Abstract
The determination of 241Am in the environment is of importance in monitoring its release and assessing its environmental impact and radiological risk. This paper aims to give an overview about the recent developments and the state-of-art analytical methods for 241Am determination in environmental samples. Thorough discussions are given in this paper covering a wide range of aspects, including sample pre-treatment and pre-concentration methods, chemical separation techniques, source preparation, radiometric and mass spectrometric measurement techniques, speciation analyses, and tracer applications. The paper focuses on some hyphenated separation methods based on different chromatographic resins, which have been developed to achieve high analytical efficiency and sample throughput for the determination of 241Am. The performances of different radiometric and mass spectrometric measurement techniques for 241Am are evaluated and compared. Tracer applications of 241Am in the environment, including speciation analyses of 241Am, and applications in nuclear forensics are also discussed.
1. Introduction
The determination of americium-241 (241Am) in the environment is of importance in monitoring its release (both in controlled and accidental releases) and in assessing environmental impact and radiological risk. Several review papers have summarized the analytical methodologies for 241Am [1,2,3,4,5]. To the best of our knowledge, these works have made good reviews about the analytical methodologies of 241Am but have not specifically focused on 241Am determination in environmental samples. New radiochemical procedures and more sensitive measurement methods have been developed and applied for the determination of 241Am in environmental samples in recent years, but they have not yet been systemically reviewed. In addition, speciation analyses of 241Am in the environment and the environmental tracer applications of 241Am have not been well-addressed so far. This paper aims to critically review technical and methodological developments for the determination of 241Am in the environment, including the development of sample pre-treatment techniques, new radiochemical procedures and automated systems, and progress in measurement techniques, especially the capabilities of mass spectrometry. Apart from the above-mentioned aspects, the methodologies for 241Am speciation analyses in the environment and tracer applications of 241Am in geological and nuclear forensics studies are also discussed in this paper.
1.1. Nuclear Physical and Chemical Properties of 241Am
Americium, with an atomic number of 95, is one of the purely man-made elements. Americium has about 30 isotopes or isomers, with mass numbers ranging from 232 to 247 and half-lives ranging from 55 s to 7370 years. Among those isotopes, 241Am (T1/2 = 432.2 years) is the most important one and is formed in reactors as a product of the irradiation of plutonium with neutrons:
241Am is an alpha emitter with the energies of alpha particles (Eα = 5.388 MeV, 1.7%; Eα = 5.468 MeV, 85.2%; Eα 5.443 MeV, 12.8%) and gamma (Eγ = 59.6 keV, 35.9%) emission [6]. The gamma emission can be used to measure 241Am directly from some samples, such as highly contaminated soil. Most typically, 241Am is measured using alpha spectrometry. In the last few years, alpha spectrometry has been increasingly replaced by the more precise, accurate, and sensitive mass spectrometry. However, due to the high self-absorption of alpha particles and the interferences in mass spectrometric measurement, radiochemical separation is needed to concentrate and separate Am from interfering elements.
Americium is known in formal valence states of 0, III, IV, V, and VI, but only state III is prevailing in ordinary redox conditions. Due to comparable electronic configurations (c.f. Am [Rn] 5f77s2 and Eu [Xe] 4f76s2) and ionic radii (Am3+: 98nm and Eu3+:94.7nm), americium has similar chemical properties with lanthanides. The closely similar behavior of Am(III) and lanthanides becomes the main obstacle in separating americium from lanthanides. The formation of complex ions in aqueous solutions with inorganic ions or organic compounds is an important property of Am. The strength of the complexes of Am(III) with inorganic ligands is as follows:
PO43− > CO32− > OH− > SiO(OH)3 > HPO42− > F− > SO42− > H2PO4− > SCN− > NO3− > Cl− > ClO4−.
In groundwaters that are rich in carbonate, carbonate complexes of Am are the prevailing species in the pH range from 6–11. Americium is also strongly associated with organic matter, such as humic acid. The basis of the separation of americium and lanthanides on an anion-exchanger relies on the strong sorption of the anion complexes of Am(III) with SCN. Am(III) can be oxidized theoretically to Am(IV) because the standard electrode potential of the Am4+/Am3+couple in basic solutions is relatively low (0.4–0.5 V). However, the species of Am(IV) are unstable unless in the presence of a strong, complex agent, e.g., phosphates, and are reduced back to the trivalent state easily. To oxidize Am(III) to Am(VI), forming AmO22+, a more powerful oxidizer such as (NH4)2S2O4 is needed [7]. The highly valent Am(V) and Am(VI) species are more stable in basic solutions compared to acidic solutions. Burns et al. [8] oxidized Am3+ to AmO2+ and stabilized the AmO2+ ions in acidic media with the presence of hypochlorite, which is possible to enable an effective group separation of lanthanides from actinides.
1.2. Sources of 241Am in the Environment
241Am is released into the environment through several different sources. First of all, as the result of atmospheric nuclear weapons testing from 1945–1980, controlled or accidental releases from nuclear reprocessing plants (Sellafield, La Hague, and Mayak), and nuclear accidents (Palomares in 1966, Thule in 1968, Chernobyl in 1986, and Fukushima in 2011), 241Am has been introduced directly to different extents into the environment [9]. Secondly, the level of 241Am in the environment has been increased due to the decay of the pure beta emitter 241Pu (T½ = 14.2 year) from nuclear denotations, either authorized or accidental releases, and should continue to increase for the next several decades. It has been estimated that, during the period of 1945–1980, about 142 PBq of 241Pu [10] was released into the environment by atmospheric nuclear weapons testing. Considering that the total deposition of 241Pu was about 108 PBq for the northern hemisphere to a reference date of 1965, about 3.0 PBq 241Am would have been generated by 2006 due to the decay of 241Pu [10]. In the same situation, the amount of 241Am released from the Chernobyl reactor was estimated to increase to 33.6 times the initial activity until 2058 [11], and the activity of 241Am should reach its maximum value approximately in 2090 [12]. Table 1 summarizes the different sources of 241Am and 241Pu in the environment. Up to this date, the majority of 241Am in the environment has originated from atmospheric nuclear weapons testing. The amount of 241Am released by either reprocessing nuclear plants or nuclear accidents accounts for a small proportion of the total inventory in the environment. It should be noted that the peak release of 241Am from the nuclear reprocessing plant at Sellafield occurred from 1971–1975, with the highest discharge of 118 TBq in 1974. After the initial peak, the discharge of 241Am from Sellafield reduced to about 8 TBq y−1 from 1978–1981 and then to about 0.04 TBq y−1 from 2005–2009 [13]. The La Hague nuclear reprocessing plant in France also reached its peak discharge of 241Am in 1974. From 1995–1999, the discharges of 241Am and 241Pu from La Hague were 0.31 TBq and 21.9 TBq, respectively. However, the total discharge of 241Am from La Hague has not been well-documented [14]. The determination of 241Am is an important topic in relation to health, nuclear waste management from nuclear reactors, the recycling and final storage of radioactive waste, the control of illicit nuclear activities, etc. Methods for the determination of 241Am in a wide variety of environmental samples include nondestructive gamma spectrometry, alpha spectrometry, mass spectrometry, etc., after chemical separation.

Table 1.
Sources of 241Am and 241Pu in the environment.
1.3. Distribution and Transfer of 241Am in the Environment
The distribution characteristics of 241Am in the environment are strongly influenced by the source and migration behavior of 241Am in different environmental compartments, such as atmospheric, terrestrial, and aquatic environments. The levels of 241Am vary with location, sample type, and the transport pathways within and between different environmental compartments. Table 2 summarizes the distributions of 241Am in some specific samples from different locations.

Table 2.
Distributions of 241Am in some specific locations.
241Am is one of the most toxic transuranic nuclides due to its long half-life, α-particle emission, and especially its tendency to deposit in several key tissues and organs, such as the skeleton and liver, if it enters the human body. 241Am in the environment can be transferred into the human body through drinking water and the food chain. The World Health Organization (WHO) suggested that the level of 241Am in drinking water should be below 1 Bq/L [37]. The maximum permissible quantity for 241Am in the human body was reported to be 11.1 kBq (8.77 × 10−8 g) [38].
Attention has been also paid to recent trends in the transfer of 241Am from the food chain to creatures, as well as from soil to plants. Baigazinov et al. [39] presented the transfer parameters of 241Am to the tissues of horses from contaminated soil and feed at the Semipalatinsk Test Site (STS). The observed maximum transfer factor for 241Am was (72 ± 22) × 10−5 day∙kg−1 fresh mass in the liver of a mare fed with leachate from contaminated soil and feed. A.S. Mamyrbayeva et al. [40] described the excretion dynamics of 241Am from the muscle, liver, and bone of broilers after a 30-day application of contaminated feed. The results showed that 241Am mainly metabolized in liver and bone, and the activity concentration of 241Am in muscle was much lower. B.M. Bolotov et al. [41] reported that the concentrations of 241Am in human hair collected from the Semipalatinsk area were lower than 0.05 Bq/kg. However, there is still a lack of data on the transfer factors of 241Am from the food chain to human beings. In contrast, a number of studies have been carried out to investigate the uptake of 241Am from the soil into plants. The experiments conducted by Sokolik et al. [42] showed that the soil-to-grass transfer factor of 241Am decreased in the order of soddy-podzolic sand < soddy-podzolic loamy sand < alluvial soddy loamy sand < peat-bog. Plant species with different physical and chemical properties usually differ in their transfer factors for 241Am due to the variability in their metabolic processes and biological factors, the distribution of roots in the soil, and rhizosphere properties. Table 3 presents the transfer factors of 241Am from soil to plants, with most values at very low levels.

Table 3.
Transfer factors for 241Am from soil to plants.
2. Sample Pre-Treatment and Pre-Concentration
2.1. Sample Pre-Treatment
The purposes of sample pre-treatment are to decompose the sample to release the target radionuclides into a homogenous solution to thus facilitate the subsequent chemical separation procedure. For soil samples and sediments, grinding, sieving, homogenizing, drying, and ashing are necessary before sample decomposition. Ashing aims to remove organic matters contained in the sample since, otherwise, they interfere with the performance of the chemical separation procedure. Wang [45] pointed out that ashing temperatures for soil samples should be carefully selected to avoid the formation of refractory fractions. An XRD analysis of soil samples revealed that plagioclase-like silicate materials were formed after high-temperature ashing, and 450 °C was recommended as an ideal ashing temperature.
The methods for sample decomposition can be divided into two categories: acid digestion and alkaline fusion. For acid digestion, aqua-regia leaching is frequently used to release actinides from the matrix and has been widely adopted by a number of laboratories to accommodate large-size samples for 241Am determination. However, it has been shown that leaching is not appropriate for soil containing refractory fractions. In the case of refractory residues associated with silicate lattices in the soil and air filter [46], HF in combination with other acids (typically HNO3 and HClO4) in an open vessel is a good choice for total dissolution of the matrix to release the entire Am content from the soil sample. Careful control of the physical and chemical conditions during total dissolution with an HF–HNO3–HClO4 system is necessary to prevent the formation of insoluble fluorides, such as AlF3 and CaF2. The process requires that the sample acid mixture is not evaporated to dryness in the initial decomposition. Practically, the addition of sufficient boric acid can prevent the formation of any insoluble fluorides. It is more efficient to perform sample decomposition in a closed system than in an open system. High-pressure microwave digestion systems with closed pressure relief containers have shown advantages of more vigorous digestion of 241Am at elevated temperatures and pressures, which not only reduces analytical time and consumption of the reagent, but also improves the operational safety [47,48,49]. However, closed microwave digestion systems might not be favorable to treat large amounts of samples.
Alkali fusion is an extremely aggressive method performed by melting the sample with a mixture of fusion flux (e.g., hydroxides, peroxides, carbonate, hydrosulfates, pyrosulfates, or lithium borates) in a graphite, nickel, zirconium, or platinum crucible at atmospheric pressure [1,50,51,52]. Due to the high operational temperature in the fusion process, the efficiency of decomposition by alkaline fusion is much higher compared to acid digestion. After cooling, the well-mixed molten cake is dissolved with diluted HNO3 or HCl. Maxwell [50] demonstrated that sodium hydroxide fusion could provide a rapid treatment for the analysis of 241Am in large soil samples (100–200 g). The main benefit of alkali fusion is the limited use of acids and the absence of HF in the pre-treatment process.
In general, acid leaching is the simplest method to treat large amounts of solid samples. However, the 241Am contained in some specific samples, such as vitrified samples, might not be completely released into the solution by acid-leaching because 241Am intrudes the crystal lattices of minerals. Incomplete sample decomposition gives rise to the underestimation of results. Total acid dissolution in many cases can dissolve the refractory fractions of samples, but it is time-consuming with high consumption of the acids and limited sample throughput. Alkali fusion can offer the complete decomposition of samples, and it is the most effective and aggressive method for decomposing solid samples containing silicates and refractory fractions. However, the extraneous salts introduced by the fusion flux are sometimes troublesome for the following chemical separation and measurement.
2.2. Pre-Concentration
The objective of pre-concentration is to concentrate the sample to a smaller volume and remove most matrix interferences. Coprecipitation is a commonly used method for the pre-concentration of Am from large-volume water samples, as well as the solution obtained after the pre-treatment of solid samples. The most frequently used reagents for the coprecipitation of Am are listed in Table 4. Generally, more than 95% of 241Am can be scavenged by coprecipitation. Sometimes, a combination of different reagents is used for the coprecipitation of 241Am. For example, calcium oxalate coprecipitation was used after ferric hydroxide coprecipitation to eliminate the interference of iron in the subsequent chemical purification for Am [53].

