine. The second and third phase (1–6 h) results in enhanced levels of cyclooxygenase and prostaglandins in rats [30]. Younis et al. [31] described the inhibition of NO with Fraxinus xanthoxyloides extracts in RAW-264.7 cells. The treatment of ANLC and ANFC exhibited anti-edematous consequences during early and late phases of edema development by hindering the discharge of inflammatory mediators. The occurrence of phytol might be accountable towards the anti-inflammatory potential by inhibiting neutrophil transfer, which is partially due to the suppression of IL-1β, TNF-α and oxidative stress levels [32].
Rheumatoid arthritis is a systemic inflammatory syndrome affecting about 1% population worldwide. Increased production of IL-1 and TNF-α plays a vital role in its pathogenesis. IL-1 and TNF-α increase the production of metalloproteinases and propagation of synovial cells, which results in the degradation of cartilage [33]. The declaration of several inflammatory mediators, such as interleukin family and-TNF-α, have been assessed in serum by Trigonella foenum seeds extract in adjuvant-induced arthritis in animal models [34]. This study demonstrated that ANLC and ANFC showed an appreciable reduction of inflammation in adjuvant-induced arthritis in rats, suggesting its role in the destruction of pro-inflammatory mediators.
Histamine production is deliberated as a part of the immune mechanism and is released under inflammation responses. Histamine plays a role during inflammation through edema, vasodilation and recruitment of eosinophils and enhanced vascular permeability. It also regulates the proliferation of T cells, leukocyte function and migration, B cell maturation and differentiation and discharge of lysosomal enzymes in neutrophils [35]. During histamine induced paw edema, NO synthase plays a vital role. Therefore, suppressing nitric oxide, inflammation can be decreased as it is directly involved in macromolecular extravasation prompted by histamine induced inflammation. So, using this approach, NO suppression by Bixa orellena extract has been validated in rats [36]. In the present study, ANLC and ANFC significantly inhibited inflammation induced by histamine, signifying that the anti-inflammatory activity detected is because of the stabilization of the mast cell membranes and the inhibition of nitric oxide synthesis.
Xylene-induced ear edema is also a well-established animal model to evaluate the anti-acute inflammatory effect of A. nitida leaves and fruit. Xylene provokes behavioral effects tempted by nociceptors. These nociceptors activate the inflammatory phase involving series of stimuli comprising inflammation of the peripheral tissues and central sensitization [37]. Our study indicates that A. nitida extracts act as anti-inflammatory agents as they reduce ear edema in rats. These findings are in coherence with previous findings [38].
The folkloric remedies from the plant kingdom signify the presence of biologically active compounds. In the present study, ANLM and ANFM were subjected to GC-MS analysis and 23 compounds from leaves were eluted between 6.30–40.05 min with RSI range 369–948 and 24 compounds from fruit were eluted between 6.36–40.05 min with RSI range 350–934, respectively. From the analysis, the therapeutically important bio-constituents were detected, which might be responsible for potential anti-inflammatory and analgesic effects. The GC-MS screening of the methanolic extract of leaves exhibited the existence of Neophytadiene, an alkene which contains significant anti-inflammatory properties and might be contributing to the anti-inflammatory potential of the ANLC fraction [39]. Likewise, the presence of 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (terpene Alcohol), phenanthrene (arene) and phytol (diterpene alcohol), which are potential therapeutic anti-inflammatory agents, might contribute to the anti-inflammatory properties of ANLC and other fractions in crude extracts [32,40,41,42]. GC-MS screening of fruit has also shown the presence of neophytadiene, 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (terpene alcohol) and Vitamin E (alcohol), which have been reported to exhibit anti-inflammatory effects, might be contributing towards the anti-inflammatory aptitude of ANFM [39,42].
The hot plate method (central type) and writhing test (peripheral type) are well-established methods in assessing analgesic potential of medicinal agents. In the hot plate method, ANLC and ANFC presented the best analgesic activity among all tested samples. Morphine excites analgesic effect by the initiation of opioid-receptors and the comparison of morphine and extracts represents the fact that both might cause similar effects to reduce pain sensations. In the writhing test, chloroform fraction of A. nitida (leaves and fruit) represented prominent analgesic activity, signifying that it has an analgesic effect on both the peripheral as well as on the central nervous systems. The presence of phytol, a diterpene alcohol and vitamin E in ANLM and ANFM revealed by GC-MS analysis, was reported to possess strong anti-nociceptive properties, reducing sensitivity to painful stimuli [32,43]. The compound phytol has been described in plant extracts, revealing antioxidant as well as anti-nociceptive effects, it was also reported to cause a substantial rise in latency in the hot plate test [44]. These anti-inflammatory and analgesic effects of extracts specifically ANLC and ANFC are encouraging, so the new medicines with improved biological actions and minimum side effects need to be developed.
