1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, which is frequently diagnosed in patients older than 60 years of age. It is a progressive neurodegenerative disorder that causes brain atrophy, neuronal death, and the loss of neuronal connections, leading to irreversible memory loss and cognitive impairment. In advanced stages of AD, complications from severe loss of brain function—such as dehydration, malnutrition, or infection—may be fatal. It has been challenging for clinicians to distinguish between the cognitive decline related to normal aging and that of AD. Currently, an absolute diagnosis of AD can only be determined by postmortem histopathological examination, although in vivo imaging is making major advances in pursuit of this diagnosis. Upon examining the postmortem brains of AD patients, the disease is shown to be associated with neuropathological features including the accumulation of amyloid β (Aβ) plaques, also known as senile plaques, and neurofibrillary tangles (NFT) of highly phosphorylated tau in the brain [
1]. The deposition of Aβ plaques in the brain increases slowly and eventually plateaus in regions of early amyloid formation, such as the temporal associative isocortex, soon after the onset of the cognitive degeneration symptoms, or even during the preclinical stages of the disease, while NFT formation continues throughout the course of the disease [
2]. Accordingly, Aβ plaque accumulation may start years before the onset of illness and even emerge well in advance of NFT formation [
3]. The Aβ fragment, Aβ42, is shown to be dominant in Aβ plaques in AD brains [
4], resulting from increased γ-secretase activity [
5]. Additionally, mutations in the APP gene, which generally lead to an increase in Aβ peptide concentration, have been shown to cause some forms of early-onset familial AD, whereas mutations in tau genes without the presence of Aβ plaques can lead to non-Alzheimer dementias with neurofibrillary pathology [
6]. Commonly used experimental animal models of AD are transgenic mice that overexpress human genes associated with familial AD (FAD) that lead to the formation of Aβ plaques. In particular, this study utilizes 5 × FAD transgenic mouse models, that contain three AD-linked mutations in human APP and two AD-linked mutations in PSEN1 genes: the Florida (I716V), Swedish (K670N/M671L), and London (V717I) mutations in APP; and the M146L and L286V mutations in PSEN1 [
7].
Although the root cause of most Alzheimer’s cases still remains unknown, numerous findings based on genetic evidence signify a higher level of Aβ deposits in the brain, which correlates with a lower concentration in cerebrospinal fluid, which is essential in the pathogenesis of AD [
8]. Therefore, imaging Aβ plaques in the brain may be helpful for AD risk prediction, the diagnosis of cerebral amyloidosis, and evaluating the efficacy of anti-amyloid treatments. Positron emission tomography (PET) studies of Aβ accumulation in AD have shown clinical utility [
9]. Neuroimaging studies have conducted extensive research on the accumulation of Aβ plaques in the brains of AD patients: some further support previous pathological findings [
1]. Successful clinical research trials utilizing [
11C]PiB for diagnosing Aβ in AD patients and assessing the therapeutic efficacy of drugs for AD have facilitated the development and translation of PET for clinical use [
10]. Clinical studies of [
11C]PiB showed that it could detect Aβ deposition in AD patients and possibly identify persons with mild cognitive impairment [
11,
12]. The highly selective binding of [
11C]PiB for insoluble amyloid plaques in the AD human brain was significantly correlated with post-mortem quantitative analyses [
13]. However, the extensive clinical use of [
11C]PiB is limited by the [
11C] agent’s short half-life and the requirement of a cyclotron facility on site. Hence, several fluorine-18-labeled PET radiotracers are now being clinically used as they offer the advantages of a longer half-life than carbon-11-labeled agents. [
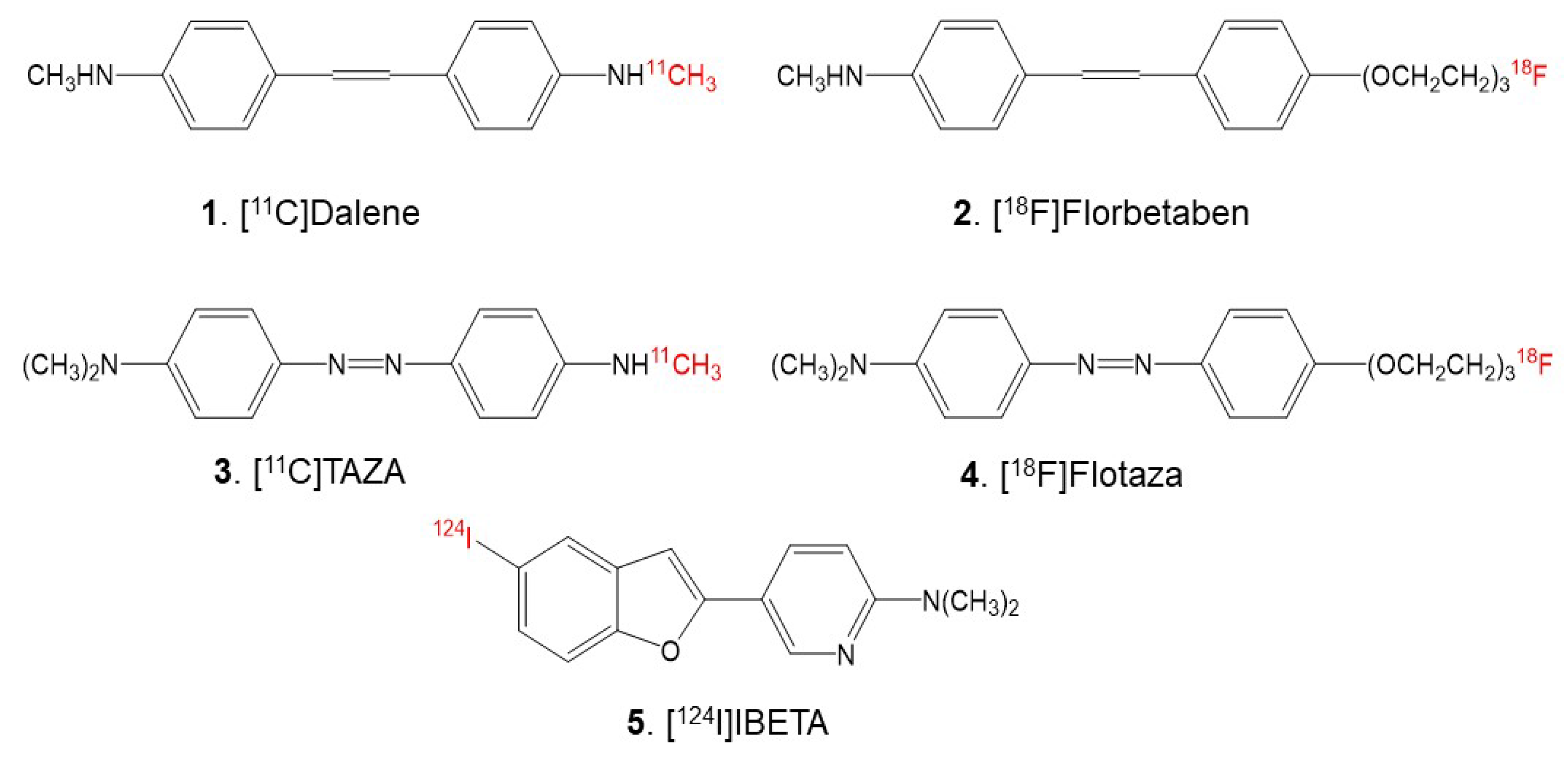
18F]Florbetabir was the first fluorine-18 agent approved for clinical use, followed by [
18F]Florbetaben (
Figure 1) and [
18F]Flutemetamol.