Table 4.
The most frequently used reagents for the coprecipitation of 241Am.
The marriage of mesoporous ceramics with self-assembled monolayer chemistry has created a powerful new class of environmental sorbent materials called self-assembled monolayer on mesoporous supports (SAMMSs). SAMMS materials offer extremely large surface areas (up to 500m2 g−1) and functionalities that have been fine-tuned to selectively capture 241Am and other actinides [64,65]. Am distribution coefficients were reported to be as high as 240,000 and 460,000 for two types of SAMMSs (Gly-UR SAMMS and Ac-Phos SAMMS, respectively) [64]. Since SAMMSs are effective in highly complex matrices such as blood, plasma, and urine, Yantasee et al. [66] used SAMMSs with an isomer of hydroxypyridinone (3,4-HOPO) for the selective pre-concentration of 241Am from blood and plasma to improve the detection limits of the analytical instruments. One appealing nanotechnology that uses magnetic nanoparticles (MNPs) conjugated with actinide-specific chelators (MNP-) for separating actinides from spent nuclear fuel solution was developed [67]. It utilized coated MNPs to selectively adsorb actinides (Np, Am, and Cm) onto their surfaces, after which the loaded particles were collected using a magnetic field. MNP-Che is an appealing technique the for pre-concentration of Am in water or solutions obtained after the pre-treatment of solid samples. The removal percentage of Am(III) by MNPs- DTPA in an acidic solution is over 90% after 30 min of sorption time.
3. Chemical Separation and Purification Procedures
Chemical separation and purification procedures are usually designed to concentrate and purify target radionuclides, which is imperative for low-level environmental radio assays. For the purification of 241Am, a variety of chemical separation procedures have been applied, including solvent extraction, ion-exchange chromatography, extraction chromatography, and combinations of two or more of these methods.
3.1. Solvent Extraction
Solvent extraction is widely used to separate 241Am in the reprocessing of spent fuel and the treatment of radioactive waste. Many extraction reagents have been involved in the separation of Am, including TTA, PMBP, TOPO, TOA, CMPO, HDEHP, and DDCP. For instance, a PMPB-TOPO extraction method was reported to purify americium in mosses and lichens [68]. Solvent extraction with PMBP in cyclohexane was used to purify americium from rare-earth elements [36]. Popov et al. [24] reported the use of 10% tri-iso-octylamine (TIOA) in xylene to separate americium from uranium, polonium, and plutonium in Bulgarian soil. One of the extractants, 2-hydroxy-2-trifluoromethyloctanoic acid (Hhfo), was synthesized and characterized for separating americium and lanthanides, and the maximum separation factor of Eu and Am by Hhfo reached 2.31 [69]. Due to the similar behaviors of Am(III) and lanthanides, it is always important to thoroughly separate americium from lanthanides to eliminate the interference of lanthanides in the measurement of Am. Three major groups of trivalent Am(III) ligands (O-donating, S-donating, and N-donating) have been proposed to separate Am from lanthanides based on the fact that americium forms slightly stronger complexes with ligands containing soft donor atoms than lanthanides. CMPOs are well-known O-donating ligands for separating Am(III). However, this type of ligand usually lacks discrimination between the same oxidation states of Am(III) and Eu(III), which results in a relatively low separation factor. Cyanex 301 is an S-donating ligand, and this sulfur-containing compound is a good example of an Am(III) chelator having a very high Eu and Am separation factor due to the preferable covalent binding of Am(III) to the relatively softer sulfur donor atom. N-donor ligands are classified as intermediates between O-donor and S-donor ligands with respect to extraction efficiency and Am(III) selectivity. A breakthrough came when Kolarik et al. synthesized the first BTP and found that it had remarkable extraction capabilities for actinides over lanthanides when contacted with high acidity in liquid–liquid extraction [70]. Panak et al. [71] investigated BTPs and BTBP, which were assumed to be promising solvents for separating Am(III) from lanthanides(III). The earliest BTPs had alkyl chains or alkyl-branched chains at the R position, such as n-Bu-BTP, isobutyl-BTP, and n-Pr-BTP. These extractants, along with other alkyl-substituted BTPs, displayed high selectivity for actinides over lanthanides in liquid–liquid extraction studies. Yuanlai et al. [72] synthesized a silica-based macroporous isobutyl-BTP/SiO2-P adsorbent by impregnating an isobutyl-BTP (2,6-di(5,6-diisobutyl-1,2,4-triazin-3-yl)pyridine) extractant into an acroporous SiO2-P support to directly separate trivalent Am from fission products (FPs) containing rare-earth (RE) groups in high-level radioactive liquid waste (HLLW). It was observed that the isobutyl-BTP/SiO2-P adsorbent exhibited good adsorption selectivity for 241Am over rare-earth (III) groups in a 0.01 M HNO3 solution and showed weak or no adsorption affinity to light and middle rare-earth (III) groups. The same group has also synthesized several other R-BTP/SiO2-P adsorbents (R = isohexyl, isoheptyl, and cyheptyl) and has investigated their fundamental properties, such as adsorption ability or stability [73,74,75]. Some other derivatives, such as CA-BTP [76,77], MOB-BTP [78], and CyMe4BTBP [79], have shown behavior more efficient for Am(III) extraction at lower concentrations. Nowadays, as great progress has been made in ion-exchange and extraction chromatography techniques, solvent extraction is no longer popular in the analysis of 241Am in the environment and is not suitable for batch-wise treatment due to its relatively high-complexity operations. However, solvent extraction still offers some attractive features when a single sample is required for analysis. For example, the separation of 241Am using solvent extraction with PMBP or TOA can be completed within a few hours.
3.2. Ion-Exchange Chromatography
Americium may be absorbed by either cation- or anion-exchange resin. The applicability of Chelex-100 cation-exchange resin was investigated for the separation of americium and samarium in aqueous solutions [80]. The maximum separation efficiency of Chelex-100 for trivalent lanthanides and actinides was achieved at pH 2.5. Increasing salinity (e.g., [Na+] and [Ca2+]), iron ([Fe3+]), and colloid concentrations in the solution resulted generally in decreasing the chemical recovery of Am. A procedure for the separation of Am from rare-earth elements was developed through step-by-step elution from KU-2 cation-exchange resin in the NH4+ form with α-hydroxyisobutyric acid (pH 4.75) [81]. Am3+ cations have a high distribution factor on cation resin at low acidities, which can be easily eluted from the cation exchangers by concentrated acids. However, insufficient selectivity was observed between Am(III) and lanthanides, which was due to the fact that Am3+ cations have a similar ionic radius and almost identical effective nuclear charges to Cm3+, Nd3+, and Sm3+. As a consequence, cation-exchange chromatography has not been widely applied for Am purification. Compared to cation exchange, anion exchange is a better choice for separating interfering alpha emitters from Am, especially relatively high levels of trivalent lanthanides. This is due to the fact that most of the matrix elements, especially lanthanides, are not able to form anion complexes under certain conditions, but anion complexes of Am(III) with SCN− have strong sorption on anion exchangers.
3.3. Extraction Chromatography
Extraction chromatography (EC) is also called solid-phase extraction. Compared to solvent extraction, EC offers a number of advantages, including fast kinetics, high selectivity, and less reagent consumption and hazardous waste generation. Earlier studies have reported the application of supported HDEHP [82] and supported TOPO [83] for the separation of Am from Pu and U. Later, the supported HDEHP was developed and produced as a commercially available resin (LN resin) by Eichrom Co. A single column consisting of tri-n-octylaime (TONA) supported by microporous polyethylene was used to simultaneously separate 241Am, 244Cm, 239+240,238Pu, 237Np, and 234,235,238U. Because Am(III) was not retained on the TNOA extraction column, the effluent from the column loading was directly electroplated for Am measurement using alpha spectrometry [84]. Mohandas et al. [85] investigated the uptake of uranium and americium from nitric acid solutions with sulphonated phosphinic acid resin. The advantage of the sulphonated phosphinic acid resin, compared to phosphinic acid resin or conventional cation-exchange resin, was its greater capacity for the uptake of U(VI) and Am(III) from high-acid media. This advantage was maintained, even in the presence of NaNO3.
A series of EC materials have been developed for the separation of actinides by Horwitz and coworkers [86,87,88] at the Argonne National Laboratory during the 1990s, and later, Eichrom Co. made these materials commercially available. Commercial EC resins, including TRU, TEVA, UTEVA, DGA, DIPEX, and DIPHONIX, can facilitate efficient Am separation, and the characteristics of these resins are compiled in Table 5. Recently, several novel EC resins [79] were synthesized by the solvent impregnation of triazine ligands (CyMe4BTBP and CyMe4BTPhen) into Amberlite XAD7 and Amberchrom CG300 polymer supports. The Amberchrom-supported CyMe4BTBP resin achieved a weight distribution ration (DAm) of 170 within 60 min and a decontamination factor (DF) of >1000 for americium over lanthanides using column chromatography. The Amberchrom CyMe4BTPhen resin achieved a DAm of 540 within 30 min and a DF for americium over lanthanides of 60–160.

Table 5.
The characteristics of different commercial extraction chromatographic resins.
3.4. Combined Procedures for 241Am Determination
Although a single resin column, such as DGA, can provide a reasonably good separation of 241Am, it is impossible to meet the requirements of all cases for 241Am analysis in the environment. In recent years, many researchers have hyphenated different chromatographic resins to develop more effective procedures for 241Am determination. These methods sequentially separate 241Am and other actinides, providing advantages of reduced analytical time and cost. The principle of these combined procedures is based on the different absorption properties of different valent radionuclides on the EC resins. Figure 1 shows a typical scheme of a combined procedure for the determination of 241Am and other actinides in a homogeneous sample solution after pre-treatment and pre-concentration.
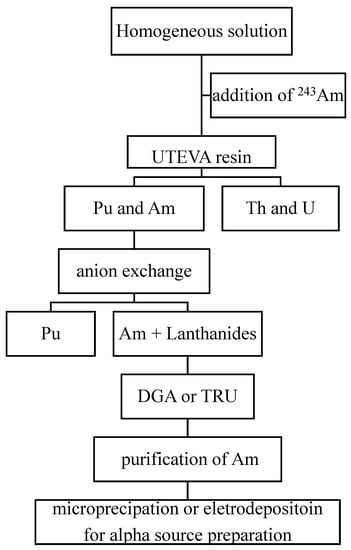
Figure 1.
Scheme of a combined procedure for the determination of 241Am from an aqueous solution.
Table 6 summarizes the major characters of combined procedures for the determination of Am in environment samples that have been developed in recent years. Those combined procedures can meet the requirements for the determination 241Am in the environment.

Table 6.
The major parameters of combined procedures for the determination of 241Am in environmental samples developed in recent years.
3.5. Automated Systems for the Separation of Am
In the past decades, semi-automated and fully automated systems have been developed to analyze either single or multiple radionuclides in both emergency and routine operational situations [103,104,105]. These systems are suitable to separate not only Am, but also other radionuclides, such as Sr, Np, U, Th, and Pu isotopes. Most automated systems are based on dynamic flow approaches. The latest review [106] summarized systematically different flow approaches for automated radiochemical analyses in environmental, nuclear, and medical applications. Dynamic-flow-based approaches, including flow injection (FI), sequential injection (SI), multi-commuted flow injection (MCFI), multi-syringe flow injection (MSFI), multi-pumping flow system (MPFS), and pressurized injection (PI) techniques, have been developed and applied to meet analytical criteria under different situations. Recent testing has shown that flow techniques can be used for 241Am analysis in many situations, as summarized in Table 7. Specific automated systems for the determination of 241Am were developed using ion chromatography [107] and capillary extraction chromatography [108] separation prior to measurement.

Table 7.
Overview of flow approaches developed for 241Am determination.
4. Source Preparation
After chemical separation and prior to measurement, the purified Am fraction needs to be prepared in a certain geometric or chemical form to facilitate the subsequent quantitation using a radiometric or spectrometric method. Depending on the measurement method selected for 241Am, the criteria for the source preparation are different, but generally, fast turnover and high chemical yield are desired.
4.1. Source Preparation for Alpha Spectrometry
To obtain a thin, flat, and uniform alpha source is critical for achieving a high detection efficiency in alpha spectrometry measurement. Due to the short range of alpha radiation in matter, the thickness of the source should be limited to a few micrometers, otherwise the alpha spectra become degraded, and poor peak resolution makes it very difficult to evaluate the spectrum. Several methods have been used for 241Am alpha-source preparation, including evaporation, electrodeposition, micro-coprecipitation, and ion implantation. The pitfall of the evaporation method is that an alpha-source surface prepared by evaporation does not have strong adherence to a plate or disk. Mirashi et al. [115] compared electrodeposition with the drop deposition method and observed that the resolution of the alpha spectra obtained that were prepared using electrodeposition were better than those using the drop deposition method. The chemical yield of electrodeposition was strongly affected by pH, the concentration of buffer solution, the amount of impurities in the electrolyte, deposition time, temperature, etc. It was observed that, when Fe(III) concentrations >0.1 mM (~30 µg Fe) were found in NH4Cl solution (5 mL), only 6% of the 241Am could be electrodeposited [116]. A variety of electrolytes or buffers have been employed for 241Am electrodeposition, such as oxalic acid-NH4Cl solution [115], NaHSO4-Na2S2O4 buffer [117], H2SO4-(NH4)2SO4 buffer [53,84], Na2SO4 [118], NaHSO4-H2SO4-NH4OH buffer [119], NH4Cl [116], (NH4)2C2O8-hydroxyl ammonium sulfate-DTPA [120], and (NH4)2C2O8-(NH4)2SO4-DTPA [121]. Among these electrolytes, the NaHSO4-Na2S2O4 buffer solution is regarded as the most robust because the electrolyte can be pre-adjusted to an optimal pH, and no further adjustment is needed in the process. Jung-Suk et al. [122] evaluated the performances of NH4Cl, (NH4)2C2O8, and (NH4)2SO4 as electrolyte solutions for the preparation of americium sources for alpha spectrometry. The recovery of americium in the (NH4)2SO4 solution was found to be relatively low compared to those in the other solutions.
