Silver Nanoparticle-Functionalised Nitrogen-Doped Carbon Quantum Dots for the Highly Efficient Determination of Uric Acid
Abstract
:1. Introduction
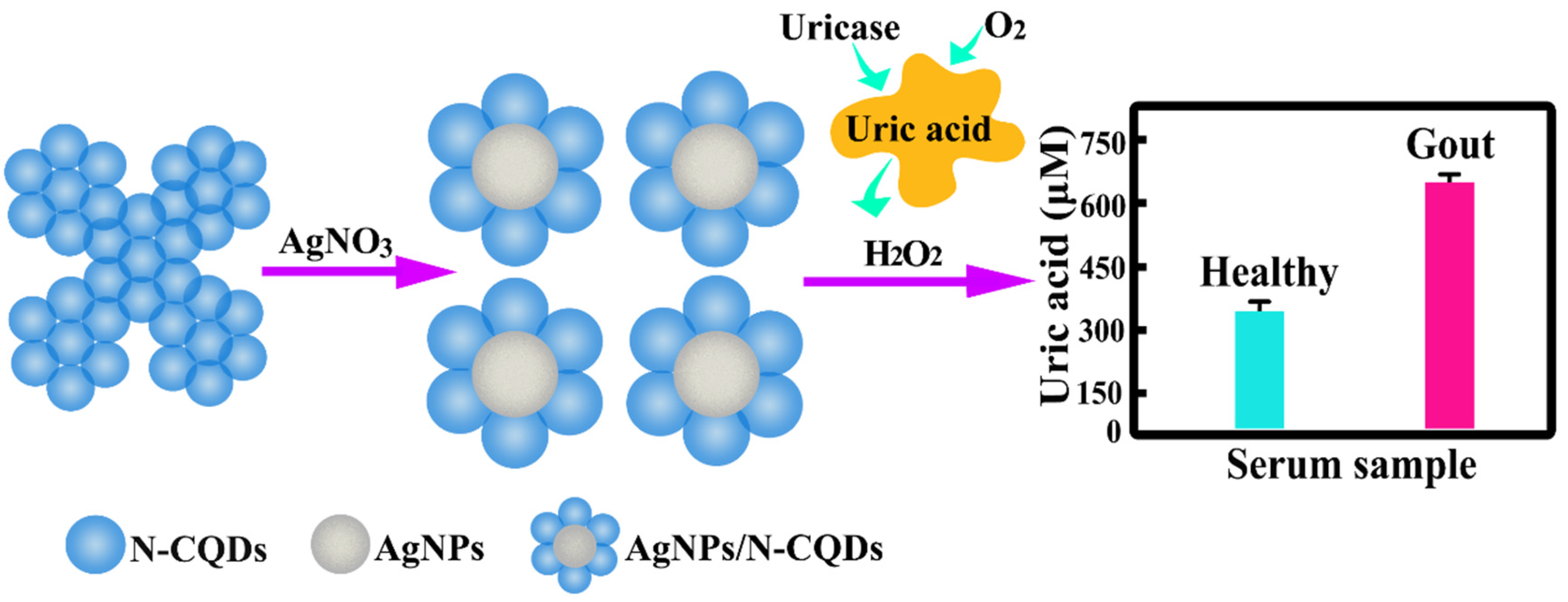
2. Results and Discussion
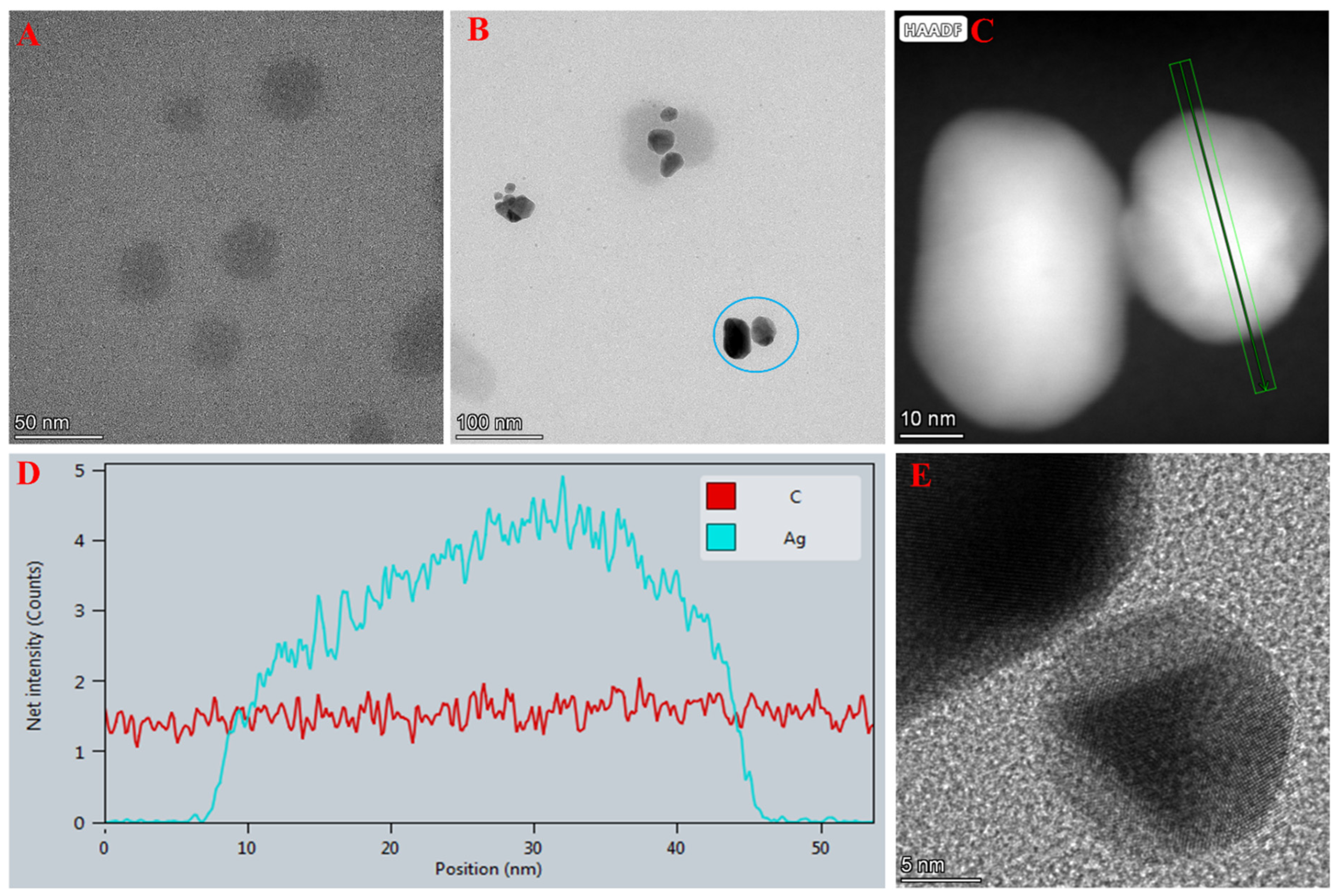
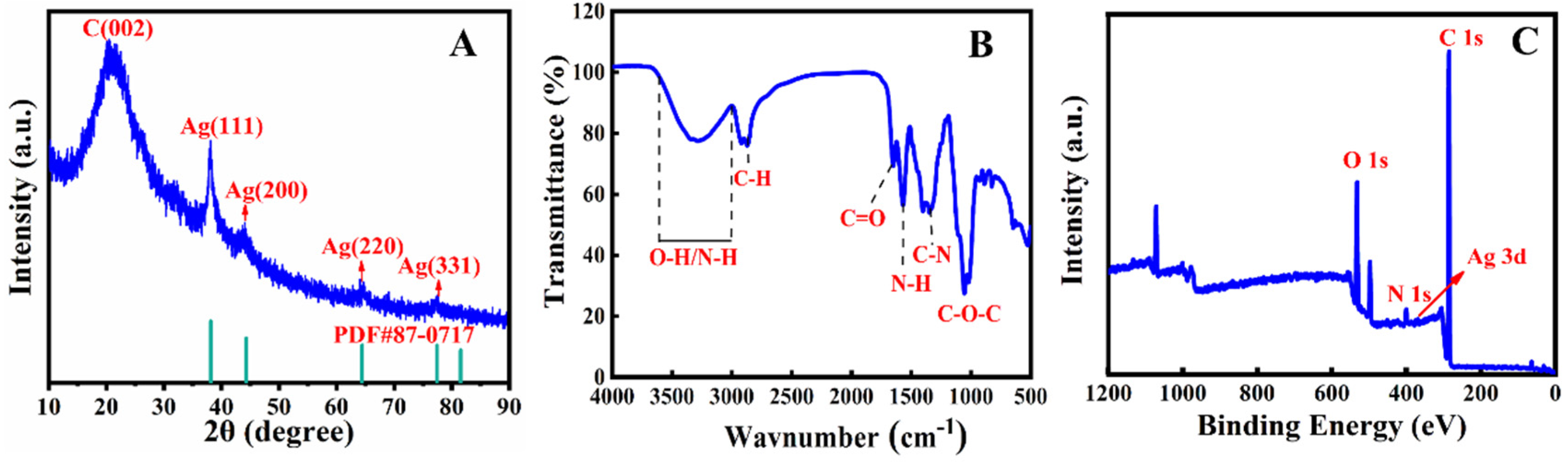
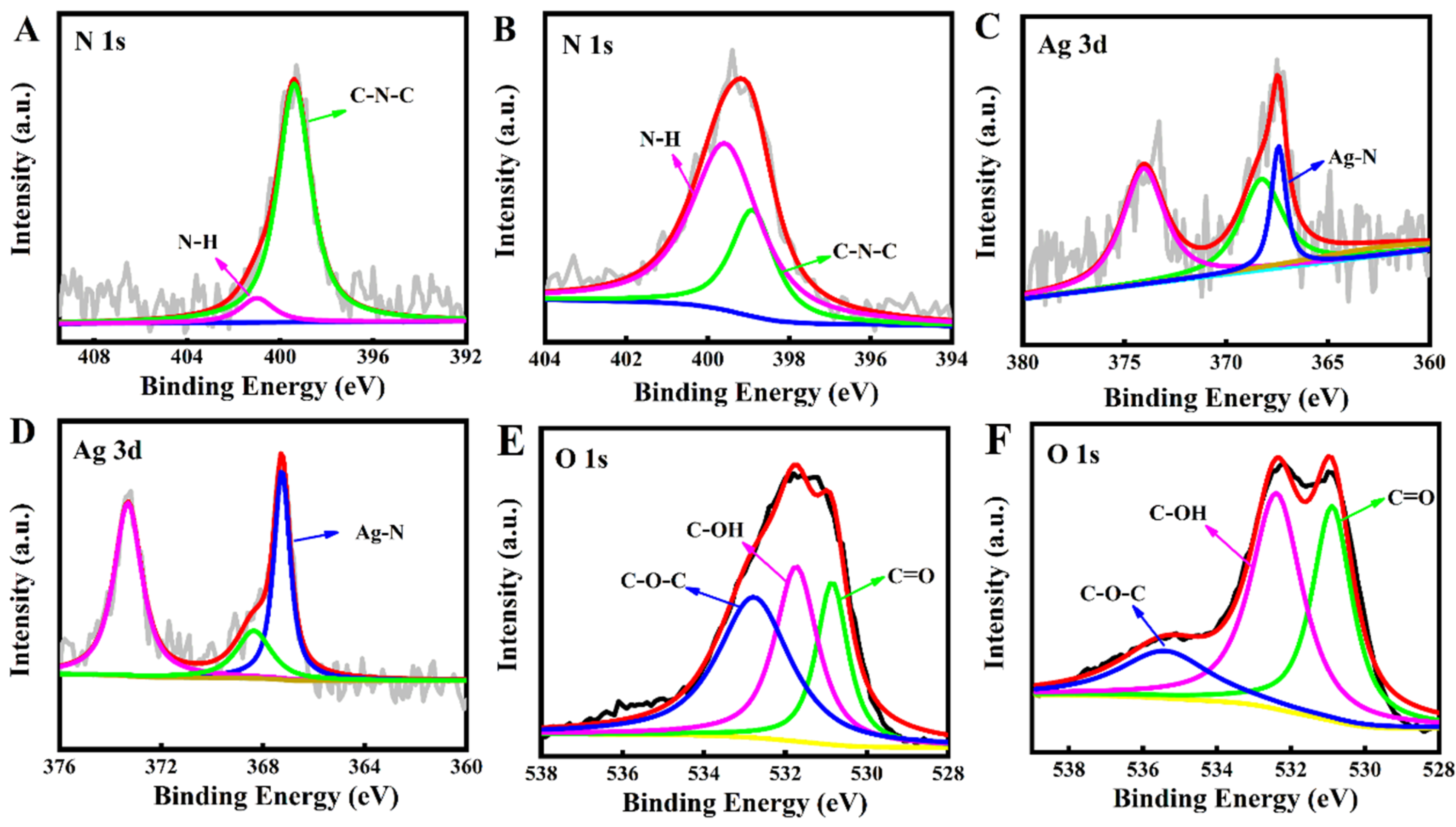
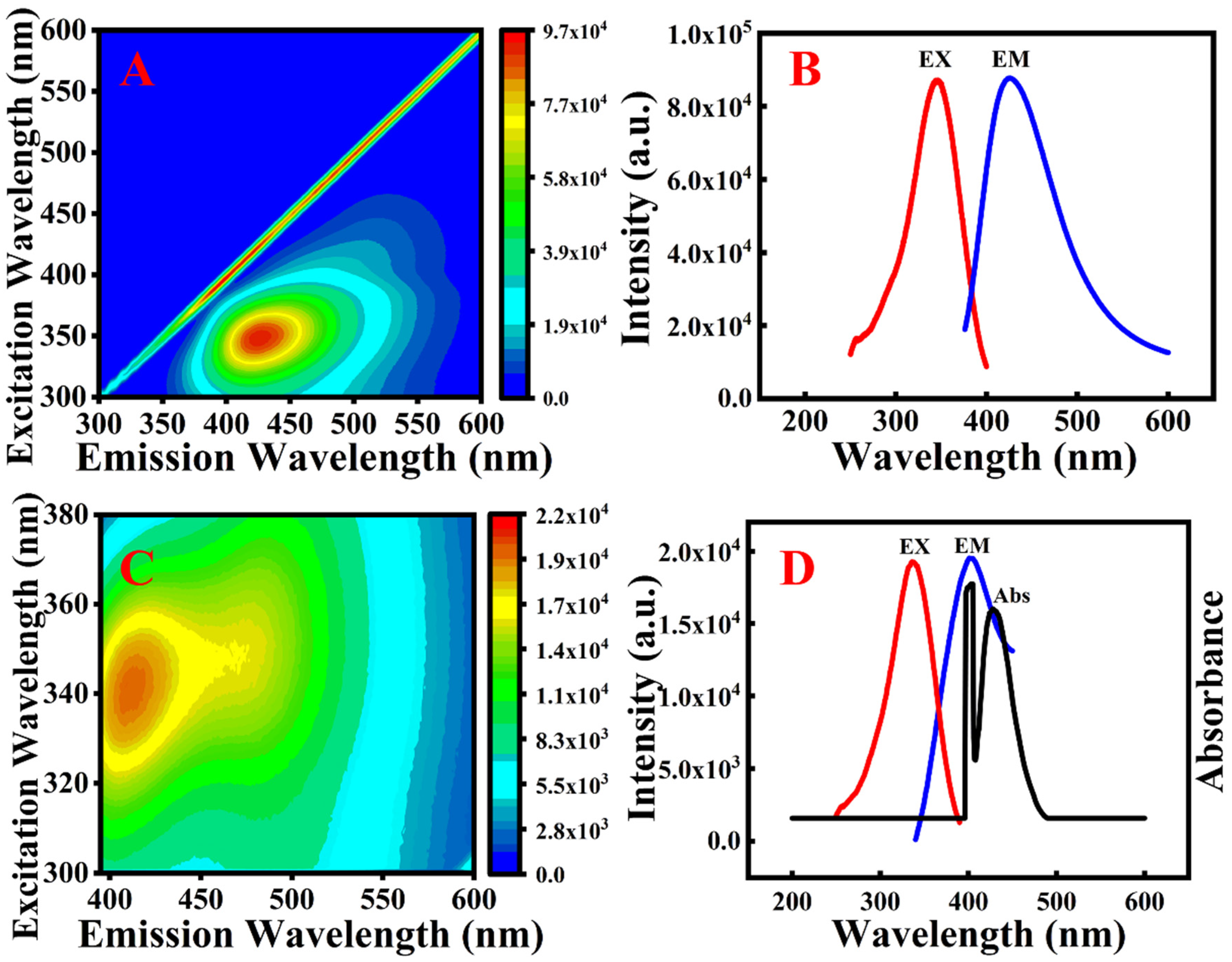
2.1. Characterisation of the AgNP/N-CQDs
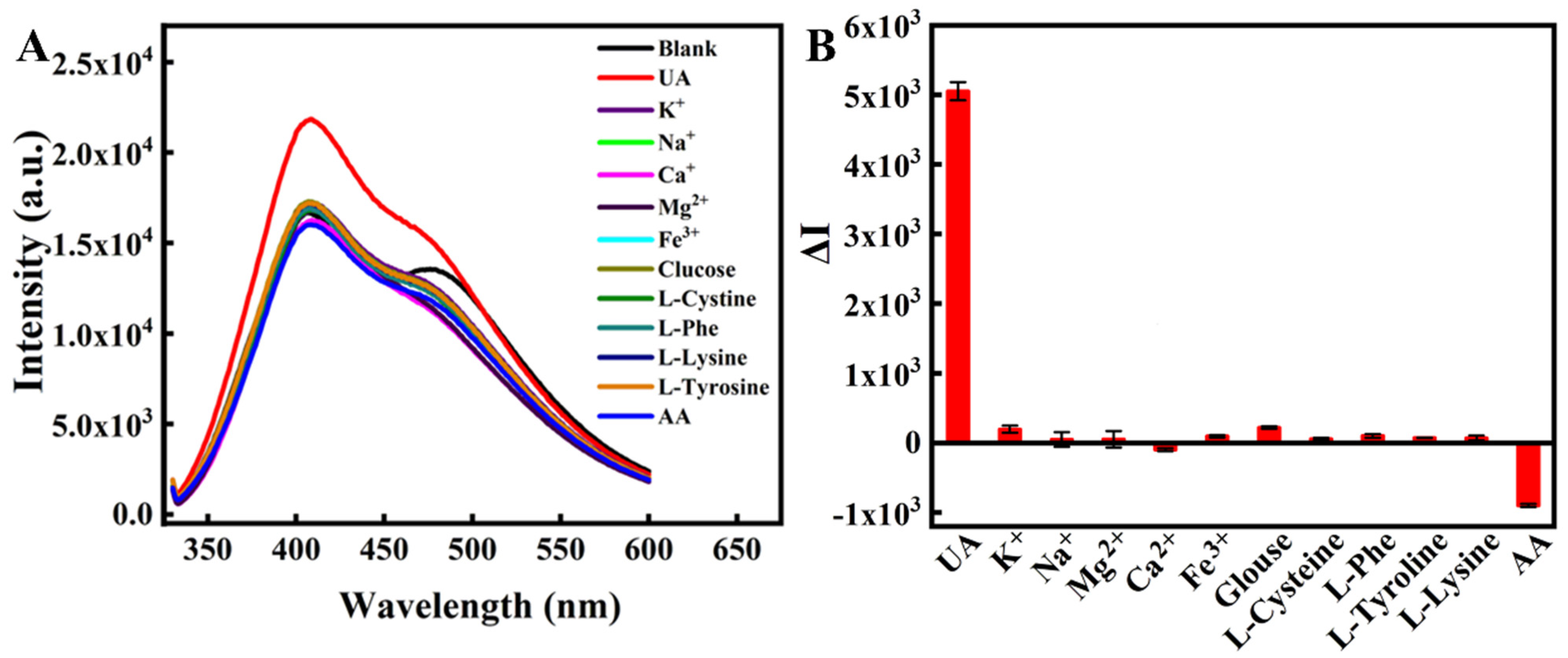
2.2. Selectivity of the Assay
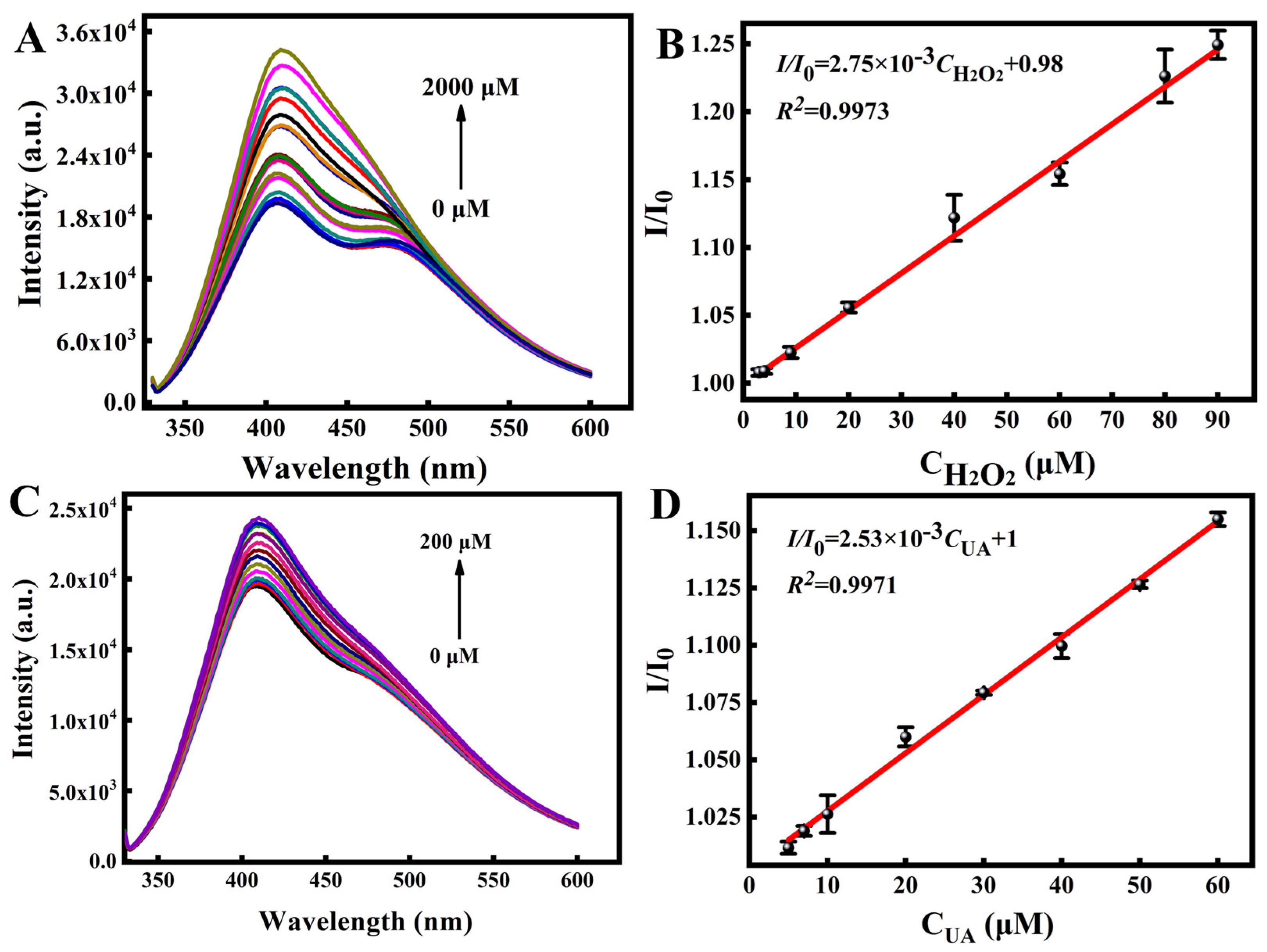
2.3. Fluorescence Response of the AgNPs/N-CQDs to Uric Acid
2.4. Biological Sample Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Measurements
3.3. Synthesis of the AgNPs/N-CQDs Composite
3.4. Detection of Uric Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, T.; Meng, Q.; Sun, Z.S.; Chen, Y.Y.; Ai, W.; Zhao, Z.; Kang, D.W.; Dong, Y.; Liang, R.P.; Wu, T.; et al. Novel human urate transporter 1 inhibitors as hypouricemic drug candidates with favorable druggability. J. Med. Chem. 2020, 63, 10829–10854. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Fang, A.J.; Wen, Y.Q.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. Rapid and highly-sensitive uric acid sensing based on enzymatic catalysis-induced upconversion inner filter effect. Biosens. Bioelectron. 2016, 86, 109–114. [Google Scholar] [CrossRef]
- Zhang, F.M.; Ma, P.Y.; Deng, X.Y.; Sun, Y.; Wang, X.H.; Song, D.Q. Enzymatic determination of uric acid using water-soluble CuInS/ZnS quantum dots as a fluorescent probe. Microchim. Acta 2018, 185, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Li, H.C.; Guo, B.; Wei, L.J.; Chen, B.; Zhang, Y.Y. Gold