Since the heteroatoms in the “azo” functionality of [
11C]TAZA (
Figure 1) result in a higher affinity to Aβ plaques and lower white matter binding in postmortem human AD brain slices in comparison to [
11C]PiB, a fluorine-18 analog of [
11C]TAZA—[
18F]Flotaza (
Figure 1)—has recently been developed and evaluated for Aβ plaque imaging [
14,
15]. [
18F]Flotaza yielded significantly higher binding in the gray matter regions where Aβ plaques were located by anti-Aβ immunostains, compared to [
11C]TAZA. Additionally, [
18F]Flotaza showed a higher gray matter to white matter ratio than [
18F]Florbetaben, a close fluoropegylated analog of [
11C]Dalene (
Figure 1), which exhibited a low amount of gray matter binding [
15].
Single-photon emission computed tomography (SPECT) studies of Aβ plaque imaging have not advanced as much as PET studies [
16]. Radioiodinated derivatives using iodine-125 and iodine-123 for imaging Aβ plaque has been pursued by several investigators [
16,
17,
18,
19]. Biodistribution studies and preliminary results in mouse models using iodine-123 pyridyl benzofuran and imidazo[1,2-a]pyridine derivatives have been reported [
17,
18]. Promising preliminary human SPECT imaging studies using [
123I]ABC577 were reported and compared with known PET imaging agents [
19]. However, further studies on the in vivo evaluation of the radiodinated SPECT agents in either transgenic mouse models or human AD have not been reported.
In our pursuit for a radioiodinated Aβ plaque imaging agent for in vivo and in vitro studies, we identified
125I-labeled 5-(5-Iodobenzofuran-2-yl)-N,N- dimethylpyridin-2-amine as a suitable candidate because of its high affinity [
17]. Previous studies with this molecule included radiosynthesis using iodine-125 and normal mice biodistribution studies. Iodine-123 radiolabeling and in vivo SPECT imaging in transgenic mice were not carried out, although preliminary human AD brain slices in vitro exhibited promising properties [
17]. Thus, our interest is in further evaluating the properties of this iodobenzofuran derivative. Deiodination has not been reported in previous studies, and it remains unclear if radio-deiodination is a concern with the in vivo imaging studies with these derivatives.
The goal of this study is to assess the feasibility of using 5-(5-[
124/125I]iodobenzofuran-2-yl)-N,N-dimethylpyridin-2-amine (acronym: [
124/125I]IBETA) (
Figure 1) for Aβ plaque imaging. Iodine-124 (longer half-life of 4.2 days)-labeled PET imaging agents may be suitable for extended PET imaging. Additionally, since PET has a higher resolution compared to SPECT, it may be useful for the identification of small brain regions in transgenic AD mice brains with Aβ plaque accumulation. Thus, we report the following: (a) the radiosynthesis of [
124/125I]IBETA; (b) the evaluation of drug effects on the binding of [
125I]IBETA in a 5 × FAD mouse model known to have robust Aβ plaques [
20] and postmortem human AD brain slices in vitro; (c) the evaluation of the binding of [
124I]IBETA to Aβ plaque in postmortem human AD and 5 × FAD mouse brain slices in vitro; and (d) preliminary PET/CT studies of [
124I]IBETA in a living 5 × FAD AD mouse model.
2. Materials and Methods
2.1. General Methods
All chemicals and solvents were purchased from Aldrich Chemical and Fisher Scientific. Deionized water was acquired from the Millipore Milli-Q Water Purification System. Iodine-125 sodium iodide, carrier free (specific activity = 643 MBq/µg) in 0.01 N NaOH, was purchased from American Radiolabeled Chemicals, Inc., St. Louis, MO, USA. Iodine-124 sodium iodide, carrier free (specific activity = >1000 GBq/µmole) in 0.01N NaOH, was purchased from 3D Imaging, LLC., Little Rock, AK, USA. Iodine-124 and iodine-125 radioactivity were counted in a Capintec CRC-15R dose calibrator while low level counting was carried out in a Capintec Caprac-R well-counter. All solvents used were provided by Fisher Scientific. Gilson high-performance liquid chromatography (HPLC) was used for semi-preparative reverse-phase column chromatography with a UV detector set at dual wavelengths of 254 nm and 280 nm as well as a radioactivity detector. A semi-preparative HPLC column of 100 × 250 mm and a 10-micron Econosil C18 reverse-phase was used. Analytical thin-layer chromatography (TLC) was used to monitor the reactions (Baker-flex, Phillipsburg, NJ, USA). Radio TLC was scanned on an AR-2000 imaging scanner (Eckart & Ziegler, Berlin, Germany). Electrospray mass spectra were obtained from a Model 7250 mass spectrometer (Micromass LCT). Proton NMR spectra were recorded on a Bruker OM EGA 500-MHz spectrometer. Brain slices were prepared at 10µm thick using the Lieca 1850 cryotome. In vitro-labeled brain sections were exposed to phosphor films (Perkin Elmer Multisensitive, Medium MS) and read using the Cyclone Phosphor Imaging System (Packard Instruments). Analysis of in vitro autoradiographs was performed using Optiquant acquisition and analysis software.
2.2. Animals
All animal studies were approved by the Institutional Animal Health Care and Use Committee of University of California, Irvine. IACUC PHS Assurance number, D16-00259 (A3416-01)
2.2.1. C57BL/6 Mice
Adult mice were used in this study (28 g). Mice were purchased from Jackson Laboratory and housed under controlled temperatures of 22 °C ± 1 °C, in a 12 h light–dark cycle, on at 6:00 AM, with water and food chow ad libitum.
2.2.2. 5 × FAD Transgenic Mice
The 5 × FAD transgenic line of mice (MMRRC hemizygous; 4 male and 4 female) were purchased from Jackson Laboratory. Female mice were 20–28 g and male mice weighed 26–38 g. All mice were housed in standard cages.