As an alternative to the electrodeposition method, the alpha source of americium can be prepared via micro-coprecipitation with rare-earth fluorides (often NdF3) [123] or hydroxide (such as cerous hydroxide, Fe(OH)3, and Sm hydroxide) [63,124,125]. Fluoride micro-coprecipitation methods have a higher selectivity for actinides and lanthanides than hydroxide methods. NdF3 micro-coprecipitation was reported to perform better than CeF3 and SmF3 [55] because NdF3 precipitated much more slowly and, thus, the precipitation was more homogeneous [123]. One of the key factors in the micro-coprecipitation process is that the sample size or the amount of rare-earth carrier must be limited so that the total mass of the precipitate does not exceed 100 µg to avoid undesirable degradation of the resultant alpha spectra. M. P. Dion [126] presented a novel method using ICP-MS, where the electron multiplier was removed, and a ‘‘collector’’ was fabricated to implant mass-selected ions for alpha spectrometry source preparation. This method produced thin, contaminant-free 241Am samples that yielded an energy resolution of 20 keV FWHM (full width at half maximum). Although electrodeposition is time-consuming and tedious (usually more than 1 h), alpha sources prepared with electrodeposition have much better resolutions and qualities than those prepared with micro-coprecipitation. The resolution of the α spectrum has been valued at about 20–30 keV for electrodeposited 241Am sources [117,119,120] but at about 40–50 keV for micro-coprecipitated sources [55,124]. Micro-coprecipitation procedures are much faster (within 30 min) and, generally, give much higher chemical yields (>98%) than electrodeposition (60–95%). Practically, electrodeposition is more favorable than micro-coprecipitation for americium alpha-source preparation to ensure the quality of the source.
4.2. Source Preparation for Mass Spectrometry
Among different mass spectrometric techniques, the most commonly used for 241Am measurement are ICP-MS, TIMS, and AMS. For ICP-MS measurement, the purified 241Am solution is preferentially prepared in weak HNO3 (around 0.5 mol L−1). This is usually achieved by evaporating the obtained americium fraction to dryness, destroying the organic matter, and re-dissolving with weak HNO3 solution. For TIMS measurement, the purified 241Am solution is typically reduced by evaporation to a very small volume (1 µL) that contains about 100–500 ng 241Am, which is loaded onto a high-purity filament (rhenium, tantalum, tungsten, etc.) and dried with electric heating. For AMS measurement, coprecipitation with NdF3 [91] or Fe(OH)3 [127] can be adapted for the source preparation. The precipitate is thereafter pressed into a target suited for AMS measurement. An optimized method using mixed titanium and iron hydroxide was developed, which showed promising results for the detection of femtogram levels of 241Am using AMS [128].
5. Alpha Spectrometry for 241Am Measurement
Alpha spectrometry is the most sensitive measuring technique to detect 241Am before high-sensitivity mass spectrometry is exploited. Alpha spectrometry can be performed using different types of detectors, such as gas ionization (Frisch grid) detectors, solid (e.g., ZnS(Ag)) and liquid scintillation detectors, magnetic spectrometers, nuclear track detectors, and semi-conduction detectors. A typical alpha spectrometer with an ion-implanted Si detector of 300–600 mm2 surface area and 100 µm thickness has a resolution of 17–25 keV in the energy range of 3–10 MeV, a counting efficiency of 15–30% for a source-to-detector height of 5–10 mm, and a background of 10−4 to 10−5 cps for a counting time of 105 s. The FWHM of an alpha spectrum in the range of 20–60 keV depends not only on the performance of the detector but also on the quality of the source.
The complete separation of 241Am from the sample matrix is imperative for obtaining sufficiently thin alpha sources. It is of particular importance for samples containing relatively high levels of trivalent lanthanides and 210Pb from the americium fraction, which can interfere significantly with the measurement of 241Am by alpha spectrometry. Excessive lanthanides can degrade the alpha spectra, and 210Pb, through its grand-daughter 210Po (major alpha energy 5.30 MeV), interferes with the measurement of 243Am (major alpha energy 5.27 MeV), which is a yield tracer for americium separation. Since americium and curium behave chemically in very similar manners, the alpha spectra of 241Am often contain peaks of curium isotopes. The alpha peaks of 241Am,244Cm, and 242Cm are clearly distinguishable in alpha spectrometry so that the isotopes of 242Cm and 244Cm do not interfere with the determination of 241Am. In the case that there is no suitable isotopic tracer available for the determination of curium, it is reasonable that the yield of curium is calculated from the americium tracer. It is noteworthy that a slight deviation (<5%) might exist between americium and curium chemical yields in the same chemical procedure.
A suitable algorithm is very important to evaluate an alpha spectrum. A method based on the geometric progression decrease in the counts in the far tail of an alpha spectrum was developed for the simultaneous detection of plutonium, americium, and curium using alpha spectrometry [129]. For evaluating precision and accuracy, synthetic mixtures were prepared from solutions of enriched isotopes, and sources were prepared by direct evaporation and electrodeposition. Precision and accuracy of about 1% were demonstrated in the measurement of the 241Am/239Pu and 241Am/233U activity ratios using a silicon surface-barrier detector. Because of the relatively small energy difference, the peaks of 241Am (5.486 MeV, 85.2% abundance; 5.443 MeV, 12.8% abundance) and 243Am (5.277 MeV, 88% abundance; 5.486 MeV, 10.6% abundance) were partially overlapped. A series of synthetic mixtures covering a wide range (0.3 to 2.0) of 241Am/243Am alpha activity ratios from high-purity 241Am and 243Am solutions was employed to evaluate two algorithms used to account for the tail contribution due to energy degradation [130]. The results showed that precision and accuracy of about 1% could be achieved for 241Am/243Am activity ratios using alpha-spectrometry. In earlier years, special efforts have been made to resolve the overlapping of the 238Pu and 241Am peaks by means of an analytical function for fitting peaks in alpha spectra from Si detectors [131]. Similar to the early work of Bortels [131], M. P. Dion [126] presented the response of a silicon detector that was modeled as a convolution of a Gaussian model with one exponential function. These methods were based on the use of complex mathematical procedures to unfold the alpha spectra of radionuclides presented in the source. The impressive progress achieved by Devol et al. [132] was that 238Pu/241Am isotopic ratios of plated alpha sources were quantified by the alpha in a combination of conversion electron spectrometry using a cooled, passivated, ion-implanted planar silicon (PIPS) detector. However, the aforementioned methods have not found wide application for the measurement of 238Pu/241Am activity ratios due to their complexity. Up to now, none of the commercial spectrometers and spectrum evaluation software is available to distinguish the overlapping of 238Pu and 241Am peaks. Developing high-resolution alpha spectrometers should be regarded as an attractive avenue to perform the accurate determination of 241Am.
Alpha spectrometry has been used for many decades for 241Am measurement and is still a popular technique. For a typical Si detector assuming a 2-day measurement time, the limit of detection (LOD) for 241Am is obtained as 0.2–0.4 mBq/sample, which refers to 25–50 mBq/kg for 10 g of sample with an 80% chemical yield. The LOD can be improved by increasing the sample size, prolonging the counting time, and improving the chemical yield. The major disadvantage of alpha spectrometry is that it is time-consuming, especially when performing with low levels of 241Am, which can take from several days to several weeks depending on the concentrations of 241Am in samples. Attention should be paid in the purification of 241Am sources to avoiding any contamination from 238Pu, which emits alpha particles in similar energy range (5.499 MeV, 70.9% abundance; 5.456 MeV, 29.0% abundance) as 241Am. Due to these disadvantages, alpha spectrometry has been increasingly replaced by mass spectrometry in the last few years.
6. Mass Spectrometry for 241Am Measurement
More precise, accurate, and sensitive measurements of 241Am concentrations and isotope ratios at trace and ultra-trace levels are very necessary for environmental samples such as biological samples, soil, dust, and water. Mass spectrometric techniques are of interest due to their high sensitivity, multi-isotope capability, and high accuracy. Specially, 241Am/243Am isotope dilution mass spectrometry is the preferred method to determine the concentrations of 241Am in environmental samples precisely and accurately. Aggarwal et al. [5] reviewed mass spectrometric techniques for the analysis of americium several years before. In light of the significant progress in mass spectrometry techniques recently, the present status and trends of mass spectrometry for the measurement of 241Am are summarized in this review.
6.1. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
The powerful ICP-MS provides a fast and sensitive detection technique for long-lived radionuclides, such as actinides and 99Tc. In the initial phase of ICP-MS instrument development, the sensitivity of ICP-MS for the measurement of 241Am was not superior to alpha spectrometry [133]. Varga et al. [134] made an attempt to determine 241Am in Chernobyl soil using ICP-MS, but the limit of detection was only 104 fg/g. Nowadays, the analysis of 241Am (T1/2 = 432 years) can be carried out using ICP-MS with higher sensitivity and a lower detection limit [135]. ICP-MS has gained popularity compared to TIMS for 241Am determination in complex biological and environmental samples because of the less stringent requirements of sample purity, the ease of liquid sample introduction, the possibility to use another element as an internal standard for mass bias correction, the employment of an external calibration procedure, and the possibility for combination with an automated system. Chartier et al. [136] compared the performances of TIMS and ICP-MS to determine americium in spent nuclear fuels after separation with high-performance liquid chromatography. The results obtained with the double-spike isotope dilution method demonstrates that ID ICPMS was accurate and reliable for the determination of 241Am/238U and 244Cm/238U in spent reactor fuels. However, matrix effects, instrumental mass bias, spectroscopic and nonspectroscopic interferences, memory, and the carry-over effect still needed to be checked, minimized, and corrected. Potential interferences for the measurement of 241Am, including isobaric and polyatomic interferences, are listed in Table 8. These interferences could possibly be reduced by emphasis on the removal of interferences [137] using double-focusing sector field ICP-MS (SF-ICP-MS) [107] and high-resolution ICP-MS (HR-ICP-MS) [93] at the required mass resolution, as well as collision cells in quadrupole ICP-MS(Q-ICP-MS).

Table 8.
Isobaric and polyatomic interferences of 241Am in ICP-MS measurement.
Zheng et al. [138] reported a rapid analytical method for determining 241Am in marine sediment using isotope dilution SF-ICP-MS combined with a high-efficiency sample introduction system (APEX-Q). A low limit of detection 0.041 mBq/g (0.32 fg/g) was achieved that was two times lower than the typical detection limit achievable by alpha spectrometry (ca. 0.1mBq). The phenomenon was observed that the isobaric interference with the determination of 241Am could be effectively removed when He-NH3 was used as a collision–reaction gas in Q-ICP-MS, while high sensitivity was still kept. Zhang and coworkers [26] developed a method for 241Am measurement using triple quadruple ICP-MS (ICP-MS/MS) with He-NH3 as a collision–reaction gas. The extremely low limit of detection of 0.091 fg/g was three times better than those using other types of ICP-MS methods. Very recently, the same group reported a novel method to determine ultra-trace levels of 241Am using ICP-MS/MS with O2/He-He as the collision–reaction gas. The polyatomic ions formed by the interfering elements (Pb, Hg, and Tl) could be completely eliminated, even when Cl− was present in the solution. The detection limit of 241Am was as low as 0.017 fg/g [139]. Theoretically, the precision of isotope ratio measurements and the LOD can be improved by more than one order of magnitude using multiple-ion-collector ICP-MS (MC-ICP-MS) compared to single-collector ICP-MS. Steven J. Goldstein [140] applied MC-ICP-MS for the isotopic measurements of 241Am in environmental samples and obtained accurate results with a low detection limit of 1.4 fg. Table 9 compares the limits of detection (LODs) obtained with different types of ICP-MS instruments for 241Am measurement.

Table 9.
Comparison of LODs obtained by different ICP-MS instruments for 241Am measurement.
6.2. Thermal Ionization Mass Spectrometry (TIMS)
TIMS is a popular mass spectrometric technique for actinide isotope analysis to obtain isotope ratios with high accuracy (measurement trueness and precision). TIMS requires the element in pure chemical form to achieve high ionization efficiency. TIMS is free from polyatomic isobaric interferences and does not suffer from any memory or carry-over effect, as with ICP-MS. Because there is an inherent limitation of isotope fractionation in TIMS that leads to preferential evaporation of the lighter isotope, it is necessary to optimize the analysis conditions with a certified reference material (CRM) and to apply a mass fractionation correction factor to arrive at accurate isotope ratios. Thus far, no such CRM is available for americium. A meaningful attempt was made wherein three gravimetric mixtures with 241Am/243Am isotope ratios at, nominally, 1:1, 20:1, and 200:1 were prepared for calibrating TIMS instruments used for americium isotope measurement through an isotope dilution (ID) approach. The ID approach yielded analytical values with expanded uncertainties of ~0.1% (k = 2) [149].