nanoclusters as switch-off fluorescent probe for detection of uric acid based on the inner filter effect of hydrogen peroxide-mediated enlargement of gold nanoparticles. Biosens. Bioelectron. 2017, 91, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; McAdams-Demarco, M.; Folsom, A.R. Serum uric acid, gout, and venous thromboembolism: The atherosclerosis risk in communities study. Thromb. Res. 2016, 144, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.X.; Tang, C.; Han, X.X.; Zhang, H.C.; Guo, F.N.; Yang, T.; Wang, J.H. Simultaneous and sensitive detection of multiple small biological molecules by microfluidic paper-based analytical device integrated with zinc oxide nanorods. Talanta 2021, 232, 122499–122507. [Google Scholar] [CrossRef]
- Sun, Y.P.; Chen, J.; Qi, H.Y.; Shi, Y.P. Graphitic carbon nitrides modifed hollow fiber solid phase microextraction for extraction and determination of uric acid in urine and serum coupled with gas chromatography-mass spectrometry. J. Chromatogr. B 2015, 1004, 53–59. [Google Scholar] [CrossRef]
- Zhou, S.; Zuo, R.X.; Zhu, Z.; Wu, D.; Vasa, K.; Deng, Y.W.; Zuo, Y.G. An eco-friendly hydrophilic interaction HPLC method for the determination of renal function biomarkers, creatinine and uric acid, in human fluids. Anal. Methods 2013, 5, 1307–1311. [Google Scholar] [CrossRef]
- Minta, D.; Moyseowicz, A.; Gryglewicz, S.; Gryglewicz, G. A promising electrochemical platform for dopamine and uric acid detection based on a Polyaniline/Iron Oxide-Tin Oxide/Reduced Graphene Oxide ternary composite. Molecules 2020, 25, 5869. [Google Scholar] [CrossRef]