2.3. Human Tissue
Human postmortem brain tissue samples were obtained from Banner Sun Health Research Institute, Sun City, AZ brain tissue repository for in vitro experiments. Age- and gender-matched AD brain and cognitively normal (CN) brain tissue samples were used for the study. Human postmortem brain slices were obtained from chunks of frozen tissue on a Leica 1850 cryotome cooled to −20 °C. Iodine-124 and Iodine-125 autoradiographic studies were carried out by exposing tissue samples on storage phosphor screens (Perkin Elmer Multisensitive, Medium MS and tritium sensitive phosphor screens). The apposed phosphor screens were read and analyzed by OptiQuant acquisition and the analysis program of the Cyclone Storage Phosphor System (Packard Instruments Co., Boston, MA, USA). Adjacent slices were used for immunostaining with anti-Aβ. All postmortem human brain studies were approved by the Institutional Biosafety Committee of the University of California, Irvine.
2.4. Synthesis
5-(5-Bromobenzofuran-2-yl)-N,N-dimethylpyridin-2-amine (Br-BETA) was synthesized using previously published methods [
17]. 5-bromobenzofuran-2-boronic acid (72 mg, 0.299 mmol) was treated with 5-iodo-N,N-dimethylpyridin-2-amine (66 mg, 0.266 mmol) in the presence of (Ph
3P)
4Pd (36 mg, 0.0312 mmol) in 2M Na
2CO
3 (aq.)/dioxane (15 mL, 2:1). The reaction mixture was stirred under reflux overnight. The mixture was removed from the heat and allowed to cool to room temperature. Then, 1M NaOH (2 mL) was added to the mixture while being stirring at room temperature. The organic phase was extracted using ethyl acetate and was then dried over MgSO
4. After filtration, the solvent was removed by vacuum rotary evaporation, and the residue was further purified using silica gel thin-layer chromatography (TLC) in hexane/ethyl acetate (4:1). The experimental yield was low (6.66%). Mass spectra (ESI):
m/
z 318 ([M + H]
+, 100%).
N,N-Dimethyl-5-(5-(tributylstannyl)benzofuran-2-yl)pyridin-2-amine (
Figure 2) was synthesized based on previously published methods [
17]. The bromobenzofuran intermediate (5.6 mg, 0.0177 mmol) was treated with bis(tributyltin) (0.0150 mL) in the presence of (Ph
3P)
4Pd (1.869 mg, 0.00162 mmol) in a dioxane/triethylamine solvent mixture (0.8 mL, 3:1). The mixture was stirred at 90
°C overnight. The product was extracted using silica gel TLC in hexane/ethyl acetate (4:1) as the product traveled faster and had a higher R
f value than the starting material. The experimental yield was low (16.0%). Mass spectra (ESI):
m/
z 528 ([M + H]
+, 100%).
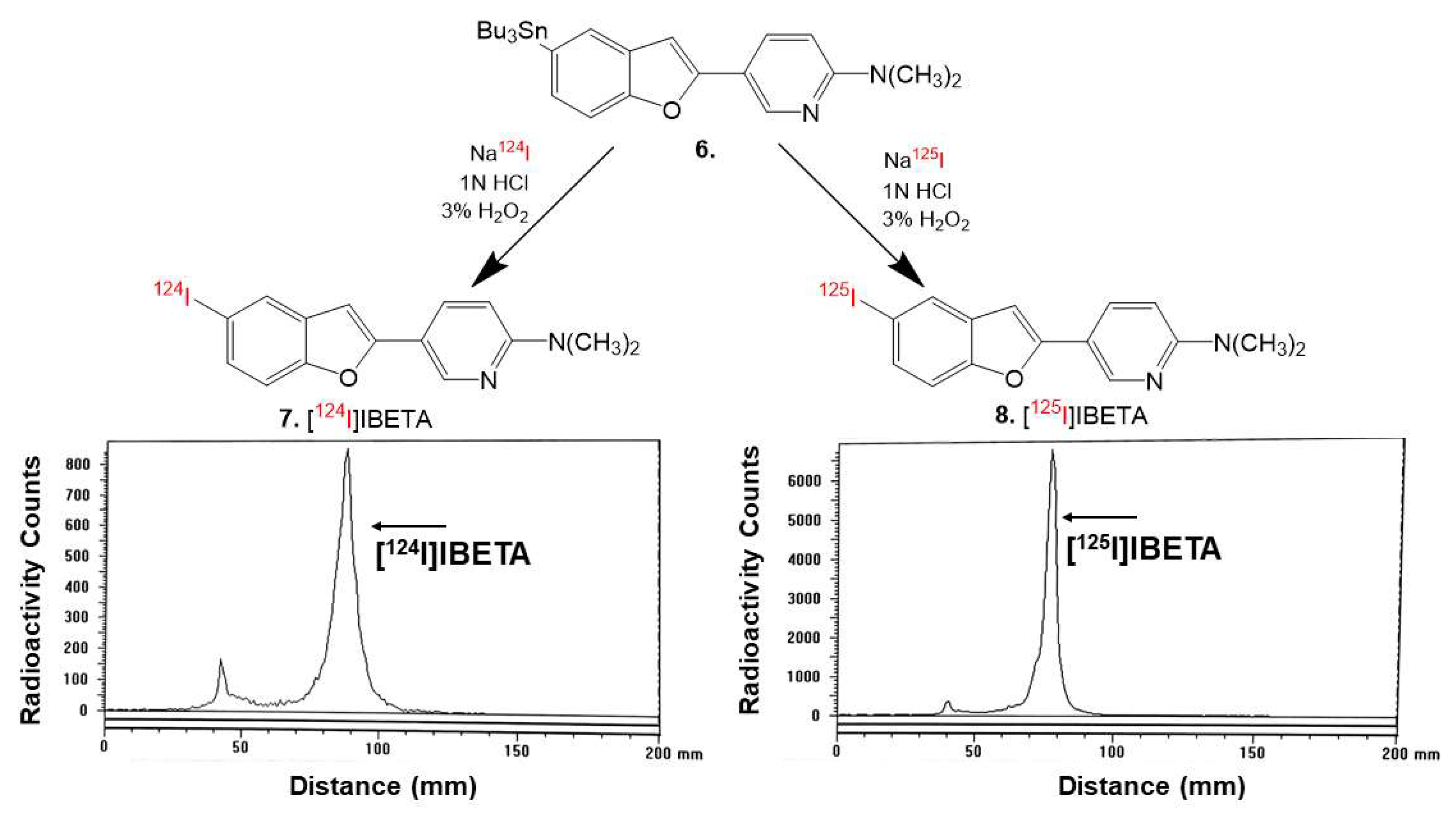
2.5. Radiosynthesis
Sodium iodide, [
124I]NaI (3D Imaging LLC) and [
125I]NaI (ARC Inc.) were used to prepare [
124I]IBETA and [
125I]IBETA by the electrophilic substitution of the tributyltin derivative using reported radioiodination methods [
21,
22]. The radiosynthesis of [
124I]IBETA using methods modified from [
22] and [
125I]IBETA using methods modified from [
15,
16] were successfully caried out. Then, 0.1 mL H
2O
2 (3%) was added to a mixture of 0.1 mL of tributyltin derivative (1 mg/0.2 mL of ethanol), 21 MBq NaI
124, and 0.1 mL of 1N HCl in a sealed vial. The reaction was allowed to proceed at room temperature for 30 min before it was terminated by the addition of sodium bisulfite. The solvent was removed by vacuum rotary evaporation and [
124I]IBETA was purified by HPLC. Radio TLC confirmed a radiochemical purity of >85% [
124I]IBETA (
Figure 2). Molar activity was estimated to be >500 TBq/mmole under the no-carrier added conditions
The same reaction was used to synthesize [
125I]IBETA from the tributyltin derivative (1.5 mg/0.7 mL of ethanol) and 3.4 MBq [
125I]NaI. The reaction was allowed to proceed at room temperature for 60 min before it was terminated by the addition of sodium bisulfite 0.1 M. The purification and isolation of [
125I]IBETA were conducted on preparative TLC. Two rounds of extraction were performed using dichloromethane. The extract was then dried using anhydrous MgSO
4. Radio TLC confirmed a radiochemical purity of >95% [
125I]IBETA (
Figure 2). Molar activity was estimated to be approximately 90 TBq/mmole under the no-carrier added conditions.