An alternative method to overcome the limitation of isotope fractionation in TIMS is to employ total evaporation (TE) or ion current integration with a multi-collector detector system, which has been applied in U and Pu isotope analyses. The advantage of the TE methodology for isotope ratio measurement is that the sum-integrated ratio from the analytical technique is close to the true value. Alexandre Quemeta et al. [150] demonstrated that TIMS measurements with the TE method combined with isotope dilution could yield expanded uncertainties (k = 2) at 0.1% and 0.81% for the 241Am/243Am ratio and the concentration of 241Am. In a more recent study, they employed TIMS with total evaporation to measure Nd, Am, and Cm isotopes, and the uncertainty estimations were below 0.2% (k = 2) [151]. Multiple collector thermal ionization mass spectrometry (MC-TIMS) was evaluated for trace and ultra-trace levels of the isotope ratio analyses of actinides. The achieved high total efficiency and low background resulted in a detection limit of <0.1 fg 241Am using filament and cavity resin bead load techniques [152]. Up to now, the application of TIMS is still limited for the measurement of americium because this technique requires time-consuming and labor-intensive source preparation and cannot be hyphenated with online chemical procedures. Nevertheless, TIMS has the potential to meet high accuracy requirements when an americium isotopic CRM become available in the future.
6.3. Accelerator Mass Spectrometry (AMS)
Accelerator mass spectrometry (AMS) is presently one of the most sensitive analytical techniques for the determination of actinides. The reason for the high sensitivity of AMS is that the stripping process and acceleration of the ions to MeV energies provide both the destruction of the molecular isobaric background and a strong reduction in tailing interferences. The application of AMS to measure 241Am in the environment has become more and more popular in recent years. An attempt to use AMS to determine 241Am was performed by Kazi et al. [91] for soil samples, and the minimum detectable activity (MDA) of 241Am was achieved as 1.4 mBq (1.12 fg), much higher than the 0.3 mBq (0.24 fg) of MDA for alpha spectrometry. Quinto et al. [153] studied a method where actinides were concentrated from small amounts of groundwater and seawater via iron hydroxide coprecipitation and were directly pressed into sputter cathodes of AMS. The detection of the injected tracers for 243Am was nearly 8 × 10−3 fg/g. A method was tested to increase the beam current of americium for AMS using mixtures of PbF2 and NdF3, and the LOD of 241Am using this method was 1.8 fg [154]. Measurements of 241Am in oxide and fluoride coprecipitation matrices using AMS were also compared, and the results indicated that the fluoride anion beam method provided more than one order of magnitude better sensitivity than the oxide anion method. The detection limits of the fluoride anion method and oxide anion method were 0.3 fg and 1.5 fg, respectively [92].
Investigations on the performances and the potential backgrounds of americium analyses with low-energy AMS showed that the sub-fg range of 241Am could be determined relative to a 243Am tracer if the samples and AMS standards were prepared identically with regard to the matrix elements [155]. Xiongxin Dai [128] described a new bioassay method for the analysis of sub-fg levels of americium in large-volume urine samples using compact AMS, and the limit of detection for 241Am in urine was 0.1–0.2 fg/L. Another impressive work was the concentration of 241Am in groundwater from the Grimsel Test Site (Switzerland), with levels as low as 1.2 × 105 atoms/mL (0.048 fg/mL) determined using AMS [127]. However, due to the high cost of an AMS facility, it is not suitable for routine measurements [156]. Complicated and expensive experimental equipment and time-consuming sample preparation still restrict the application of AMS in the measurement of 241Am.
6.4. Quality Control and Uncertainty for 241Am Determination
Since the radioactivity level of 241Am in most environmental samples is extremely low, the quality control in determining 241Am is increasingly important. Quality control over the accuracy of the data was assured by participating in comparison runs and by analyzing CRMs for 241Am [157]. CRMs represent important benchmarks in identifying methodologies, detecting training needs, upgrading the quality of laboratories’ performances, and assessing the validity of analytical methods. Polona Tavčar [6] reported the certified value of 241Am in some CRMs, including IAEA300 (sediment from the Baltic Sea), IAEA135 (marine sediment from the Irish Sea), soil-6 (soil from Austria), IAEA375 (soil collected after the Chernobyl accident), IAEA385 (Irish Sea sediment), IAEA368 (ocean sediment from Mururoa Atoll), and NIST-SRM 4350b (Columbia River sediment). Due to the decay of 241Pu, the certified value of 241Am presented in the CRMs should be corrected for in-growth from 241Pu.
To properly evaluate the uncertainty of concentrations of 241Am in environmental samples, the uncertainty components should be identified. Those components come from gravimetric links, measurement repeatability, 241Am decay and in-growth, background, and the accuracy and purity of trace 243Am. Zhang [26] suggested that the uncertainty of the 241Am concentrations in CRMs and other samples mainly came from the uncertainty of the atomic ratio of 243Am and 241Am measured with mass spectrometry (the same situation happened in measurements with alpha spectrometry). The uncertainty of the 241Am concentrations in environmental samples was generally less than 30%.
7. Speciation Analyses of 241Am in Environmental Samples
As the behavior of 241Am in the environment is strictly connected with its physico-chemical forms, the speciation of 241Am is very important to predict its transfer and to estimate its mobility and bioavailability. 241Am released to the environment can be present in different species, ranging from simple ions and complexes to colloids, particles, and fragments. The following information can be obtained via a speciation analysis of 241Am: (1) the confinement to particles of various sizes; (2) the distribution among various geochemical fractions (exchangeable, oxidizable, reducible, sulfide, etc.); (3) the distribution among the cation, anion, and molecular forms; and (4) the chemical characteristics of the radionuclide (its host compound, nearest ligand shell, degree of oxidation, etc.) [158].
7.1. Soluble Species of 241Am in Natural Water
In general, the trivalent state (Am(III)) is the only prevalent oxidation state in ocean- and groundwater [159]. However, under most environmental conditions, americium may exist as complex species in addition to Am3+. The soluble species of 241Am in natural water are summarized in Table 10 [160]. Hui et al. [161] modeled the speciation distribution and solubility of americium in Chinese Beishan groundwater using PHREEQC software. The results indicated that americium mainly occurred as [AmCO3]− and [AmSiO(OH)3]2+ in neutral conditions, whereas AmOHCO3·0.5H2O and [Am(OH)3] became predominant in alkaline conditions. At the Australian legacy radioactive waste disposal site, the soluble species of americium in natural water were dominated primarily by cationic species, including Am3+, [AmCO3]+, [Am(OH)]2+, [Am(OH)2]+, and [Am(OH)3] [162]. The pH, as well as the concentration and types of ligands, in water affect the distribution of species compositions of 241Am.

Table 10.
Summary of soluble species of Am in natural water.
7.2. Particle- and Colloid-Associated 241Am in Natural Water
According to the definition presented by Salbu et al. [163], particles are defined as entities with diameters larger than 0.45 μm, while colloids or pseudo-colloids are defined as localized heterogeneities ranging in size from 1 nm to 0.45 μm. The americium species is known to be readily stuck to particles and exist in colloid and pseudo-colloid forms. 241Am can form colloidal fractions in natural water fairly easily, especially in its lower oxidation states (Am(III)). This is due to the low solubility of some of its compounds and its tendency to hydrolyze even under relatively acidic conditions. Pseudo-colloids of 241Am in water are ionic species associated with colloids of other origins, such as organic fractions and mineral oxides (e.g., silica). The pH, HCO3− content, metal concentration (aluminum), and presence of humic acids have all been identified as parameters influencing the formation of americium particulates. It was reported that americium was not readily able to be in particulate form (>0.45 μm) in most well- and streamwater in the Sarzhal region of the Semipalatinsk Nuclear Test Site [164]. Large proportions of americium (87%) were observed to be associated with mobile colloids in the submicron size range at the Australian legacy radioactive waste disposal site [162]. It was proved that, in acid, ion-poor water, only 17% of the 241Am was present as particles [165]. This implied that 87% of the 241Am existed in colloid, pseudo-colloid, and soluble forms. In more ion-rich water with neutral pH, a high amount of 241Am was found in particulate form, amounting to 67% in streamwater. Molero et al. [166] investigated the distribution of particulate americium in Spanish Mediterranean coastal waters by measuring concentrations of 241Am in suspended particulate matter after filtering (<0.22 μm) large volumes (200–300 L) of seawater samples. The results indicated that particulate americium constituted, on average, 45% of the total concentration in seawater, while soluble americium represented 55% of the total concentration. It is interesting to observe that the suspended particulate matter was enriched in 241Am by a factor of eight compared to 239Pu. This further confirmed the particle-reactive behavior of americium in natural water systems.
7.3. Fractionation Analyses of 241Am in Soil and Sediment
The most commonly used procedure for 241Am fractionation analysis in soil and sediment is sequential extraction. This is based on the method developed by Tessier [167] for the speciation of particulate trace metals in soil and sediment. This sequential extraction procedure can determine the fractionation of 241Am as several desired geochemical fractions that are leachable by reagents with different chemical compositions and strengths, such as exchangeability, binding to carbonates, binding to Fe-Mn oxides, and binding to organic and residual matter. Table 11 shows typical sequential extraction reagents and conditions used for the fractionation of 241Am.

Table 11.
Typical sequential extraction reagents and conditions for the fractionation of 241Am in soil and sediment.
The fractionation results of 241Am are very different with respect to different solid samples. In sea sediment, americium is always associated mainly with carbonates and organic matter, and the insoluble fractions are generally high. The distributions of 241Am fractions in sea sediments of different origins are presented in Table 12. In agricultural soil in the UK and western Europe, most of the 241Am was associated with organic matter [168]. In floodplain soil of the Yenisei River, americium was observed to be mostly associated with highly mobile organic matter, such as fulvo acids [159], while >70% of the 241Am was associated with organic matter in the Yenisei river sediment [169]. The distribution of 241Am in sediment traps from Lake Michigan showed that 75% of the 241Am was distributed in the Fe and Mn oxide fractions [170]. Americium was associated in Rocky Flats soil in the following order: sesquioxide (45%) > water soluble fraction (16%) > refractory silicate (14%) > carbonate (12%) > organic fraction (8%) > exchangeable fraction (6%) [171]. For samples from a former radioactivity laboratory during dismantling activities, most of the americium in solid residue was associated with carbonates (~18%), oxides (~41%), and residual phases (~32%). However, americium in tank mud samples demonstrated a more uniform distribution among carbonates (~29%) and organic (~36%) and residual (~24%) matter [172]. Lujaniena et al. [173] observed that 241Am was associated with acid solubles (41%) and residues (38.5%) in aerosol samples collected during the Chernobyl accident. As for contaminated soil samples from nuclear weapons test sites, a significant portion of the 241Am is associated with the residue phase. For example, Yanmei et al. [174] reported that 53–83.6% of americium remained in the residue phase in contaminated soil from western China. Similar results also occurred in contaminated soil from the Semipalatinsk Nuclear Test Site. These results indicated that americium released from nuclear weapons testing had limited mobility, and thus, its transfer and migration in the environment was not significant. Comparing the migration of 241Am and 239,240Pu in successive layers of Chinese forest, grassland, and desert soils, the migration behaviors of 241Am and 239,240Pu were rather similar; both velocities were less than 0.3cm/y in diverse types of soils [25].

Table 12.
Distributions of 241Am in different fractions in sea sediment.
8. Tracer Applications of 241Am in the Environment
8.1. 241Am as a Time-Marker for Sediment Dating
As an alternative to 137Cs, which has wide-spread applications for benchmarking sedimentation rate, applications of 241Am for sediment dating [177,178,179] or sedimentation rate in aquatic environments [180] have been reported occasionally. This method assumes that the 241Am level of fresh nuclear weapons test debris is essentially zero, and its presence in older deposits is through the in-growth of 241Pu in weapon-fallout-derived release. According to the reconstruction data of the cumulative 241Pu and 241Am inventories for the northern hemisphere since 1954, the distribution of 241Am was dominated by a peak in 1963, with ca. 20% of the inventory being attributed to that year [181]. In many cases, the value of 137Cs as a dating tool has been significantly reduced by the evident mobility of this isotope and its consequent limitation. However, 241Am is a preferable time-marker for a growing dataset from lakes, as well as regional and coastal seas, with a wide range of pH values, suggesting that 241Am is considerably less mobile than 137Cs and can provide a useful means of sediment-dating from the early 1960s, where the 137Cs signal has been significantly decayed. When the specific activity of 241Am in different layers of a sediment core are determined, it is reasonable to use the peak of 241Am activity to benchmark the year 1963 and to calculate the average accumulation rate. The half-life (432 years) of 241Am ensures that it remains detectable in lake sediment for several centuries, while 137Cs decays away during this period. The main challenge in applying 241Am as a time-marker lies in its low concentration. 241Am has not been widely adopted, partially as a result of difficulties in the rapid and reliable measurement of its trace levels.
8.2. 241Am Signatures in Nuclear Forensics
Nuclear forensics has been defined as the “analysis of intercepted illicit nuclear or radioactive material and any associated material to provide evidence for nuclear attribution”. This scientific analysis aims at providing clues about the intended use of the material and its history, providing investigative leads and possibly leading to a source attribution [182]. Many characteristic parameters (so-called signatures), including the ratio of 241Am with other isotopes (241Pu and 239+240Pu), often provide important information related to the source term and history of the materials.