- Liu, M.W.; He, Y.; Zhou, J.; Ge, Y.L.; Zhou, J.G.; Song, G.W. A “naked-eye’’ colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal. Chim. Acta 2020, 1103, 134–142. [Google Scholar] [CrossRef]
- Dey, N.; Bhattacharya, S. Nanomolar level detection of uric acid in blood serum and pest-infested grain samples by an amphiphilic probe. Anal. Chem. 2017, 89, 10376–10383. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, R.; Biswas, S. A pyrene-functionalized metal-organic framework for nonenzymatic and ratiometric detection of uric acid in biological fluid via conformational change. Inorg. Chem. 2019, 58, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Jiao, Y.; Hu, F.; Yang, Y.L. Green synthesis of carbon dots from pork and application as nanosensors for uric acid detection. Spectrochim. Acta A 2018, 190, 360–367. [Google Scholar] [CrossRef]
- Deng, J.H.; Lu, Q.J.; Hou, Y.X.; Liu, M.L.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. Nanosensor composed of nitrogen-doped carbon dots and gold nanoparticles for highly selective detection of cysteine with multiple signals. Anal. Chem. 2015, 87, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Yang, Y.; Liu, W.; Ding, F.; Zhao, Q.B.; Zou, P.; Wang, X.X.; Rao, H.B. Colorimetric and fluorometric determination of uric acid based on the use of nitrogen-doped carbon quantum dots and silver triangular nanoprisms. Microchim. Acta 2018, 185, 281–289. [Google Scholar] [CrossRef]