4. Materials and Methods
4.1. Plant Sampling
The leaves and fruits of A. nitida were collected from Charbagh town (34.8358° N, 72.4436° E) in the district of Swat, Khyber Pakhtunkhwa province, Pakistan. The samples were identified by Dr. Mir Ajab Khan, Professor at the Department of Plant Sciences, and a specimen was submitted after authentication (127963) to the Herbarium of Pakistan Museum of Natural History, Islamabad, Pakistan.
4.2. Extract Preparation
The leaves and fruits of A. nitida were shade-dried at room temperature for two weeks and powdered in a Willy Mill to 80-mesh size. The fine powder (1 kg each) was macerated in 3L of commercial grade methanol (97%) for 10 days with frequent shaking. After maceration the extract was filtered with Whatman No.1 filter paper. The process was repeated thrice, and the filtrate obtained was mixed before evaporation with rotary evaporator under vacuum. A part of the leaves (ANLM) and fruits (ANFM) methanolic extracts were suspended in distilled water and fractionated by adding solvents in increasing polarity: n-hexane (ANLH and ANFH), chloroform (ANLC and ANFC), ethyl acetate (ANLE and ANFE) and residual (ANLA and ANFA) were used as an aqueous extract [45]. It is experimentally proven that extraction solvents play an important role in the extraction of important bioactive compounds from plant. n-hexane and chloroform tend to extract nonpolar compounds, while ethyl acetate has a partial polar and partial nonpolar extraction ability. On the other hand, aqueous extract consists of polar compounds. So, by utilizing this range of solvents we can extract polar, amphoteric and nonpolar compounds present in plants, which might be responsible for the diverse pharmacological activities.
4.3. Animals
Rats (Sprague-Dawley) of either sex, weighing (170–220 g) at 6 weeks old were kept at 25 ± 2 °C with a 12 h light/dark cycle at the Animal Facility of Quad-i-Azam University Islamabad, Pakistan (QAUIP). Rats were provided with healthy feed and laboratory conditions. The ethical approval for the use of rats for research and care (275-BCH) was acquired from the Ethics Committee of QAUIP. In each experiment, rats were divided into normal control, inflammation/pain-induced control, positive controls and experimental groups with seven rats in each treatment, and test samples were administered orally in each group.
4.4. Acute Toxicity Study
The acute toxicity of all the extract/fractions was investigated according to Guideline 425 of the Organization for Economic Co-operation and Development (OECD, 2001). For this, rats were treated at single dose of 2000 mg/kg, i.p., and saline (10 mL/kg) separately for each/fraction of both samples (leaves and fruits). The doses were prepared in normal saline and administered orally. The toxicity was examined after 24 h, following the behavior and clinical symptoms for 15 days.
4.5. Assessment of Anti-Inflammatory Activity
4.5.1. Carrageenan-Induced Hind Paw Edema Test
Rats were divided into different groups comprising of 7 rats in each [46]. Group I was administered with 0.9% saline and the other groups were administered with samples (leaves/fruit) and two positive controls, including diclofenac potassium and fluoxetine at different concentrations. After 30 min, 0.1 mL (1%) of carrageenan was injected in the sub-plantar region of the right hind paw of each rat. The edema development was recorded with plethysmometer after 1, 2, 3 and 4 h of injection to rats and the percentage of inhibition was determined by the given formula. Data were analyzed using seven replicates by two-way ANOVA followed by Bonferroni’s test in GraphPad Prism with F value 102 for fruit and 99.6 for leaves, respectively.
4.5.2. Freunds’ Complete Adjuvant Induced Arthritis
To investigate the chronic inflammation Freunds’ complete adjuvant-induced arthritis model [47] was used. Rats were administered with extract/fractions as above while arthritis was induced with a 0.1 mL (1 mg) intradermal injection of Mycobacterium tuberculosis (heat-killed) into the sub-plantar region of the left hind paw of rats. Diclofenac potassium and fluoxetine served as positive controls. The inhibition of edema development was determined after 1, 2, 3 and 4 h, as previously described [47]. Data were analyzed using seven replicates by two-way ANOVA followed by Bonferroni’s test in GraphPad Prism with F value 106 for fruit and 105 for leaves, respectively.