The ‘age’ of plutonium material, as one of the key signatures which refers to the time elapsed since the last purification from its progeny, is the first parameter to be determined when deducing the history of plutonium material in nuclear forensics. The age of plutonium material can be determined in principle using four parent–daughter relations: 238Pu–234U, 239Pu–235U, 240Pu–236U, and 241Pu–241Am. Actually, the most important method for determining the age of plutonium is based on the 241Pu/241Am ratio due to the relatively short half-life of 241Pu (14.2 years). Therefore, the measurement of trace 241Am in plutonium material plays an important role in the age estimation of plutonium material. Figure 2 shows the decay chain of 241Pu.
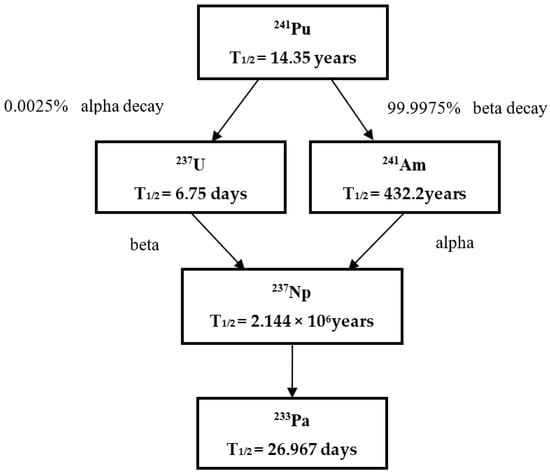
Figure 2.
The decay chain of 241Pu.
Many methods have been developed for the dating of plutonium in recent years. The nondestructive method using gamma spectrometry is based on measuring the specific activities of 241Am and 237U, which approach equilibrium with 241Pu to obtain the 241Pu/241Am ratio [183]. This method needs at least 100 mg of plutonium material for the measurement and showed excellent results for samples with ages ranging from 8–20 years [184]. M. Wallenius [185] determined the 241Pu/241Am ratio using TIMS and described a methodology allowing for the accurate determination of several almost ’30-year-old’ plutonium materials. Zhang et al. [186] developed a method to determine the 241Pu/241Am ratio in plutonium material using alpha spectrometry and TIMS. This method attempted to avoid the use of an isotope dilution analysis so that it could be applicable for labs that could not obtain suitable isotope tracers, such as 243Am and 242Pu isotopes. Yan Chen [187] compared different detection methods, such as MC-ICP-MS, alpha spectrometry, and liquid scintillation counting (LSC), for age determination from the 241Pu/241Am ratios. The study showed that MC-ICP-MS provided the most accurate and precise ‘age’, with a typical precision of 1.5−3% for an ng amount of Pu material. However, the combination of alpha spectrometry with LSC produced 5−10% negatively biased results. The reason for this difference was attributed to the high uncertainty of the LSC measurement of 241Pu. Mats Eriksson [188] analyzed the americium signatures of five isolated, hot particles from the Thule accident (1968) using alpha spectrometry and ICP-MS. From the activity ratio of 241Pu/241Am, the ages of weapon-grade plutonium materials were estimated to be from the late 1950s to the early 1960s. This was in good agreement with [189], who estimated the time of production of the material to be 1960 ± 4.
All of the above-mentioned methods have relied on the hypothesis that there were no daughters (241Am, 235U, 234U, and 236U) existing in the plutonium material at the initial time. Zsolt Varga described a method for the preparation and validation of plutonium age-dating reference materials. The age values obtained for the test samples using the four different parent–daughter pairs (chronometers) were in excellent agreement and were also consistent with the known production date [190]. However, for US weapon-grade plutonium, 241Am/241Pu often gives significantly larger values for age than U isotopes such as 235U and 236U. The only reasonable explanation for this observation is that, when the uranium isotopes are removed from the plutonium sample for the last time, some americium is left in the material. In fact, it is still a challenge to identify freshly produced plutonium material through parent–daughter pairs. H.T. Zhang [186] reported a minimum reachable age and estimated that it was possible to distinguish only 134 days of newly produced plutonium materials from aged ones through the 241Pu/241Am ratio.
The inventory of 241Am and the isotopic ratios in environmental samples can provide clues to attribute the origins of 241Am and other isotopes. Several successful cases have been reported recently. Lichens and mosses from coastal zones of the Canadian Arctic and Alaska were sampled, and the analysis of the isotopic ratio exhibited the dominant contribution of the global fallout (SNAP 9A satellite re-entry fallout) for the presence of 241Am and plutonium isotopes [28]. Pierre-Andre Pittet [191] analyzed 241Am, 241Pu, 239+240Pu, and 90Sr in a few selected soil samples obtained near the nuclear reprocessing plant of La Hague. The results revealed the presence of previous environmental contamination originating from several incidents at the La Hague site involving atmospheric transfer and leaks in flooded waste pits. The radioactivity of 241Am and plutonium isotopes in soil cores from the Gambier Island (French Polynesia) were higher than the global fallout at this latitude, thus confirming that the dominant source of these radionuclides was from the local fallout during the 1970s of the French atmospheric tests of Moruroua and Fangataufa (located nearly 400 km from Gambier) [192].
9. Conclusions and Perspectives
As a result of atmospheric nuclear weapons testing and controlled or uncontrolled releases from nuclear reprocessing plants and nuclear accidents, 241Am has been introduced to different extents into the environment. The measurement of 241Am in the environment is of importance in monitoring its release and in assessing the environmental impact and radiological risk; thus, effective analytical techniques are needed. The separation of trivalent americium is always an issue in actinide chemistry due to lack high-efficiency procedures for americium and of high selectivity between americium and lanthanides. The most impressive progress might be the development and wide application of commercial extraction chromatographic resins, which continue to play an important role in the chemical separation of americium. Combined procedures based on different resins are aimed at finding more efficient and effective procedures for 241Am determination. Automated systems focus on increasing the analytical speed and throughput and reducing the lab intensity. Alpha spectrometry has been used for many decades for the measurement 241Am and continues be a popular technique due to its low cost. ICP-MS has shown higher sensitivity than alpha spectrometry and has begun to be applied more often for the determination of 241Am in the environment in recent years. More research works may be needed on the speciation of 241Am in soils and sediments, as 241Am is potentially a more soluble isotope than 239Pu. The tracer applications of 241Am in the environment have made remarkable progress. 241Am as a time-marker has been applied for sediment dating. The ratios of 241Am with other isotopes (241Pu and 239+240Pu) are important signatures, providing important information related to the history of materials and the origins of sources in nuclear forensics.
Author Contributions
Conceptualization, H.Z., X.H. and J.Q.; resources, H.Z., X.H., J.Q. and J.L.; writing—original draft preparation, H.Z.; writing—review and editing, X.H., J.Q. and J.L.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of China grant number [2015FY110800].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMS | Accelerator-based mass spectrometry |
| BTBP | 2,6-bis(5,6-dialkyl-1,2,4-triazinyl-3-yl)dipyridine |
| BTP | 2,6-bis(5,6-dialkyl-1,2,4-triazinyl-3-yl)pyridine |
| CA-BTP | bis-2,6-(5,6,7,8-tetrahydro-5,9,9-trimethyl-5,8-methano-1,2,4-benzotriazin-3-yl) pyridine |
| CMPO | octyl, phenyl-N, N-di-iso-butyl carbamoylmethyl phosphine oxide |
| CRM | certified reference material |
| CyMe4BTBP | 6,6′-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrobenzo [1,2,4]triazin-3-yl)-2,2′-bipyridine |
| CyMe4BTPhen | 2,9-bis(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo [1,2,4]triazin-3-yl)-1,10-phenanthroline |
| DAm | weight distribution ration |
| DDCP | dibutyl-N,N′-diethylcarbamylphosphonate |
| DF | decontamination factor |
| DGA | di glycol amide |
| DIPEX | a commercial resin consisting of bis(2-ethylhexyl)methanediphosphonic acid |
| DIPHONIX | a commercial resin containing sulfonic and gem-diphosphonic acid groups |
| DTPA | 2-hydorxy-1,3-diaminopropane tetra-acetic acid |
| EC | extraction chromatography |
| FI | flow injection |
| FPs | fission products |
| FWHW | full width at half maximum |
| HDEHP | di-2-ethylhexylphosphoric acid |
| IAEA | international atomic energy agency |
| ICP-MS | inductively coupled plasma mass spectrometry |
| ID | isotope dilution |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MC | multiple-ion collector |
| MCFI | multi-commuted flow injection |
| MNPs | magnetic nanoparticles |
| MOB-BTP | 3,3′-dimethoxy-phenyl-bis-1,2,4-triazinyl-2,6-pyridine |
| MPFS | multi-pumping flow system |
| MSFI | multi-syringe flow injection |
| PI | pressurized injection |
| PIPS | passivated ion-implanted planar silicon |
| PMBP | 1-benzyl-3-methyl-4-benzoyl acetyl acetone |
| Q-ICP-MS | quadrupole ICP-MS |
| RE | rare-earth |
| SAMMS | self-assembled monolayer on mesoporous supports |
| SF | sector field |
| STS | Semipalatinsk Test Site |
| SI | sequential injection |
| TBP | tributyl phosphate |
| TE | total evaporation |
| TEVA | tetravalent actinide |
| TIMS | thermal ionization mass spectrometry |
| TIOA | tri-iso-octylamine |
| TOA | trioctylamine |
| TONA | tri-n-octylaime |
| TOPO | trioctyl phosphine oxide |
| TRU | trans-uranium |
| TTA | triethylene tetramine |
| UTEVA | uranium and tetravalent actinides |
| WHO | World Health Organization |
| XRD | X-ray diffraction |
References
- Warwick, P.; Croudace, I.; Carpenter, R. Review of analytical techniques for the determination of Americium-241 in soils and sediments. Appl. Radiat. Isot. 1996, 47, 627–642. [Google Scholar] [CrossRef]
- Hou, X.; Roos, P. Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal. Chim. Acta 2008, 608, 105–139. [Google Scholar] [CrossRef] [PubMed]
- Vajda, N.; Kim, C. Determination of Transuranium Isotopes (Pu, Np, Am) by Radiometric Techniques: A Review of Analytical Methodology. Anal. Chem. 2011, 83, 4688–4719. [Google Scholar] [CrossRef]
- Vajda, N.; Kim, C. Determination of Am-241 isotope: A review of analytical methodology. J. Radioanal. Nucl. Chem. 2010, 284, 341–366. [Google Scholar] [CrossRef]
- Aggarwal, S.K. A review on the mass spectrometric studies of americium: Present status and future perspective. Mass Spectrom. Rev. 2018, 37, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Tavcar, P.; Jakopic, R.; Benedik, L. Sequential determination of 241Am, 237Np, Pu radioisotopes and 90Sr in soil and sediment samples. Acta Chim. Slov. 2005, 52, 60–66. [Google Scholar]
- Osváth, S.; Vajda, N.; Molnár, Z. Development of a complex method for the determination of actinoides. J. Radioanal. Nucl. Chem. 2009, 281, 461–465. [Google Scholar] [CrossRef]
- Burns, J.; Shehee, T.; Clearfield, A.; Hobbs, D. Separation of Americium from Curium by Oxidation and Ion Exchange. Anal. Chem. 2012, 84, 6930–6932. [Google Scholar] [CrossRef]
- Thakur, P.; Mulholland, G.P. Determination of 237Np in environmental and nuclear samples: A review of the analytical method. Appl. Radiat. Isot. 2012, 70, 1747–1778. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation[R]; United Nations Scientific Committee on the Effects of Atomic Radiation: Vienna, Austria, 2000; ISBN 92-1-142238-8. [Google Scholar]
- Pazukhin, E.M.; Drozd, I.P.; Tokarevskii, V.V. Chernobyl accident and the problem of 241Am. Radiochemistry 1995, 36, 590–597. [Google Scholar]