- Kalaiyarasan, G.; Veerapandian, M.; Jebamercy, G.; Balamurugan, K.; Joseph, J. Amygdalin-functionalized carbon quantum dots for probing β-glucosidase activity for cancer diagnosis and therapeutics. ACS Biomater. Sci. Eng. 2019, 5, 3089–3099. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.W.; Zhou, S.Q. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Qu, X.C.; Niu, Q.Q.; Tian, C.Q.; Fan, C.J.; Ye, B.F. A geen synthesis of highly fluorescent nitrogen-doped graphene quantum dots for the highly sensitive and selective detection of Mercury(II) ions and biothiols. Anal. Methods 2016, 8, 1565–1571. [Google Scholar] [CrossRef]
- Deng, Y.C.; Tang, L.; Feng, C.Y.; Zeng, G.M.; Wang, J.J.; Lu, Y.; Liu, Y.N.; Yu, J.F.; Chen, S.; Zhou, Y.Y. Construction of plasmonic Ag and nitrogen-doped graphene quantum dots codecorated ultrathin graphitic carbon nitride nanosheet composites with enhanced photocatalytic activity: Full-spectrum response ability and mechanism insight. ACS Appl. Mater. Inter. 2017, 9, 42816–42828. [Google Scholar] [CrossRef]
- Chen, S.; Hai, X.; Chen, X.W.; Wang, J.H. In situ growth of silver nanoparticles on graphene quantum dots for ultrasensitive colorimetric detection of H2O2 and glucose. Anal. Chem. 2014, 86, 6689–6694. [Google Scholar] [CrossRef]
- Gutierrez, S.; Wing, G.; Yoshida, M.; Yacaman, J. HAADF study of Au-Pt core-shell bimetallic nanoparticles. Appl. Phys. A 2004, 79, 481–487. [Google Scholar] [CrossRef]
- Sinduja, B.; John, S.A. Silver nanoparticles capped with carbon dots as a fluorescent probe for the highly sensitive “off–on” sensing of sulfide ions in water. Anal. Bioanal. Chem. 2019, 411, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Hou, J.Z.; Huo, D.Q.; Wang, X.F.; Li, J.W.; Xu, G.L.; Bian, M.H.; He, Q.; Hou, C.J.; Yang, M. Green emitting carbon dots for sensitive fluorometric determination of cartap based on its aggregation effect on gold nanoparticles. Microchim. Acta 2019, 186, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.; Liu, Y.H.; Zhu, M.G.; Gao, E.; Zhao, B.; Yang, X.P. Ultrabright full color carbon dots by fine-tuning crystal morphology controllable synthesis for multicolor bioimaging and sensing. ACS Appl. Mater. Inter. 2019, 11, 27259–27268. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Borah, S.C.; Kumari, R.; Saikia, R.; Deshmukh, A.B.; Shelke, M.V.; Sengupta, P.; Szunerits, S.; Boukherroub, R. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf B Biointerfaces 2013, 105, 128–136. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zhang, J.T.; Xiong, Z.G.; Yong, Y.; Zhao, X.S. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Jia, J.; Lu, W.J.; Li, L.; Gao, Y.F.; Jiao, Y.; Han, H.; Dong, C.; Shuang, S.M. Orange emission N doped carbon dots as fluorescent and colorimetric dual-mode probe for nitrite detection and cellular imaging. J. Mater. Chem. B 2020, 8, 2123–2127. [Google Scholar] [CrossRef]
- Yue, X.Y.; Zhou, Z.J.; Wu, Y.M.; Jie, M.S.; Li, Y.; Guo, H.B.; Bai, Y.H. A green carbon dots-based fluorescent sensor for selective and visual detection of nitrite triggered by the nitrite-thiol reaction. New J. Chem. 2020, 44, 8503–8511. [Google Scholar] [CrossRef]
- Li, W.J.; Kong, C.Y.; Qin, G.P.; Ruan, H.B.; Fang, L. P-type conductivity and stability of Ag-N codoped ZnO thin films. J. Alloy. Compd. 2014, 609, 173–177. [Google Scholar] [CrossRef]