2.6. In Vitro Mice Brain Autoradiography
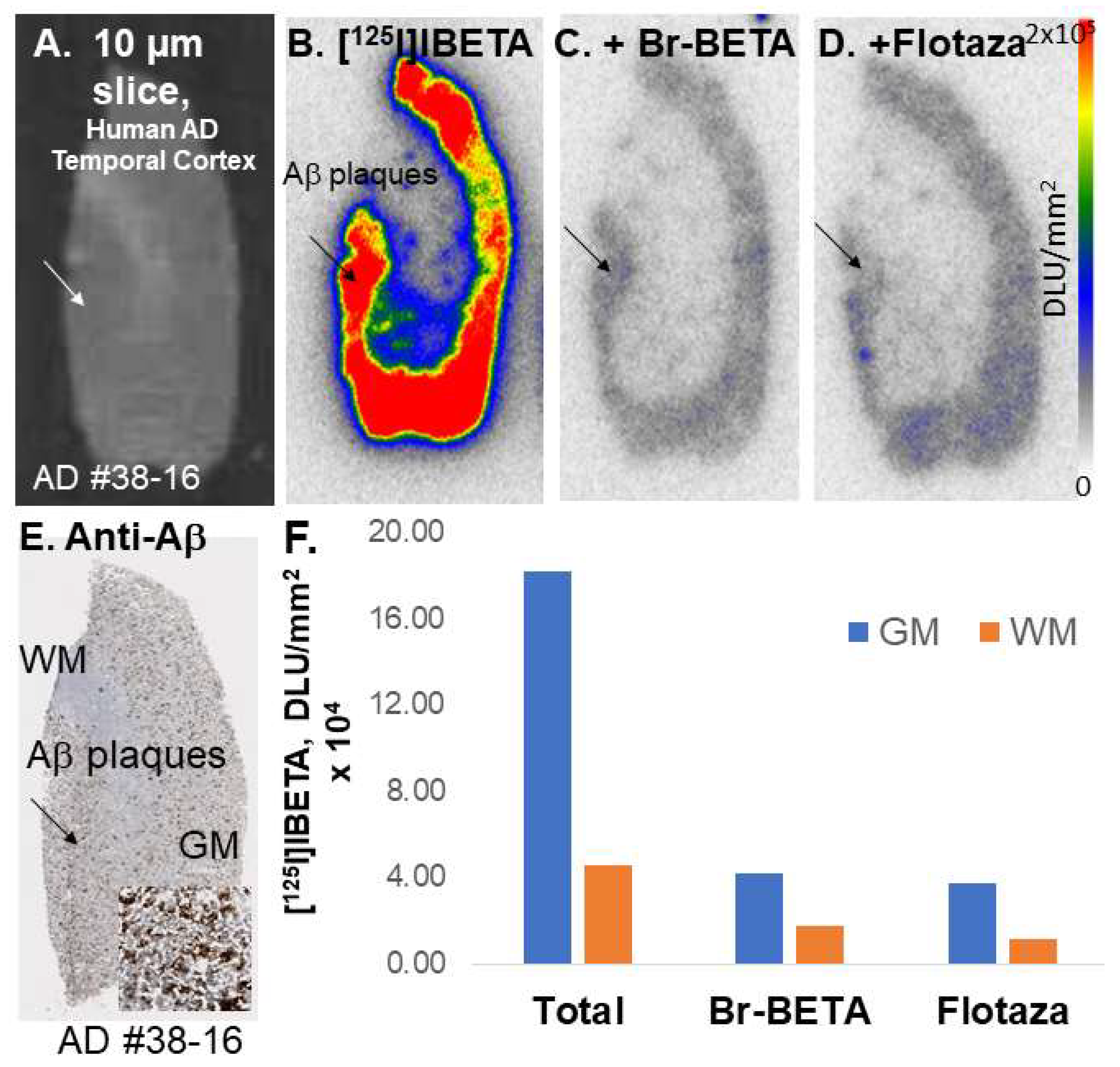
All experiments were carried out in accordance with the Institutional Animal Care and Use Committee at the University of California, Irvine, and were consistent with Federal guidelines. Male and female hemizygous 5 × FAD seven-month-old mice obtained from MMRRC JAX were used for in vitro and in vivo studies. Horizontal brain slices were sectioned (10 μm thickness) on a Leica 1850 Cryostat and collected on Fisher slides. The slides contained three to four brain sections each, were placed in separate glass chambers (six slides per chamber), and were preincubated in PBS buffer for 15 min. The preincubation buffer was discarded. The brain slices were treated with [124I]IBETA and [125I]IBETA in 40% ethanol PBS buffer pH 7.4 (60 mL, 5 kBq/mL). The chambers were incubated at 25 °C for 1.25 h. The nonspecific binding of [125I]IBETA was measured in separate chambers using Flotaza and Br-BETA (0.1 µM). The slices were then washed with cold PBS buffer, 90% ethanolic PBS buffer twice, PBS buffer, and cold water. The brain sections were air-dried and exposed overnight on a phosphor film (Multisensitive Medium MS, PerkinElmer, Waltham, MA). The apposed phosphor screens were read and analyzed by OptiQuant acquisition and the Cyclone Storage Phosphor System (Packard Instruments Co., Boston, MA, USA). The regions of interest (ROIs) were drawn on the slices and the extent of binding of [124I]IBETA and [125I]IBETA was measured in DLU/mm2.