- Muravitsky, A.V.; Razbudey, V.F.; Tokarevsky, V.V.; Vorona, P.N. Time-dependent 241Am activity in the environment from decay of 241Pu released in the Chernobyl accident. Appl. Radiat. Isot. 2005, 63, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Leonard, K.; Hughes, L. Artificial radionuclides in the Irish Sea from Sellafield: Remobilisation revisited. J. Radiol. Prot. 2013, 33, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Cundy, A.B.; Croudace, I.W.; Warwick, P.E.; Oh, J.; Haslett, S.K. Accumulation of COGEMA-La Hague-derived Reprocessing Wastes in French Salt Marsh Sediments. Environ. Sci. Technol. 2002, 36, 4990–4997. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.B.; Scott, R.D. Sellafield waste radionuclides in Irish sea intertidal and salt marsh sediments. Environ. Geochem. Hlth. 1993, 15, 173–184. [Google Scholar] [CrossRef]
- Ikaheimonen, T.; Ilus, E.; Klemola, S.; Dahlgaard, H.; Ryan, T.; Eriksson, M. Plutonium and americium in the sediments off the Thule air base, Greenland. J. Radioanal. Nucl. Chem. 2002, 252, 339–344. [Google Scholar] [CrossRef]
- Aarkrog, A.; Boelskifte, S.; Dahlgaard, H.; Duniec, S.; Holm, E.; Smith, J.N. Studies of transuranics in an arctic marine environment. J. Radioanal. Nucl. Chem. 1987, 115, 39–50. [Google Scholar] [CrossRef]
- Aarkrog, A. Inventory of Nuclear Releases in the World. In Radioecology and the Restoration of Radioactive-Contaminated Sites; Luykx, F.F., Frissel, M.J., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1996; Volume 13, pp. 31–43. ISBN 978-94-009-0301-2. [Google Scholar]
- Ahn, J.; Carson, C.; Jensen, M.; Juraku, K.; Nagasaki, S.; Tanaka, S. Reflections on the Fukushima Daiichi Nuclear Accident: Toward Social-Scientific Literacy and Engineering Resilience; Springer Nature: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Duffa, C.; Renaud, P.; Goutelard, F. Activities and transfers of Pu and Am in rice samples from Camargue, France. J. Radioanal. Nucl. Chem. 2002, 252, 247–248. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sakaguchi, A.; Ochiai, S.; Takada, T.; Hamataka, K.; Murakami, T.; Nagao, S. Isotopic Pu, Am and Cm signatures in environmental samples contaminated by the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2014, 132, 31–46. [Google Scholar] [CrossRef]
- Lujanienė, G.; Valiulis, D.; Byčenkienė, S.; Aakalys, J.; Povinec, P.P. Plutonium isotopes and 241Am in the atmosphere of Lithuania: A comparison of different source terms. Atmos. Environ. 2012, 61, 419–427. [Google Scholar] [CrossRef]
- Thakur, P.; Mulholland, G.P. Determination of Pu, Am, U and Cs in large soil samples in the vicinity of the USDOE Waste Isolation Pilot Plant. J. Radioanal. Nucl. Chem. 2011, 288, 499–506. [Google Scholar] [CrossRef]
- Popov, L.; Mihailova, G.; Naidenov, I. Determination of activity ratios of 238,239+240,241Pu, 241Am, 134,137Cs, and 90Sr in Bulgarian soils. J. Radioanal. Nucl. Chem. 2010, 285, 223–237. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Z.; Guo, Q.; Zheng, J.; Li, S.; Lin, J.; Tan, Z.; Huang, W. Distinctive distributions and migrations of 239+240Pu and 241Am in Chinese forest, grassland and desert soils. Chemosphere 2018, 212, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Fang, S.; Hou, X.; Zhang, L.; Dang, H.; Yi, X.; Zhai, S.; Wang, W.; Xu, J. Determination of ultra-low level 241Am in soil and sediment using chemical separation and triple quadrupole inductively coupled plasma mass spectrometry measurement with He-NH3 as collision-reaction gas. Spectrochim. Acta Part B At. Spectrosc. 2021, 178, 106113. [Google Scholar] [CrossRef]
- Holgye, Z.; Schlesingerova, E.; Tecl, J.; Filgas, R. 238Pu, 239,240Pu, 241Am, 90Sr and 137Cs in soils around nuclear research centre Rez, near Prague. J. Environ. Radioact. 2004, 71, 115–125. [Google Scholar] [CrossRef]
- Cwanek, A.; Mietelski, J.W.; Bokas, E.; Olech, M.A.; Anczkiewicz, R.; Misiak, R. Sources and variation of isotopic ratio of airborne radionuclides in Western Arctic lichens and mosses. Chemosphere 2020, 239, 124783. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Desideri, D.; Guerra, F.; Meli, M.A.; Testa, C. Concentration and vertical distribution of plutonium and americium in Italian mosses and lichens. J. Radioanal. Nucl. Chem. 1997, 222, 3–9. [Google Scholar] [CrossRef]
- Wan Mahmood, Z.U.Y.; Shahar, H.; Ahmad, Z.; Wo, Y.M.; Abu Bakar, A.S. Behavior and distribution of 239+240Pu and 241Am in the east coast of Peninsular Malaysia marine environment. J. Radioanal. Nucl. Chem. 2010, 286, 265–272. [Google Scholar] [CrossRef]
- Mulsow, S.; Coquery, M.; Dovlete, C.; Gastaud, J.; Ikeuchi, Y.; Pham, M.K.; Povinec, P.P. Radionuclide concentrations in underground waters of Mururoa and Fangataufa Atolls. Sci. Total Environ. 1999, 237–238, 287–300. [Google Scholar] [CrossRef]
- Lee, S.; Povinec, P.P.; Wyse, E.; Pham, M.K.; Hong, G.; Chung, C.; Kim, S.; Lee, H. Distribution and inventories of 90Sr, 137Cs, 241Am and Pu isotopes in sediments of the Northwest Pacific Ocean. Mar. Geol. 2005, 216, 249–263. [Google Scholar] [CrossRef]
- Ugur, A.; Yener, G. Plutonium isotopes, 241Am and 137Cs activity concentrations in marine sediments of Gokova Bay, Aegean Turkish Coast. J. Radioanal. Nucl. Chem. 2002, 252, 47–51. [Google Scholar] [CrossRef]
- Mihai, S.; Hurtgen, C. Plutonium and americium in sediment samples along the Romanian sector of the Danube river and the Black Sea coast. J. Radioanal. Nucl. Chem. 1997, 222, 275–278. [Google Scholar] [CrossRef]
- Kim, C.K.; Morita, S.; Seki, R.; Takaku, Y.; Ikeda, N.; Assinder, D.J. Distribution and behaviour of 99Tc, 237Np, 239,240Pu, and 241Am in the coastal and estuarine sediments of the Irish Sea. J. Radioanal. Nucl. Chem. 1992, 156, 201–213. [Google Scholar] [CrossRef]
- Desideri, D.; Giuliani, S.; Meli, M.A.; Testa, C.; Triulzi, C.; Vaghi, M. Presence of 137Cs, Pu isotopes and 241Am in ligurian and Tyrrhenian Seas sediments. J. Radioanal. Nucl. Chem. 2004, 260, 9–12. [Google Scholar] [CrossRef]
- Caroli, S.; Forte, M.; Nuccetelli, C.; Rusconi, R.; Risica, S. A short review on radioactivity in drinking water as assessed by radiometric and Inductively Coupled Plasma-Mass Spectrometry techniques. Microchem. J. 2013, 107, 95–100. [Google Scholar] [CrossRef]
- Liu, N.; Liao, J.; Yang, Y.; Luo, S.; Luo, Q.; An, Z.; Duan, Y.; Liu, M.; Zhao, P. Biosorption of 241Am by Saccharomyces cerevisiae: Preliminary investigation on mechanism. J. Radioanal. Nucl. Chem. 2008, 275, 173–180. [Google Scholar] [CrossRef]
- Baigazinov, Z.; Lukashenko, S.N.; Panitsky, V.; Kadyrova, N.Z.; Karatayev, S.S.; Mamyrbayeva, S.; Baigazy, S.; Bazarbaeva; Kabdyrakova, A.B.; Kunduzbaeva, E.; et al. The transfer of 239 + 240Pu, 241Am, 137Cs and 90Sr to the tissues of horses. J. Environ. Radioact. 2020, 222, 106322. [Google Scholar] [CrossRef]
- Mamyrbayeva, A.S.; Baigazinov, Z.A.; Lukashenko, S.N.; Panitskiy, A.V.; Karatayev, S.S.; Baigazy, S.A.; Bazarbayeva, A.B.; Zhadyranova, A.A.; Kenzhina, L.B.; Mukhamediyarov, N.; et al. The excretion of 241Am and 137Cs from the broilers organs after long-term application. J. Environ. Radioact. 2021, 229–230, 106543. [Google Scholar] [CrossRef]
- Bolotov, B.M.; Gaitinov, A.C.; Polyakov, A.I.; Chuburkov, Y.T.; Perelygin, V.P.; Drobina, T.P.; Kravets, L.I.; Timokhin, S.N.; Mietelski, J.M.; Szeglowski, Z.; et al. On the determination of and content in human tissues. Radiat. Meas. 2003, 36, 541–545. [Google Scholar] [CrossRef]
- Sokolik, G.A.; Ovsiannikova, S.V.; Ivanova, T.G.; Leinova, S.L. Soil-plant transfer of plutonium and americium in contaminated regions of Belarus after the Chernobyl catastrophe. Environ. Int. 2004, 30, 939–947. [Google Scholar] [CrossRef]
- Lehto, J.; Vaaramaa, K.; Leskinen, A. 137Cs, 239,240Pu and 241Am in boreal forest soil and their transfer into wild mushrooms and berries. J. Environ. Radioact. 2013, 116, 124–132. [Google Scholar] [CrossRef]
- Wang, Z.T.; Zheng, J.; Tagami, K.; Uchida, S. Newly derived transfer factors for Th, Am, Pu, and Cl since publication of IAEA TRS No. 472: A review. J. Radioanal. Nucl. Chem. 2015, 306, 11–20. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, G.; Zheng, J.; Cao, L.; Yu, H.; Zhu, Y.; Tagami, K.; Uchida, S. Effect of Ashing Temperature on Accurate Determination of Plutonium in Soil Samples. Anal. Chem. 2015, 87, 5511–5515. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.J.; Hensley, C.A.; Armenta, C.E.; Peters, R.J. Environmental and Human Monitoring of Americium-241 Utilizing Extraction Chromatography and α-Spectrometry. Anal. Chem. 1997, 69, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Kahn, B. Total dissolution of environmental and biological samples by closed-vessel microwave digestion for radiometric analysis. J. Radioanal. Nucl. Chem. 2001, 250, 85–91. [Google Scholar] [CrossRef]
- Mietelski, J.W.; Kubica, B.; Gaca, P.; Tomankiewicz, E.; Błażej, S.; Tuteja-Krysa, M.; Stobiński, M. 238Pu, 239 + 240Pu, 241Am, 90Sr and 137Cs in mountain soil samples from the Tatra National Park (Poland). J. Radioanal. Nucl. Chem. 2008, 275, 523–533. [Google Scholar] [CrossRef]
- Luisier, F.; Alvarado, J.; Steinmann, P.; Krachler, M.; Froidevaux, P. A new method for the determination of plutonium and americium using high pressure microwave digestion and alpha-spectrometry or ICP-SMS. J. Radioanal. Nucl. Chem. 2009, 281, 425–432. [Google Scholar] [CrossRef]
- Maxwell, S. Rapid method for determination of plutonium, americium and curium in large soil samples. J. Radioanal. Nucl. Chem. 2008, 275, 395–402. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Culligan, B.; Hutchison, J.B.; Mcalister, D.R. Rapid fusion method for the determination of Pu, Np, and Am in large soil samples. J. Radioanal. Nucl. Chem. 2015, 305, 599–608. [Google Scholar] [CrossRef]
- Maxwell, S.; Culligan, B.; Kelsey-Wall, A.; Shaw, P. Rapid radiochemical method for determination of actinides in emergency concrete and brick samples. Anal. Chim. Acta 2011, 701, 112–118. [Google Scholar] [CrossRef]
- Reis, A.; Temba, E.; Kastner, G.; Monteiro, R. Combined procedure using radiochemical separation of plutonium, americium and uranium radionuclides for alpha-spectrometry. J. Radioanal. Nucl. Chem. 2011, 287, 567–572. [Google Scholar] [CrossRef]
- Sidhu, R. A robust procedure for the determination of plutonium and americium in seawater. J. Radioanal. Nucl. Chem. 2003, 256, 501–504. [Google Scholar] [CrossRef]
- Joshi, S.R. Lanthanum fluoride coprecipitation technique for the preparation of actinides for alpha-particle spectrometry. J. Radioanal. Nucl. Chem. 1985, 90, 409–414. [Google Scholar] [CrossRef]
- Rao, R.R.; Cooper, E.L. Separation of low levels of actinides by selective oxidation/reduction and co-precipitation with neodymium fluoride. J. Radioanal. Nucl. Chem. 1995, 197, 133–148. [Google Scholar] [CrossRef]
- Kimura, T. Simultaneous determination of neptunium, plutonium, americium and curium using coprecipitation with bismuth phosphate. J. Radioanal. Nucl. Chem. 1990, 139, 297–305. [Google Scholar] [CrossRef]
- Sajeniouk, A.D. Routine radiochemical method for the determination of 90Sr, 238Pu, 239 + 240Pu, 241Am and 244Cm in environmental samples. J. Radioanal. Nucl. Chem. 2005, 264, 337–342. [Google Scholar] [CrossRef]
- Crespo, M.T.; Gascón, J.L.; Aceña, M.L. Techniques and analytical methods in the determination of uranium, thorium, plutonium, americium and radium by adsorption on manganese dioxide. Sci. Total Environ. 1993, 130–131, 383–391. [Google Scholar] [CrossRef]
- Suarez-Navarro, J.; Pujol, L.; de Pablo, M. Rapid determination of gross alpha-activity in sea water by coprecipitation. J. Radioanal. Nucl. Chem. 2002, 253, 47–52. [Google Scholar] [CrossRef]
- Montaña, M.; Camacho, A.; Vallés, I.; Serrano, I. Experimental analysis of the mass efficiency curve for gross alpha activity and morphological study of the residue obtained by the co-precipitation method. Appl. Radiat. Isot. 2012, 70, 1541–1548. [Google Scholar] [CrossRef]