4.5.3. Histamine Induced Paw Edema Method
Previously reported histamine induction method was used to evaluate the antihistamine potential of the samples [48]. Chlorpheniramine maleate (antihistamine) served as the positive control drug. After 30 min of dosage, 0.1 mL (1 mg) of histamine was injected into the sub-plantar region of the left hind paw of each rat. Paw volume was measured using plethysmometer at 1, 2, 3 and 4 h of histamine injection and percent inhibition was measured as formerly described. Data were analyzed using seven replicates by two-way ANOVA followed by Bonferroni’s test in GraphPad Prism with F value 43.8 for fruit and 42.2 for leaves, respectively.
4.5.4. Xylene-Induced Ear Edema Method
In this assay, edema inhibition was investigated as described previously [49]. Diclofenac potassium and fluoxetine served as positive controls. For edema development, 0.03 mL of xylene was applied on the left ear of each rat after 30 min of extract/fraction treatment. In this case, the left ear served as the control. After 15 min, the rats were euthanized using ketamine. Using a corkborer, 6 mm circular sections of both ears were taken, and the percent of inhibition was calculated. Data were analyzed using seven replicates by one way ANOVA followed by Tukey’s test in GraphPad Prism with F value 97 for fruit and 1271 for leaves, respectively.
4.6. Assessment of Analgesic Activity
4.6.1. Hotplate Analgesic Test
The hot plate procedure was used to determine analgesic activity as described previously [46]. Animals exhibiting latency time greater than 15 s on a Harvard Apparatus set at 55 ± 2 °C were excluded in this experiment. The animals were grouped and treated with the extract/fraction as described previously along with positive control drugs (morphine and aspirin) [50,51]. After 30 min of dosage, the latency time was recorded for each rat on a hotplate maintained at 55 ± 2 °C, showing the number of lickings and jumpings. The cut-off time was set at 30 sec in order to avoid tissue damage. The latency time was recorded after 30, 60, 90 and 120 min and percent analgesia was measured by given formula. Data were analyzed using seven replicates by two-way ANOVA followed by Bonferroni’s test in GraphPad Prism with F value 61 for fruit and 104 for leaves, respectively.
4.6.2. Acetic Acid-Induced Writhing Test
The scheme used for this test was previously described by Khan et al. [52]. Rats were treated with extract/fractions or positive control drugs (morphine and aspirin) as given above. Acetic acid (1%) was administered after 30 min of treatment and the writhing movements were documented after 5 min of acetic acid induction over a period of 10 min [53]. Data were analyzed using seven replicates by one way ANOVA followed by Tukey’s test in GraphPad Prism with F value 583 for fruit and 622 for leaves, respectively. The percentage of inhibition was determined by the formula:
4.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
GC-MS analysis of ANLM and ANFM was performed on a “Thermo GC-Trace Ultra Ver; 5.0” gas chromatography attached with a “Thermo MS DSQ II” for mass evaluation. Components were detached on a “ZB 5-MS Capillary Standard Non-Polar Column” with 600 m length with a 0.25-μm film thickness. During the experiment, temperature was elevated from 70 to 260 °C at a rate of 6 °C/min. The flow-rate of carrier gas, helium, was 10 mL/min, whereas injection volume of the samples was 1 μL. The identification of the chemical components was performed on the basis of comparison of their respective retention time and mass spectra with those attained from authentic sample and/or NIST/NBS and Wiley spectral libraries [54] and determined the mass spectral match on the basis of the Match Factor (SI) or Reverse Match Factor (RSI) thresholds as reported by Gujar et al. [55].
4.8. Statistical Analysis
All data are expressed as the Mean ± SD with statistical differences (p < 0.05) between groups using one-way ANOVA following Tukey’s multiple comparison test while two-way ANOVA with Bonferroni’s multiple comparisons test was applied to the rest of the experimental models using GraphPad Prism version 8.1.0 software. Sample size and animal number for each experimental group was calculated using the ARRIVE guidelines [56].