2.7. In Vitro Postmortem Human Brain Autoradiography
All experiments were carried out in accordance with the Institutional Review Board at the University of California, Irvine, and were consistent with Federal guidelines. Human AD (n = 6) post-mortem brain tissues were obtained from Banner Health Research Institute, Sun City, Arizona. Brain slices were sectioned (10 μm thickness) on a Leica 1850 Cryostat and collected on Fisher slides. The slides contained three to four brain sections each were placed in separate glass chambers (six slides per chamber) and were preincubated in PBS buffer for 15 min. The preincubation buffer was discarded. The brain slices were treated with [124I]IBETA and [125I]IBETA in 40% ethanol PBS buffer pH 7.4 (60 mL, 5 kBq/mL). The chambers were incubated at 25 °C for 1.25 h. Nonspecific binding was measured in separate chambers using Flotaza and Br-BETA (0.1 µM) in the presence of [125I]IBETA. The slices were then washed with cold PBS buffer, 90% ethanolic PBS buffer twice, PBS buffer, and cold water. The brain sections were air-dried and exposed overnight on a phosphor film (Multisensitive Medium MS, PerkinElmer, Waltham, MA, USA). The apposed phosphor screens were read and analyzed by OptiQuant acquisition and the Cyclone Storage Phosphor System (Packard Instruments Co., Boston, MA, USA). ROIs were drawn on the slices and the extent of binding of [124I]IBETA and [125I]IBETA was measured in DLU/mm2.
2.8. Immunohistochemistry
The immunostaining of all brain sections was carried out by University of California, Irvine, pathology services using Ventana BenchMark Ultra protocols. To determine the localization of Aβ accumulation in the subject brains, neighboring slices of postmortem human AD and mouse brain slices were immunostained with anti-Aβ Biolegend 803015 (Biolegend, CA, USA), which is reactive to amino acid residue 1–16 of Aβ. Immunostained sections were scanned using the Ventana Roche instrumentation and the images were analyzed using QuPath software.
2.9. Mice PET and CT Scanning
Animals had free access to food and water during housing. All animals were fasted for 18–24 h prior to PET imaging. In preparation for the scans, the mice were induced into anesthesia with 3% isoflurane. Inveon preclinical dedicated PET (Siemen’s Inc) was used for the MicroPET studies, which has a resolution of 1.45 mm [
23]. The Inveon PET and MM CT scanners were placed in the “docked mode” for combined PET/CT experiments (Siemens Medical Solutions, Knoxville, TN, USA). A Sigma Delta anesthetic vaporizer (DRE, Louisville, KY, USA) was used to induce and maintain anesthesia during injections and PET/CT acquisitions.
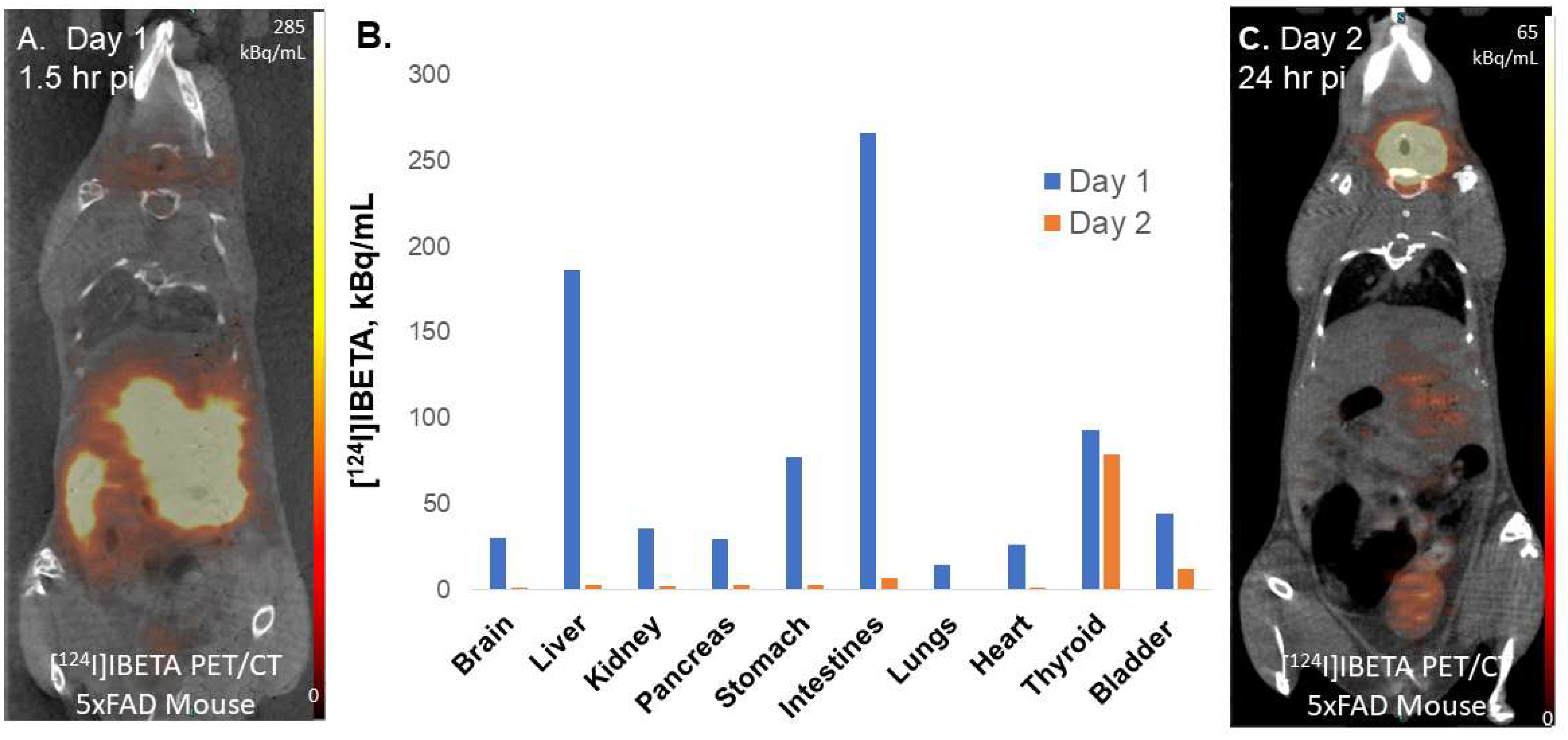
[124I]IBETA was taken in 10% alcoholic sterile saline (0.9% NaCl injection, United States Pharmacopeia) for injections into mouse models for PET/CT studies. [124I]IBETA was injected retro-orbitally (0.9 MBq) under 2% isoflurane anesthesia. The mice underwent 15 min PET scans (90 min and 24 h post-injection) in a supine position accompanied by a 7 min CT scan for attenuation correction. The CT images were reconstructed with a cone beam algorithm (bilinear interpolation, Shepp-Logan filter) into 480 × 480 × 632 image arrays with a 206 μm pixel size. Following the reconstruction, the CT images were spatially transformed to match the PET images. In addition to being reconstructed into an image, the CT data were used for the attenuation correction of the PET images.