- Singhal, R.K.; Basu, H.; Reddy, A.V.R. Removal of environmental level of 239 + 240Pu and 241Am from groundwater by using humic coated colloidal suspension of goethite (α-FeO(OH)). J. Radioanal. Nucl. Chem. 2013, 295, 1345–1351. [Google Scholar] [CrossRef]
- Kikunaga, H.; Kasamatsu, Y.; Takamiya, K.; Ohtsuki, T.; Yuki, H.; Yokoyama, A.; Nakanishi, T.; Mitsugashira, T. Development of a rapid source preparation method for high-resolution α-particle spectrometry. Appl. Radiat. Isot. 2009, 67, 539–543. [Google Scholar] [CrossRef]
- Fryxell, G.E.; Lin, Y.; Fiskum, S.; Birnbaum, J.C.; Wu, H.; Kemner, K.; Kelly, S. Actinide Sequestration Using Self-Assembled Monolayers on Mesoporous Supports. Environ. Sci. Technol. 2005, 39, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fiskum, S.K.; Yantasee, W.; Wu, H.; Mattigod, S.V.; Vorpagel, E.; Fryxell, G.E.; Raymond, K.N.; Xu, J. Incorporation of Hydroxypyridinone Ligands into Self-Assembled Monolayers on Mesoporous Supports for Selective Actinide Sequestration. Environ. Sci. Technol. 2005, 39, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Yantasee, W.; Sangvanich, T.; Creim, J.A.; Pattamakomsan, K.; Wiacek, R.J.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C. Functional Sorbents for Selective Capture of Plutonium, Americium, Uranium, and Thorium in Blood. Health Phys. 2010, 99, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Zhang, H.; Martin, L.; Todd, T.; Qiang, Y. Conjugates of Magnetic Nanoparticle—Actinide Specific Chelator for Radioactive Waste Separation. Environ. Sci. Technol. 2013, 47, 11942–11959. [Google Scholar] [CrossRef]
- Testa, C.; Desideri, D.; Guerra, F.; Meli, M.A.; Roselli, C.; Jia, G. The importance of separation chemistry for the determination of radionuclides in environmental samples. J. Radioanal. Nucl. Chem. 1998, 229, 23–32. [Google Scholar] [CrossRef]
- Seike, M.; Eguchi, M.; Shinohara, A.; Yoshimura, T. Solvent extraction behaviors of Americium(III) and Europium(III) using 2-hydroxy-2-methyloctanoic acid and 2-hydroxy-2-trifluoromethyloctanoic acid. J. Radioanal. Nucl. Chem. 2015, 303, 1413–1416. [Google Scholar] [CrossRef]
- Kolarik, Z.; Müllich, U.; Gassner, F. Selective extraction of Am(III)over Eu(III) by 2,6-ditriazolyl- and 2,6-ditriazinylpyridine. Solvent Extr. Ion Exch. 1999, 17, 23–32. [Google Scholar] [CrossRef]
- Panak, P.J.; Geist, A. Complexation and Extraction of Trivalent Actinides and Lanthanides by Triazinylpyridine N-Donor Ligands. Chem. Rev. 2013, 113, 1199–1236. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, S.; Ito, T.; Hitomi, K.; Kuraoka, E.; Usuda, S.; Ishii, K. Adsorption behavior of trivalent americium and rare earth ions onto a macroporous silica-based isobutyl-BTP/SiO2-P adsorbent in nitric acid solution. J. Radioanal. Nucl. Chem. 2014, 299, 149–155. [Google Scholar] [CrossRef]
- Liu, R.; Wei, Y.; Xu, Y.; Usuda, S.; Kim, S.; Yamazaki, H.; Ishii, K. Evaluation study on properties of isohexyl-BTP/SiO2-P resin for direct separation of trivalent minor actinides from HLLW. J. Radioanal. Nucl. Chem. 2012, 292, 537–544. [Google Scholar] [CrossRef]
- Usuda, S.; Wei, Y.; Liu, R.; Li, Z.; Xu, Y.; Wu, Y.; Kim, S. Challenges to develop single-column MA(III) separation from HLLW using R-BTP type adsorbents. Sci. China Chem. 2012, 55, 1732–1738. [Google Scholar] [CrossRef]
- Usuda, S.; Wei, Y.; Xu, Y.; Li, Z.; Liu, R.; Kim, S.; Wakui, Y.; Hayashi, H.; Yamazaki, H. Development of a simplified separation process of trivalent minor actinides from fission products using novel R-BTP/SiO2-P adsorbents. J. Nucl. Sci. Technol. 2012, 49, 334–342. [Google Scholar] [CrossRef]
- Tevepaugh, K.N.; Coonce, J.; Tai, S.; Delmau, L.H.; Carrick, J.D.; Ensor, D.D. Chromatographic separation of americium from europium using bis-2,6-(5,6,7,8-tetrahydro-5,9,9-trimethyl-5,8-methano-1,2,4-benzotriazin-3-yl) pyridine. J. Radioanal. Nucl. Chem. 2017, 314, 371–376. [Google Scholar] [CrossRef]
- Tevepaugh, K.N.; Carrick, J.D.; Tai, S.; Coonce, J.G.; Delmau, L.H.; Ensor, D.D. Separation of Americium from Europium using Camphor-BisTriazinyl Pyridine: A Fundamental Study. Solvent Extr. Ion Exch. 2016, 34, 13–25. [Google Scholar] [CrossRef]
- Hill, T.G.; Chin, A.L.; Tai, S.; Carrick, J.D.; Ensor, D.D.; Delmau, L.H. Separation of americium from europium using 3,3′-dimethoxy-phenyl-bis-1,2,4-triazinyl-2,6-pyridine. Sep. Sci. Technol. 2018, 53, 1848–1855. [Google Scholar] [CrossRef]
- Mahmoud, J.; Higginson, M.; Thompson, P.; Gilligan, C.; Livens, F.; Heath, S.L. Rapid separation of americium from complex matrices using solvent impregnated triazine extraction chromatography resins. J. Chromatogr. A 2022, 1669, 462950. [Google Scholar] [CrossRef]
- Kiliari, T.; Pashalidis, I. Americium and samarium determination in aqueous solutions after separation by cation-exchange. J. Radioanal. Nucl. Chem. 2014, 299, 721–724. [Google Scholar] [CrossRef]
- Odintsov, A.A.; Pazukhin, E.M.; Khan, V.E. Procedure for Simultaneous Determination of Uranium and Transuranium Elements in Groundwater and Liquid Radioactive Wastes from the Shelter. Radiochemistry 2005, 47, 510–515. [Google Scholar] [CrossRef]
- Jia, G.; Desideri, D.; Guerra, F.; Meli, M.A.; Testa, C. Determination of plutonium and americium in moss and lichen samples. J. Radioanal. Nucl. Chem. 1997, 220, 15–19. [Google Scholar] [CrossRef]
- Desideri, D.; Meli, M.A.; Roselli, C.; Testa, C.; Boulyga, S.F.; Becker, J.S. Determination of 236U and transuranium elements in depleted uranium ammunition by α-spectrometry and ICP-MS. Anal. Bioanal. Chem. 2002, 374, 1091–1095. [Google Scholar] [CrossRef]
- Desideri, D.; Feduzi, L.; Meli, M.; Roselli, C. Sequential determination of Am, Cm, Pu, Np and U by extraction chromatography. Microchem. J. 2011, 97, 264–268. [Google Scholar] [CrossRef]
- Mohandas, J.; Srinivasa Rao, V.; Vijayakumar, N.; Kumar, T.; Velmurugan, S.; Narasimhan, S.V. Uptake of actinides by sulphonated phosphinic acid resin from acid medium. J. Radioanal. Nucl. Chem. 2014, 302, 1185–1188. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Chiarizia, R.; Dietz, M.L.; Diamond, H.; Nelson, D.M. Separation and preconcentration of actinides from acidic media by extraction chromatography. Anal. Chim. Acta 1993, 281, 361–372. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Dietz, M.L.; Chiarizia, R.; Diamond, H.; Maxwell, S.L.; Nelson, M.R. Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: Application to the characterization of high-level nuclear waste solutions. Anal. Chim. Acta 1995, 310, 63–78. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Chiarizia, R.; Dietz, M.L. DIPEX: A new extraction chromatographic material for the separation and preconcentration of actinides from aqueous solution. React. Funct. Polym. 1997, 33, 25–36. [Google Scholar] [CrossRef]
- Macsik, Z.; Groska, J.; Vajda, N.; Vogt, S.; Kis-Benedek, G.; Kim, C.S.; Maddison, A.; Donohue, D. Improved radioanalytical method for the simultaneous determination of Th, U, Np, Pu and Am(Cm) on a single TRU column by alpha spectrometry and ICP-MS. Radiochim. Acta 2013, 101, 241–252. [Google Scholar] [CrossRef]
- Yi, X.W.; Li, D.M.; Dang, H.J.; Li, M.; Zhang, H.T. Separation of Transuranium Elements With UTEVA Extraction Chromatography. J. Nucl. Radiochem. 2010, 32, 22–26. [Google Scholar]
- Kazi, Z.H.; Cornett, J.R.; Zhao, X.; Kieser, L. Americium and plutonium separation by extraction chromatography for determination by accelerator mass spectrometry. Anal. Chim. Acta 2014, 829, 75–80. [Google Scholar] [CrossRef]
- Kazi, Z.H.; Cornett, R.J.; Zhao, X.; Kieser, W.E. Comparison of the measurement of Pu and Am isotopes by AMS using fluoride and oxide anion beams. J. Anal. Atom. Spectrom. 2015, 3, 2224–2235. [Google Scholar] [CrossRef]
- Xiao, G.; Saunders, D.; Jones, R.L.; Caldwell, K.L. Determination of 241Am in urine using sector field inductively coupled plasma mass spectrometry (SF-ICP-MS). J. Radioanal. Nucl. Chem. 2014, 301, 285–291. [Google Scholar] [CrossRef][Green Version]
- Chiariza, R.; Horwitz, E.P.; Alexandrators, S.D.; Gula, M.J. Diphonix® Resin: A Review of Its Properties and Applications. Sep. Sci. Technol. 1997, 32, 1–35. [Google Scholar] [CrossRef]
- Croudace, I.W.; Warwick, P.E.; Greenwood, R.C. A novel approach for the rapid decomposition of ActinideTM resin and its application to measurement of uranium and plutonium in natural waters. Anal. Chim. Acta 2006, 577, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Dulanská, S.; Antalík, I.; Labaška, M.; Remenec, B.; Mátel, A. Rapid determination of 239,240Pu, 238Pu, 241Am and 90Sr in high contaminated samples waste using combined SPE sorbents AnaLig® Pu-02, AnaLig® Sr-01 and DGA® Resin. J. Radioanal. Nucl. Chem. 2013, 295, 1635–1639. [Google Scholar] [CrossRef]
- Dulanská, S.; Bilohuščin, J.; Remenec, B.; Galanda, D.; Mátel, L.U. Determination of 239Pu, 241Am and 90Sr in urine using pre-filter material and combined sorbents AnaLig® Pu-02, AnaLig® Sr-01, DGA® Resin. J. Radioanal. Nucl. Chem. 2015, 304, 127–132. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Culligan, B.K. New column separation method for emergency urine samples. J. Radioanal. Nucl. Chem. 2009, 279, 105–111. [Google Scholar] [CrossRef][Green Version]
- Lee, M.H.; Jeon, Y.S.; Song, K. Determination of activity concentrations and activity ratios of plutonium, americium and curium isotopes in radioactive waste samples. J. Radioanal. Nucl. Chem. 2009, 280, 457–465. [Google Scholar] [CrossRef]
- Michel, H.; Levent, D.; Barci, V.; Barci-Funel, G.; Hurel, C. Soil and sediment sample analysis for the sequential determination of natural and anthropogenic radionuclides. Talanta 2008, 74, 1527–1533. [Google Scholar] [CrossRef]
- Luo, M.; Xing, S.; Yang, Y.; Song, L.; Ma, Y.; Wang, Y.; Dai, X.; Happel, S. Sequential analyses of actinides in large-size soil and sediment samples with total sample dissolution. J. Environ. Radioact. 2018, 187, 73–80. [Google Scholar] [CrossRef]
- Luo, M.; Liu, D.; Yang, Y.; Dai, X.; Wu, Y.; Shi, K. Simultaneous determination of actinides and 90Sr in large-size soil and sediment samples. J. Environ. Radioact. 2022, 247, 106854. [Google Scholar] [CrossRef]
- Grate, J.W.; Egorov, O.B.; Fiskum, S.K. Automated extraction chromatographic separations of actinides using separation-optimized sequential injection techniques. Analyst 1999, 124, 1143–1150. [Google Scholar] [CrossRef]
- Charlton, J.J.; Sepaniak, M.J.; Sides, A.K.; Schaaff, T.G.; Mann, D.K.; Bradshaw, J.A. The automation and optimization of solid phase extraction inductively coupled plasma mass spectrometry analysis for the high throughput determination of aqueous levels of U, Th, Np, Pu, and Am. J. Anal. Atom. Spectrom. 2013, 28, 711–718. [Google Scholar] [CrossRef]
- Guérin, N.; Nadeau, K.; Potvin, S.; Hardy, J.; Larivière, D. Automated pressurized injection system for the separation of actinides by extraction chromatography. J. Radioanal. Nucl. Chem. 2013, 295, 1803–1811. [Google Scholar] [CrossRef]
- Qiao, J. Dynamic Flow Approaches for Automated Radiochemical Analysis in Environmental, Nuclear and Medical Applications. Molecules 2020, 25, 1462. [Google Scholar] [CrossRef] [PubMed]
- Truscott, J.B.; Jones, P.; Fairman, B.E.; Evans, E.H. Determination of actinides in environmental and biological samples using high-performance chelation ion chromatography coupled to sector-field inductively coupled plasma mass spectrometry. J. Chromatogr. A 2001, 928, 91–98. [Google Scholar] [CrossRef]