- Zhuo, S.J.; Fang, J.; Wang, J.; Zhu, C.Q. One-step hydrothermal synthesis of silver-doped carbon quantum dots for highly selestive detection of uric acid. Methods Appl. Fluoresc. 2020, 8, 015005. [Google Scholar] [CrossRef]
- Zheng, Q.T.; Xiong, L.; Yu, L.; Wu, D.; Yang, C.X.; Xiao, Y.X. An enzyme-free fluorescent sensing platform for the detection of uric acid in human urine. J. Lumin. 2021, 236, 118076–118083. [Google Scholar] [CrossRef]
- Li, F.; Rui, J.H.; Yan, Z.Y.; Qiu, P.; Tang, X.M. A highly sensitive dual-read assay using nitrogen-doped carbon dots for the quantitation of uric acid in human serum and urine samples. Microchim. Acta 2021, 188, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.N.; Li, Y.H. AIEgens-based fluorescent covalent organic framework in construction of chemiluminescence resonance energy transfer system for serum uric acid detection. Microchim. Acta 2021, 188, 254–263. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhu, G.B.; Cao, W.D.; Liu, Z.J.; Pan, C.G.; Hu, W.J.; Zhao, W.Y.; Sun, J.F. A novel ratiometric fluorescent probe for the detection of uric acid in human blood based on H2O2-mediated fluorescence quenching of gold/silver nanoclusters. Talanta 2019, 191, 46–53. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.D.; Chu, X.; Liu, Y.; Zhang, Y.; Xiao, Q. Nitrogen, cobalt Co-doped fluorescent magnetic carbon dots as ratiometric fluorescent probes for cholesterol and uric acid in human blood serum. ACS Omega 2019, 4, 9333–9342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Zhu, L.J.; Lu, Q.J.