5. Conclusions
The anti-inflammatory effect of A. nitida extract/fractions were evaluated in the present study. Results obtained show that the chloroform fraction of A. nitida leaves and fruit have significant anti-inflammatory and analgesic properties. The synergistic effects of nonpolar and other phytoconstituents might be responsible for the anti-inflammatory and analgesic activity of the leaves and fruits of A. nitida. Taken together, these findings provide evidence for the anti-inflammatory and analgesic activity of A. nitida and could explain the benefits of the traditional use of this plant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27144582/s1, Table S1. Effect of A. nitida leaves extract and its fractions on carrageenan induced paw edema in rats; Table S2. Effect of A. nitida fruit extract and its fractions on carrageenan induced paw edema in rats; Table S3. Effect of A. nitida leaves extract and its fractions on Freunds’ complete adjuvant induced arthritis; Table S4. Effect of A. nitida fruit extract and its fractions on Freunds’ complete adjuvant induced arthritis; Table S5. Effect of A. nitida leaves extract and its fractions on histamine induced paw edema in rats; Table S6. Effect of A. nitida fruit extract and its fractions on histamine induced paw edema in rats; Table S7. Effect of A. nitida leaves extract and its fractions on xylene induced ear edema in rats; Table S8. Effect of A. nitida fruit and extract its fractions on xylene induced ear edema in rats; Table S9. Effect of A. nitida leaves extract and its fractions on hot plate test in rats; Table S10. Effect of A. nitida fruit extract and its fractions on hot plate test in rats; Table S11. Effect of A. nitida leaves extract and its fractions on acetic acid induced writhing in rats; Table S12. Effect of A. nitida fruit extract and its fractions on acetic acid induced writhing in rats.
Author Contributions
All authors contributed to the in vivo analysis. Specifically, M.S. drafted the manuscript; S.A.S. and S.A. carried out plant collection; H.I. and M.Z.B. conceived the study design; M.R.K. supervised the study; M.U.T. and M.U.I. carried out GC analysis; S.A.S. collection and identification of plant samples; S.S.A., S.M.A. and G.E.-S.B. revised the manuscript and provided funding. The authors declare that all data were generated in-house and that no paper mill was used. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taif University Researchers Supporting Project number (TURSP-2020/38), Taif University, Taif, Saudi Arabia, and Higher Education Commission Pakistan.
Institutional Review Board Statement
The Primate Facility of Quaid-i-Azam University Islamabad, Pakistan, was used for animals. The Institutional Ethics and Biosafety Committee approved the study with reference number 275-BCH and all safety measures were carried out to reduce the animal suffering.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Taif University Researchers Supporting Project number (TURSP-2020/38), Taif University, Taif, Saudi Arabia; Quad-i-Azam University Islamabad, Pakistan; and National University of Medical Sciences, Pakistan for providing facilities and support for this project.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Gou, K.-J.; Zeng, R.; Dong, Y.; Hu, Q.-Q.; Hu, H.-W.-Y.; Maffucci, K.G.; Dou, Q.-L.; Yang, Q.-B.; Qin, X.-H.; Qu, Y. Anti-inflammatory and analgesic effects of Polygonum orientale L. extracts. Front. Pharmacol. 2017, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M. Aromatherapy & immunity: How the use of essential oil aids immune potentiality: Part 3 immune responses to inflammation and essential oils useful in inhibiting them. Int. J. Aromather. 2001, 11, 220–224. [Google Scholar]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; De la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Richardson, J.D.; Vasko, M.R. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine induces microglia activation and the release of proinflammatory mediators in rat brain via H1R or H4R. J. Neuroimmune Pharmacol. 2020, 15, 280–291. [Google Scholar] [CrossRef]
- Snekhalatha, U.; Anburajan, M.; Venkatraman, B.; Menaka, M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Z. Für Rheumatol. 2013, 72, 375–382. [Google Scholar] [CrossRef]
- Tripathi, K. Cardiovascular Drugs. In Essentials of Medical Pharmacology, 5th ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2003; pp. 453–454. [Google Scholar]