2.10. Image Analysis
All in vivo images were analyzed using Inveon Research Workplace (IRW) software (Siemens Medical Solutions, Knoxville, TN, USA) and PMOD Software (PMOD Technologies, Zurich, Switzerland). Whole-body PET/CT images were analyzed using the IRW software for [
124I]IBETA uptake and any other CT anomalies in the whole-body images [
24]. For additional brain quantitative analysis, brain images were also analyzed using PMOD, with PET images co-registered to a mouse brain MRI template [
24,
25]. The magnitude of [
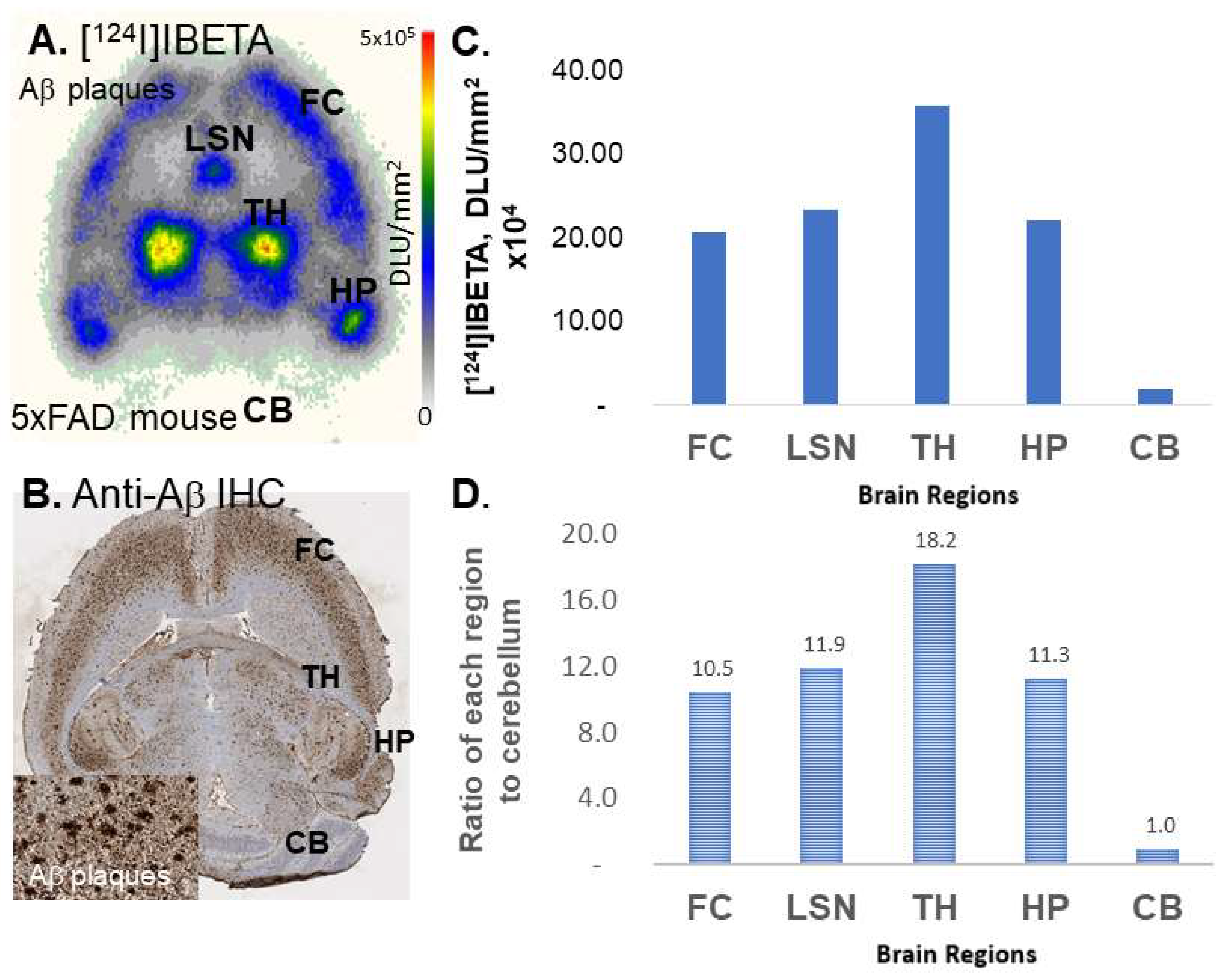
124I]IBETA in each volume of interest, VOI (in kBq/mL), was measured. Cerebellum was used as a reference region in order to calculate the ratio of the target brain regions to the reference region.
4. Discussion
Imaging biomarkers show much potential for illness diagnosis, disease progression monitoring, therapeutic response tracking, and advancing our current understanding of the physiology and pathology of AD. Fluorine-18 has been an ideal radionuclide due to its half-life of 110 min, which allows adequate time to radiolabel the drug of interest and localize it in vivo. Nonetheless, iodine-124 and iodine-125 have a much longer half-life than the traditional fluorine-18 radionuclide, which is often prepared from a cyclotron on-site due to its short half-life. The recent development of novel 123/125-iodinated pyridyl benzofuran Aβ radiotracers showed a high affinity for Aβ plaques in vitro [
17]. For further investigation, our study examined the effects of different drugs on the binding of [
125I]IBETA in vitro, which had not been reported in the previous publication. However, iodine-125 radiolabeled drugs are more commonly utilized in laboratory experiments and biodistribution studies due to their long half-life (60 days) and low energy level of emitting radiation. In the pursuit of a radioiodinated analog of [
123/125I]IBETA, we evaluated [
124I]IBETA as a possible candidate for Aβ imaging in vitro and in vivo, which can potentially be used in PET preclinical imaging for Aβ aggregates in human AD brain. This study is similar to our previous efforts on the extended imaging of dopamine receptors with iodine-124-labeled epidepride [
22].