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. A dual-signal colorimetric and ratiometric fluorescent nanoprobe for enzymatic determination of uric acid by using silicon nanoparticles. Microchim. Acta 2019, 186, 754–761. [Google Scholar] [CrossRef]
- Qi, W.J.; Zhao, M.Y.; Fu, Y.L.; He, H.K.; Tian, X.; Wu, D.; Zhang, Y.; Hu, P.P. Fluorescent detection of uric acid through photoinduced electron transfer using luminol-terbium(III) nanoparticles synthesized via aggregation-induced fluorescence strategy. Dye. Pigment. 2020, 172, 107797–107802. [Google Scholar] [CrossRef]

| Method | Probe | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| Fluorescence | Ag-CQDs | 0.005–100 | 3.5 × 10−4 | [29] |
| Fluorescence | Eu-BDC@FM | 0–200 | 0.6 | [30] |
| Fluorescence | N-CDs | 0.5–150 | 0.06 | [31] |
| Fluorescence | TPE@SNW-1 | 10.0–150 | 4.94 | [32] |
| Fluorescence | Au/AgNCs | 5.0–50 | 5.1 | [33] |
| Fluorescence | N, Co-CDs | 0.01–100 | 3.4 × 10−3 | [34] |
| Fluorescence | SiNPs | 10.0–800 | 0.75 | [35] |
| Fluorescence | Luminol-TbNPs | 0.1–50 | 2.8 × 10−2 | [36] |
| Fluorescence | AgNPs/N-CQDs | 2.0–60 | 0.53 | This work |
| Sample | Sample Number | Original Amount (μmol/L) | RSD (%) |
|---|---|---|---|
| Healthy serum | #1 | 412 ± 26 | 6.3 |
| #2 | 324 ± 7 | 2.2 | |
| #3 | 210 ± 16 | 7.6 | |
| #4 | 293 ± 17 | 5.8 | |
| #5 | 296 ± 8 | 2.7 | |
| #6 | 241 ± 18 | 7.5 | |
| #7 | 268 ± 8 | 3.0 | |
| #8 | 215 ± 3 | 1.4 | |
| #9 | 286 ± 3 | 1.0 | |
| #10 | 273 ± 6 | 2.2 | |
| Gout serum | #1 | 688 ± 5 (657) a | 0.73 |
| #2 | 522 ± 7 (502) a | 1.3 | |
| #3 | 798 ± 13 (775) a | 1.6 | |