- Raffa, R.B. Pharmacology of oral combination analgesics: Rational therapy for pain. J. Clin. Pharm. 2001, 26, 257–264. [Google Scholar] [CrossRef]
- Afilalo, M.; Morlion, B. Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician 2013, 16, 27–40. [Google Scholar] [CrossRef]
- Thomas, M.C.; Diuretics, A.C.E. Inhibitors and NSAIDs—The triple whammy. Med. J. Aust. 2000, 172, 184–185. [Google Scholar] [CrossRef]
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18,820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [Green Version]
- León, J.A.M.; Santisteban, A.G.; Fuentes, D.P.; Puebla, Y.G.; Rodríguez, E.T. In vitro anti-inflammatory activity of aqueous, ethanolic and ethereal extracts of rhizomes, leaves and stems of Anredera vesicaria. J. Anal. Pharm. Res. 2018, 7, 459–461. [Google Scholar]
- Tubon, I.; Zannoni, A.; Bernardini, C.; Salaroli, R.; Bertocchi, M.; Mandrioli, R.; Vinueza, D.; Antognoni, F.; Forni, M. In Vitro Anti-Inflammatory Effect of Salvia sagittata Ethanolic Extract on Primary Cultures of Porcine Aortic Endothelial Cells. Oxidative Med. Cell Longev. 2019, 2019, 6829173. [Google Scholar] [CrossRef]
- Zhang, H.L.; Gan, X.Q.; Fan, Q.F.; Yang, J.J.; Zhang, P.; Hu, H.B.; Song, Q.S. Chemical constituents and anti-inflammatory activities of Maqian (Zanthoxylum myriacanthum var. pubescens) bark extracts. Sci. Rep. 2017, 7, 45805. [Google Scholar] [CrossRef] [Green Version]
- Naz, R.; Ayub, H.; Nawaz, S.; Islam, Z.U.; Yasmin, T.; Bano, A.; Wakeel, A.; Zia, S.; Roberts, T.H. Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complement. Altern. Med. 2017, 17, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, S.; Chopra, R. The wealth of India. CSIR India 1948, 1, 8. [Google Scholar]
- Stewart, R.R. Flora of West Pakistan: An Annotated Catalogue of the Vascular Plants of West Pakistan and Kashmir; Fakhri Printing Press: Karachi, Pakistan, 1972; pp. 1–1028. [Google Scholar]
- Lv, H.; She, G. Naturally occurring diarylheptanoids—A supplementary version. Rec. Nat. Prod. 2012, 6, 321–333. [Google Scholar]
- Jin, W.; Cai, X.F.; Na, M.; Lee, J.J.; Bae, K. Diarylheptanoids from Alnus hirsuta inhibit the NF-kB activation and NO and TNF-alpha production. Biol. Pharm. Bull. 2007, 30, 810–813. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.W.; Kim, J.H.; Jeong, D.W.; Ahn, K.H.; Toh, S.H.; Surh, Y.J. Inhibition of cyclooxygenase-2 expression by diarylheptanoids from the bark of Alnus hirsuta var. sibirica. Biol. Pharm. Bull. 2000, 23, 517–518. [Google Scholar] [CrossRef] [Green Version]
- Tung, N.H.; Kwon, H.J.; Kim, J.H.; Ra, J.C.; Ding, Y.; Kim, J.A.; Kim, Y.H. Anti-influenza diarylheptanoids from the bark of Alnus japonica. Bioorg. Med. Chem. Lett. 2010, 20, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, H.J.; Jung, S.Y.; Yook, C.S.; Jin, C.; Lee, Y.S. A new diarylheptanoid glycoside from the stem bark of Alnus hirsuta and protective effects of diarylheptanoid derivatives in human HepG2 cells. Chem. Pharm. Bull. 2010, 58, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, I.N.; Ahmad, V.U.; Zahoor, A.; Ahmed, A.; Khan, S.S.; Khan, A.; Hassan, Z. Two new diarylheptanoids from Alnus nitida. Nat. Prod. Commun. 2010, 5, 1787–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.A.; Shah, N.A.; Ullah, S.; Alam, M.M.; Badshah, H.; Ullah, S.; Mumtaz, A.S. Documenting the indigenous knowledge on medicinal flora from communities residing near Swat River (Suvastu) and in high mountainous areas in Swat-Pakistan. J. Ethnopharmacol. 2016, 182, 67–79. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, M.R.; Shah, S.A.; Majid, M.; Ismail, H.; Maryam, S.; Batool, R.; Younis, T. Investigations on anti-inflammatory and analgesic activities of Alnus nitida Spach (Endl). stem bark in Sprague Dawley rats. J. Ethnopharmacol. 2017, 198, 407–416. [Google Scholar] [CrossRef]
- Fukawa, K.; Kawano, O.; Hibi, M.; Misaki, N.; Ohba, S.; Hatanaka, Y. A method for evaluating analgesic agents in rats. J. Pharmacol. Methods 1980, 4, 251–259. [Google Scholar]
- Arayne, M.S.; Sultana, N.; Mirza, A.Z.; Zuberi, M.H.; Siddiqui, F.A. In vitro hypoglycemic activity of methanolic extract of some indigenous plants. Pak. J. Pharm. Sci. 2007, 20, 268–273. [Google Scholar]
- Majid, M.; Khan, M.R.; Shah, N.A.; Ul Haq, I.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complement. Altern. Med. 2015, 15, 349. [Google Scholar] [CrossRef] [Green Version]