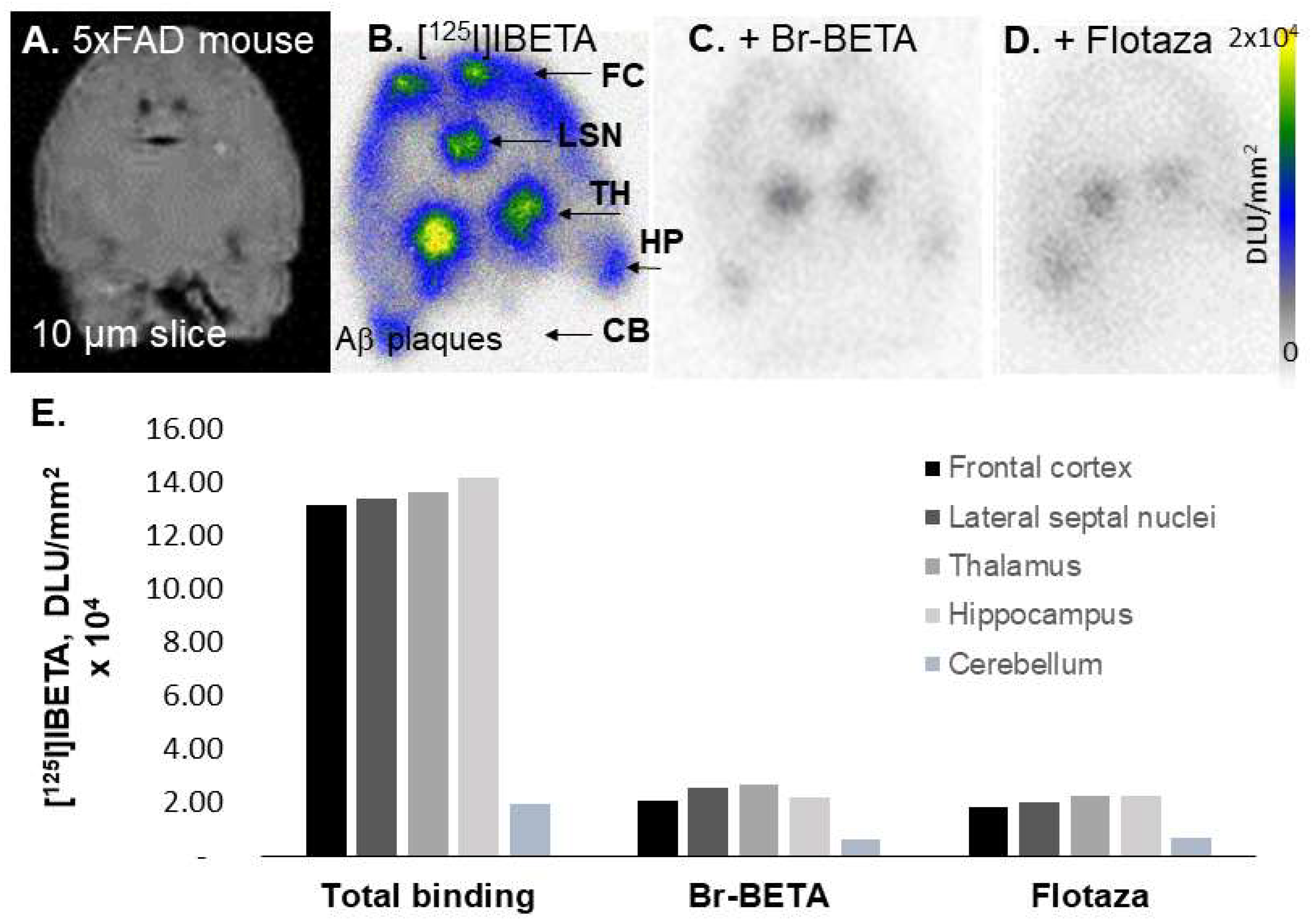
In our in vitro studies using 5 × FAD mouse brains, the bromo analog of I-BETA (Br-BETA) and Flotaza—an unlabeled fluorine analog of a new PET radiotracer for imaging Aβ plaques ([
18F]Flotaza [
15])—displaced >85% of [
125I]IBETA binding, suggesting a good correspondence in the binding sites of these two Aβ ligands. Since the binding of [
125I]IBETA was almost abolished completely in the presence of its bromo analog, a radiobrominated analog of Br-BETA, which can be labeled with
75Br (T
1/2 = 97 min),
76Br (T
1/2 = 16 h), or
77Br (T
1/2 = 3.2 days), could be developed as potential PET radiotracer for Aβ plaques with longer physical half-life options [
29]. In vitro studies using postmortem human AD brains, Br-BETA and Flotaza also displaced much [
125I]IBETA binding (>80%), suggesting low non-specific binding in vitro. The strong correlation of [
125I]IBETA uptake in vitro and anti-Aβ immunostains signifies the high affinity of this radioligand to Aβ plaques in AD brain.
[
124I]IBETA revealed a higher binding for Aβ plaques in the 5 × FAD mouse models, in comparison to its 125-iodinated analog, as shown by a higher ratio of regions to CB (>10). The higher specific activity of [
124I]IBETA may lead to greater ratios compared to [
125I]IBETA. The high uptake of [
124I]IBETA in the TH and FC regions correlated well with anti-Aβ immunostains for Aβ plaques, hence confirming the binding of [
124I]IBETA to regions that contained high Aβ accumulation. The uptake in the brain region in vivo PET/CT [
124I]IBETA scans was consistent with in vitro binding in the 5 × FAD mouse brain slices, with high uptake in the TH and FC regions. According to the scans, [
124I]IBETA successfully penetrated the blood–brain barrier upon injection and most radioactivity was cleared from the brain at 24 h post-injection. The biodistribution of radioactivity presents a similar behavior of [
124I]IBETA in vivo compared to the previously reported distribution of [
125I]IBETA [
17].