| #4 | 770 ± 17 (797) a | 2.2 | |
| #5 | 530 ± 12 (522) a | 2.3 | |
| #6 | 548 ± 19 (523) a | 3.5 | |
| #7 | 502 ± 13 (501) a | 2.6 | |
| #8 | 608 ± 16 (584) a | 2.6 | |
| #9 | 658 ± 12 (664) a | 1.8 | |
| #10 | 610 ± 20 (584) a | 3.3 |
| Sample | Original Amount (μmol/L) | Spiked Amount (μmol/L) | Recovered Amount (μmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 150 | 483 | 107 | 2.4 | ||
| Healthy serum | 323 ± 14 | 300 | 612 | 96.3 | 2.7 |
| 450 | 786 | 103 | 3.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Du, S.; Tian, F.; Long, X.; Xie, S.; Tang, S.; Bao, L. Silver Nanoparticle-Functionalised Nitrogen-Doped Carbon Quantum Dots for the Highly Efficient Determination of Uric Acid. Molecules 2022, 27, 4586. https://doi.org/10.3390/molecules27144586
Zhang Q, Du S, Tian F, Long X, Xie S, Tang S, Bao L. Silver Nanoparticle-Functionalised Nitrogen-Doped Carbon Quantum Dots for the Highly Efficient Determination of Uric Acid. Molecules. 2022; 27(14):4586. https://doi.org/10.3390/molecules27144586
Chicago/Turabian StyleZhang, Qianchun, Shuxin Du, Fengling Tian, Xixi Long, Siqi Xie, Shan Tang, and Linchun Bao. 2022. "Silver Nanoparticle-Functionalised Nitrogen-Doped Carbon Quantum Dots for the Highly Efficient Determination of Uric Acid" Molecules 27, no. 14: 4586. https://doi.org/10.3390/molecules27144586
APA StyleZhang, Q., Du, S., Tian, F., Long, X., Xie, S., Tang, S., & Bao, L. (2022). Silver Nanoparticle-Functionalised Nitrogen-Doped Carbon Quantum Dots for the Highly Efficient Determination of Uric Acid. Molecules, 27(14), 4586. https://doi.org/10.3390/molecules27144586