- Younis, T.; Khan, M.R.; Sajid, M. Protective effects of Fraxinus xanthoxyloides (Wall.) leaves against CCl 4 induced hepatic toxicity in rat. BMC Complement. Altern. Med. 2016, 16, 407. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.O.; Sousa, F.B.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.; Sousa, D.P.; Aragao, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fund. Clin. Pharm. 2014, 28, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Noguchi, N. Dynamics of antioxidant action of vitamin E. Acc. Chem Res. 2004, 37, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.; Kavitha Ch, N.; Babu, S.M.; Reddy, V.P.; Latha, A.K. Effect of ethanol extract of Trigonella foenum graecum (Fenugreek) seeds on Freund’s adjuvant-induced arthritis in albino rats. Inflammation 2012, 35, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Benly, P. Role of histamine in acute inflammation. J. Pharm. Sci. Res. 2015, 7, 373–376. [Google Scholar]
- Yong, Y.K.; Zakaria, Z.A.; Kadir, A.A.; Somchit, M.N.; Ee Cheng Lian, G.; Ahmad, Z. Chemical constituents and antihistamine activity of Bixa orellana leaf extract. BMC Complement. Altern. Med. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharm. Rev. 2001, 53, 597–652. [Google Scholar]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar]
- Carretero, M.E.; Lopez-Perez, J.L.; Abad, M.J.; Bermejo, P.; Tillet, S.; Israel, A.; Noguera, P.B. Preliminary study of the anti-inflammatory activity of hexane extract and fractions from Bursera simaruba (Linneo) Sarg. (Burseraceae) leaves. J. Ethnopharmacol. 2008, 116, 11–15. [Google Scholar] [CrossRef]
- Jananie, R.; Priya, V.; Vijayalakshmi, K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. NY Sci. J. 2011, 4, 16–20. [Google Scholar]
- Yang, L.; Qin, L.H.; Bligh, S.W.; Bashall, A.; Zhang, C.F.; Zhang, M.; Wang, Z.T.; Xu, L.S. A new phenanthrene with a spirolactone from Dendrobium chrysanthum and its anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 3496–3501. [Google Scholar] [CrossRef]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Kim, J.H.; Gao, X.; Zhou, J.-L.; Lee, I.; Chung, K.; Chung, J.M. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 2006, 122, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.; de Oliveira, G.A.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [Green Version]
- Al-Qurainy, F.; Khan, S.; Ali, M.A.; Al-Hemaid, F.M.; Tarroum, M.; Ashraf, M. Authentication of Ruta graveolens and its adulterant using internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. Pak. J. Bot. 2011, 43, 1613–1620. [Google Scholar]
- Muhammad, N.; Saeed, M.; Khan, H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement. Altern. Med. 2012, 12, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latha, R.M.; Geetha, T.; Varalakshmi, P. Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen. Pharm. 1998, 31, 601–606. [Google Scholar] [CrossRef]
- Shah, S.M.M. A possible anti-inflammatory mechanism of ethyl acetate extracts of Teucrium stocksianum Bioss. BMC Complement. Altern. Med. 2015, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Núñez Guillén, M.E.; da Silva Emim, J.A.; Souccar, C.; Lapa, A.J. Analgesic and anti-inflammatory activities of the aqueous extract of Plantago major L. Int. J. Pharm. 1997, 35, 99–104. [Google Scholar] [CrossRef]
- Singh, V.P.; Jain, N.K.; Kulkarni, S.K. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001, 915, 218–226. [Google Scholar] [CrossRef]
- Yahagi, T.; Yakura, N.; Matsuzaki, K.; Kitanaka, S. Inhibitory effect of chemical constituents from Artemisia scoparia Waldst. et Kit. on triglyceride accumulation in 3T3-L1 cells and nitric oxide production in RAW 264.7 cells. J. Nat. Med. 2014, 68, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Saeed, M.; Gilani, A.H.; Muhammad, N.; Haq, I.U.; Ashraf, N.; Rehman, N.U.; Haleemi, A. Antipyretic and anticonvulsant activity of Polygonatum verticillatum: Comparison of rhizomes and aerial parts. Phytother. Res. 2013, 27, 468–471. [Google Scholar] [CrossRef]
- Singh, N.; Rajini, P. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kim, J.Y.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta Med. 2005, 71, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.; Anderson, T.; Cavagnino, D.; Patel, A. Comparative analysis of mass spectral matching for confident compound identification using the advanced electron ionization source for GC-MS. Thermoscientific 2018, 10598, 1–7. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).