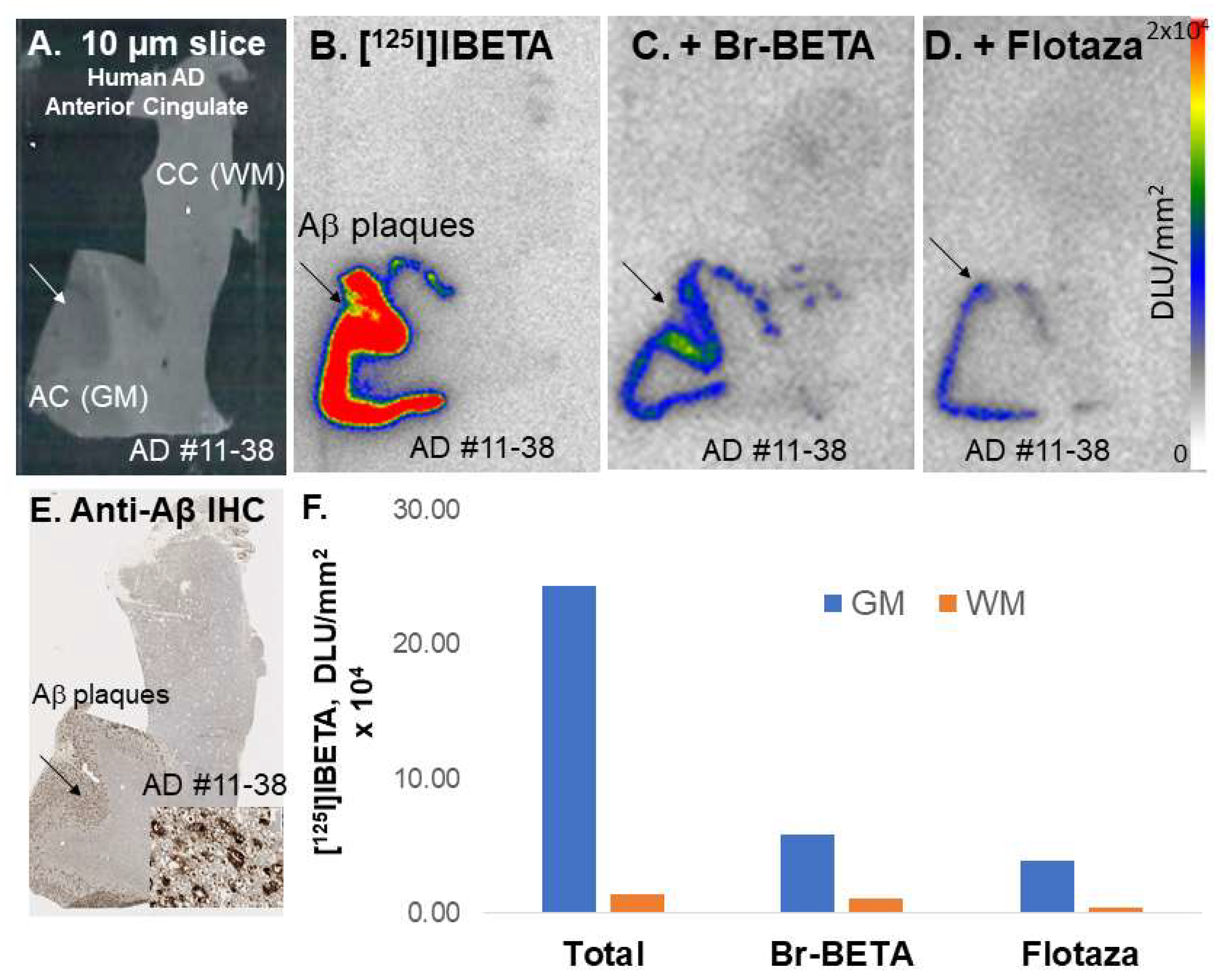
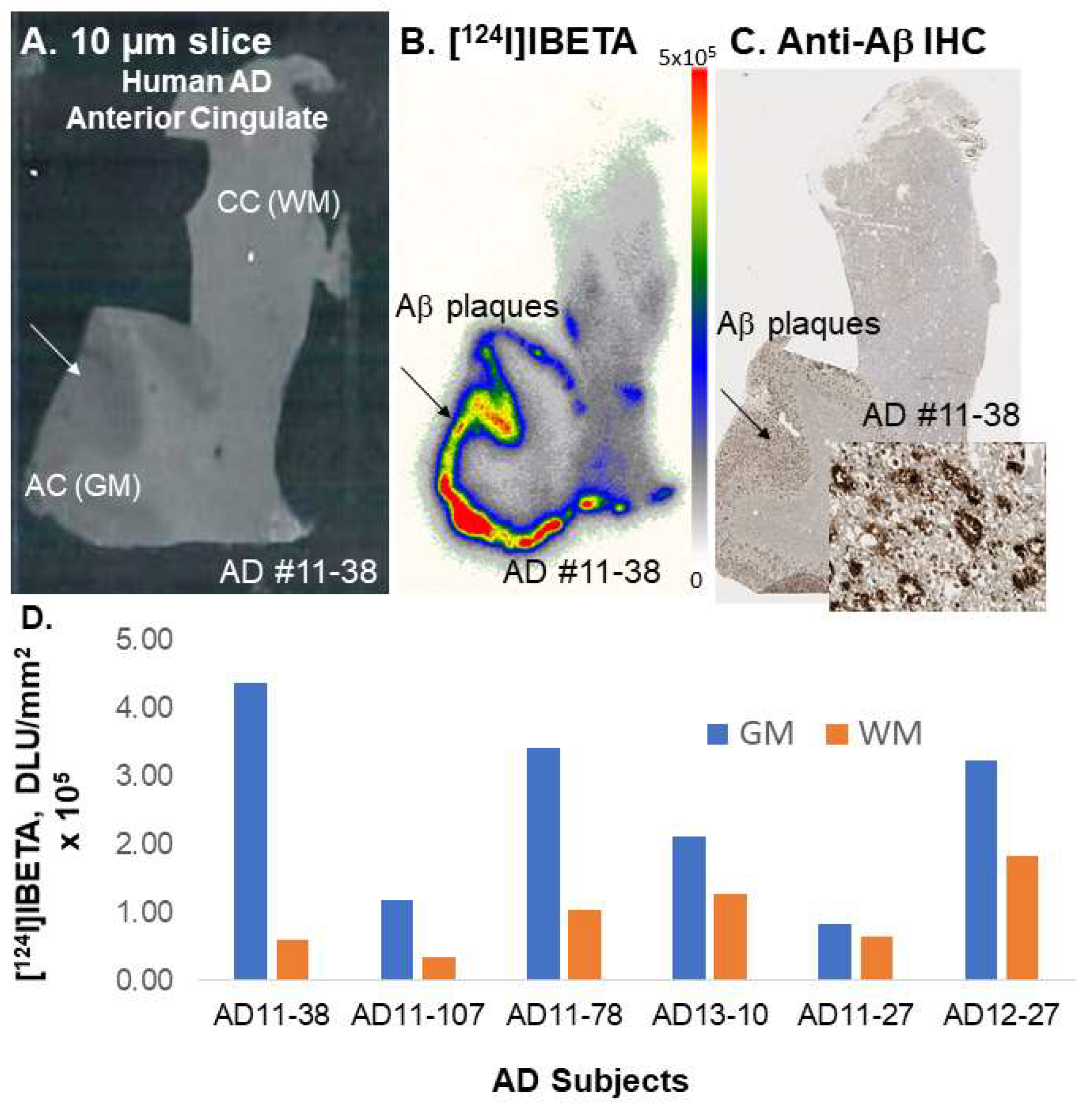
A higher uptake of [
124I]IBETA for Aβ plaques was observed in the GM than in the WM of postmortem human AD brain slices. The resulting AC to CC ratios were consistent with Aβ plaque localization in in vitro mouse brains. Nonetheless, one of the human AD subjects (AD11-38) showed much higher uptake in AC to CC (>8) in comparison to the other subjects. Thus, additional studies on the incubation condition for [
124I]IBETA, as well as more subjects, might be necessary to address the discrepancy in [
124I]IBETA binding in postmortem human AD brain slices. Furthermore, the binding of [
124I]IBETA in brain slices containing AC and CC correlated well with the localization of Aβ aggregation consistent with this mouse model [
20], which was also confirmed by anti-Aβ BioLegend 803015 immunohistochemical studies.