- Peterson, D.S.; Montoya, V.M. Separation of Actinides Using Capillary Extraction Chromatography-Inductively Coupled Plasma Mass Spectrometry. J. Chromatogr. Sci. 2009, 47, 545–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.; Chung, K.H.; Jung, Y.; Jang, M.; Kang, M.J.; Choi, G. A rapid and efficient automated method for the sequential separation of plutonium and radiostrontium in seawater. J. Radioanal. Nucl. Chem. 2015, 304, 321–327. [Google Scholar] [CrossRef]
- Wang, W.; Evans, R.D.; Evans, H.E. A rapid, automated system for the separation, preconcentration and measurement of 90Sr, and U, Am and Pu isotopes. Talanta 2021, 233, 122507. [Google Scholar] [CrossRef]
- Higginson, M.; Palmer, K.; King, J.; Dawkins, B.; Huggins, T.; Ingman, L.; Taylor, F.; Xu, N.; Kaye, P. Development of automated separations for actinides analysis. J. Radioanal. Nucl. Chem. 2019, 320, 689–698. [Google Scholar] [CrossRef]
- Egorov, O.; O’Hara, M.; Grate, J.; Ruzicka, J. Sequential injection renewable separation column instrument for automated sorbent extraction separations of radionuclides. Anal. Chem. 1999, 71, 345–352. [Google Scholar] [CrossRef]
- Grate, J.W.; Egorov, O.B. Investigation and Optimization of On-Column Redox Reactions in the Sorbent Extraction Separation of Americium and Plutonium Using Flow Injection Analysis. Anal. Chem. 1998, 70, 3920–3929. [Google Scholar] [CrossRef]
- Egorov, O.B.; Grate, J.W.; O’Hara, M.J.; Farmer, O.T., III. Extraction chromatographic separations and analysis of actinides using sequential injection techniques with on-line inductively coupled plasma mass spectrometry (ICP MS) detection. Analyst 2001, 126, 1594–1601. [Google Scholar] [CrossRef]
- Mirashi, N.N.; Aggarwal, S.K. Studies for simultaneous quantitative electrodeposition of plutonium and americium for alpha-spectrometry. J. Radioanal. Nucl. Chem. 2009, 279, 777–781. [Google Scholar] [CrossRef]
- Irlweck, K. Effects of Fe(III) ions on the electrodeposition of trace amounts of plutonium and americium. J. Radioanal. Nucl. Chem. 1996, 214, 429–437. [Google Scholar] [CrossRef]
- Bajo, S.; Eikenberg, J. Electrodeposition of actinides for alpha-spectrometry. J. Radioanal. Nucl. Chem. 1999, 242, 745–751. [Google Scholar] [CrossRef]
- Tran, Q.; Pierre, S.; de Sanoit, J.; Pomorski, M.; Bergonzo, P. Electro-Precipitation of Actinides on Boron-Doped Diamond Thin Films for Solid Sources Preparation for High-Resolution Alpha-Particle Spectrometry. Appl. Sci. 2019, 9, 1473. [Google Scholar] [CrossRef]
- Glover, S.E.; Filby, R.H.; Clark, S.B.; Grytdal, S.P. Optimization and characterization of a sulfate based electrodeposition method for alpha-spectroscopy of actinide elements using chemometric analysis. J. Radioanal. Nucl. Chem. 1998, 234, 213–220. [Google Scholar] [CrossRef]
- Janda, J.; Sládek, P.; Sas, D. Electrodeposition of selected alpha-emitting radionuclides from oxalate-ammonium sulfate electrolyte and measured by means of solid-state alpha spectrometry. J. Radioanal. Nucl. Chem. 2010, 286, 687–691. [Google Scholar] [CrossRef]
- Lee, K.B.; Man Lee, J.; Soon Park, T.; Oh, P. Preparation and activity measurement of electrodeposited alpha-emitting sources. Appl. Radiat. Isot. 2006, 64, 1260–1264. [Google Scholar] [CrossRef]
- Oh, J.; Warwick, P.E.; Croudace, I.W.; Lee, S. Evaluation of three electrodeposition procedures for uranium, plutonium and americium. Appl. Radiat. Isot. 2014, 87, 233–237. [Google Scholar] [CrossRef]
- Hindman, F.D. Actinide separations for α spectrometry using neodymium fluoride coprecipitation. Anal. Chem. 1986, 58, 1238–1241. [Google Scholar] [CrossRef]
- Sill, C.W. Precipitation of actinides as fluorides or hydroxides for high-resolution alpha spectrometry. Nucl. Chem. Waste Manag. 1987, 7, 201–215. [Google Scholar] [CrossRef]
- Lozano, J.C.; Fernandez, F.; Gomez, J.M.G. Preparation of Alpha-spectrometric sources by coprecipitation with Fe(OH)3: Application to actinides. Appl. Radiat. Isot. 1997, 48, 383–389. [Google Scholar] [CrossRef]
- Dion, M.P.; Liezers, M.; Farmer, O.T.; Miller, B.W.; Morley, S.; Barinaga, C.; Eiden, G. Improving alpha spectrometry energy resolution by ion implantation with ICP-MS. J. Radioanal. Nucl. Chem. 2015, 303, 877–884. [Google Scholar] [CrossRef]
- Quinto, F.; Blechschmidt, I.; Garcia Perez, C.; Geckeis, H.; Geyer, F.; Golser, R.; Huber, F.; Lagos, M.; Lanyon, B.; Plaschke, M.; et al. Multiactinide Analysis with Accelerator Mass Spectrometry for Ultratrace Determination in Small Samples: Application to an in Situ Radionuclide Tracer Test within the Colloid Formation and Migration Experiment at the Grimsel Test Site (Switzerland). Anal. Chem. 2017, 89, 7182–7189. [Google Scholar] [CrossRef]
- Dai, X.; Christl, M.; Sheila, K.; Synal, H. Determination of Atto- to Femtogram Levels of Americium and Curium Isotopes in Large-Volume Urine Samples by Compact Accelerator Mass Spectrometry. Anal. Chem. 2016, 88, 2832–2837. [Google Scholar] [CrossRef]
- Aggarwal, S.K.; Duggal, R.K.; Jain, H.C. Alpha spectrum evaluation method for the simultaneous determination of plutonium, americium and curium. J. Radioanal. Nucl. Chem. 1986, 107, 263–277. [Google Scholar] [CrossRef]
- Aggarwal, S.K.; Alamelu, D.; Shah, P.M.; Mirashi, N.N. Determination of 241Am/243Am ratios. J. Radioanal. Nucl. Chem. 2007, 273, 771–774. [Google Scholar] [CrossRef]
- Bortels, G.; Collaers, P. Analytical function for fitting peaks in alpha-particle spectra from Si detectors. Int. J. Radiat. Appl. Instrum. Part A Appl. Radiat. Isot. 1987, 38, 831–837. [Google Scholar] [CrossRef]
- Devol, T.; Ringberg, A.; Dewberry, R. Isotopic analysis of plutonium using a combination of alpha and internal conversion electron spectroscopy. J. Radioanal. Nucl. Chem. 2002, 254, 71–79. [Google Scholar] [CrossRef]
- Afonin, M.; Simonoff, M.; Donard, O.; Michel, H.; Ardisson, G. Pu and Am determination in the environment—Method development. Czechoslov. J. Phys. 2003, 53, A15–A23. [Google Scholar] [CrossRef]
- Varga, Z.; Surányi, G.; Vajda, N.; Stefánka, Z. Determination of plutonium and americium in environmental samples by inductively coupled plasma sector field mass spectrometry and alpha spectrometry. Microchem. J. 2007, 85, 39–45. [Google Scholar] [CrossRef]
- Becker, J.S. Inductively coupled plasma mass spectrometry (ICP-MS) and laser ablation ICP-MS for isotope analysis of long-lived radionuclides. Int. J. Mass Spectrom. 2005, 242, 183–195. [Google Scholar] [CrossRef]
- Chartier, F.; Aubert, M.; Pilier, M. Determination of Am and Cm in spent nuclear fuels by isotope dilution inductively coupled plasma mass spectrometry and isotope dilution thermal ionization mass spectrometry after separation by high-performance liquid chromatography. Fresenius’ J. Anal. Chem. 1999, 364, 320–327. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Cao, L.; Tagami, K.; Uchida, S. Method for Ultratrace Level 241Am Determination in Large Soil Samples by Sector Field-Inductively Coupled Plasma Mass Spectrometry: With Emphasis on the Removal of Spectral Interferences and Matrix Effect. Anal. Chem. 2016, 88, 7387–7394. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yamada, M. Isotope Dilution Sector-Field Inductively Coupled Plasma Mass Spectrometry Combined with Extraction Chromatography for Rapid Determination of 241Am in Marine Sediment Samples: A Case Study in Sagami Bay, Japan. J. Oceanogr. 2008, 64, 541–550. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Zhang, H.; Fang, S.; Li, C.; Yi, X.; Dang, H.; Xu, Y.; Wang, W.; Xu, J. Determination of ultra-trace level 241Am in soil by triple-quadrupole inductively coupled plasma-mass spectrometry with mass-shift mode combined with chemical separation. J. Anal. Atom. Spectrom. 2022, 37, 1044–1052. [Google Scholar] [CrossRef]
- Goldstein, S.J.; Hinrichs, K.A.; Nunn, A.J.; Gurganus, D.W.; Amato, R.S.; Oldham, W.J. Sequential chemical separations and multiple ion counting ICP-MS for 241Pu—241Am—237Np dating of environmental collections on a single aliquot. J. Radioanal. Nucl. Chem. 2018, 318, 695–701. [Google Scholar] [CrossRef]
- Hang, W.; Zhu, L.; Zhong, W.; Mahan, C. Separation of actinides at ultra-trace level from urine matrix using extraction chromatography-inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 2004, 19, 966–972. [Google Scholar] [CrossRef]
- Epov, V.N. Comparative study of three sample preparation approaches for the fast determination of americium in urine by flow injection ICP-MS. Can. J. Anal. Sci. Spectrosc. 2005, 50, 14–22. [Google Scholar]
- Guerin, N.; Calmette, R.; Johnson, T.; Lariviere, D. Multi-dimensional extraction chromatography of actinides for alpha and mass spectrometry. Anal. Methods-UK 2011, 3, 1560–1567. [Google Scholar] [CrossRef]
- Agarande, M.; Benzoubir, S.; Bouisset, P.; Calmet, D. Determination of 241Am in sediments by isotope dilution high resolution inductively coupled plasma mass spectrometry (ID HR ICP-MS). Appl. Radiat. Isot. 2001, 55, 161–165. [Google Scholar] [CrossRef]
- Varga, Z. Application of inductively coupled plasma sector field mass spectrometry for low-level environmental americium-241 analysis. Anal. Chim. Acta 2007, 587, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, Y.; Lin, J.; Li, Z.; Tan, Z. Rapid method for sequential determination of Pu and Am in soil and sediment samples by sector-field inductively coupled plasma mass spectrometry. J. Radioanal. Nucl. Chem. 2021, 328, 137–147. [Google Scholar] [CrossRef]
- Habibi, A.; Vivien, C.; Boulet, B.; Cossonnet, C.; Gurriaran, R.; Gleizes, M.; Cote, G.; Larivière, D. A rapid sequential separation of actinides and radiostrontium coupled to ICP-MS and gas proportional counting. J. Radioanal. Nucl. Chem. 2016, 310, 217–227. [Google Scholar] [CrossRef]
- Boulyga, S.F. Mass spectrometric analysis of long-lived radionuclides in bio-assays. Int. J. Mass Spectrom. 2011, 307, 200–210. [Google Scholar] [CrossRef]
- Mathew, K.J.; Ottenfeld, C.F.; Keller, R.C.; Kuhn, K.J.; Fulwyler, J.B. Preparation of 241Am/243Am gravimetric mixtures and development of Am isotopic and assay measurement techniques using thermal ionization mass spectrometry. Int. J. Mass Spectrom. 2020, 458, 116430. [Google Scholar] [CrossRef]
- Quemet, A.; Ruas, A.; Dalier, V.; Rivier, C. Americium isotope analysis by Thermal Ionization Mass Spectrometry using the total evaporation method. Int. J. Mass Spectrom. 2018, 431, 8–14. [Google Scholar] [CrossRef]
- Quemet, A.; Angenieux, M.; Ruas, A. Nd, Am and Cm isotopic measurements after simultaneous separation in transmutation irradiated samples. J. Anal. Atom. Spectrom. 2021, 36, 1758–1767. [Google Scholar] [CrossRef]
- Bürger, S.; Riciputi, L.R.; Bostick, D.A.; Turgeon, S.; Mcbay, E.H.; Lavelle, M. Isotope ratio analysis of actinides, fission products, and geolocators by high-efficiency multi-collector thermal ionization mass spectrometry. Int. J. Mass Spectrom. 2009, 286, 70–82. [Google Scholar] [CrossRef]
- Quinto, F.; Golser, R.; Lagos, M.; Plaschke, M.; Schäfer, T.; Steier, P.; Geckeis, H. Accelerator Mass Spectrometry of Actinides in Ground—and Seawater: An Innovative Method Allowing for the Simultaneous Analysis of U, Np, Pu, Am, and Cm Isotopes below ppq Levels. Anal. Chem. 2015, 87, 5766–5773. [Google Scholar] [CrossRef]
- Cornett, R.J.; Kazi, Z.H.; Zhao, X.L.; Chartrand, M.G.; Charles, R.J.; Kieser, W.E. Actinide measurements by AMS using fluoride matrices. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 361, 317–321. [Google Scholar] [CrossRef]
- Christl, M.; Dai, X.; Lachner, J.; Kramer-Tremblay, S.; Synal, H. Low energy AMS of americium and curium. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 331, 225–232. [Google Scholar] [CrossRef]
- Srncik, M.; Hrnecek, E.; Steier, P.; Wallner, G. Determination of U, Pu and Am isotopes in Irish Sea sediment by a combination of AMS and radiometric methods. J. Environ. Radioact. 2011, 102, 331–335. [Google Scholar] [CrossRef]
- Agapkina, G.I.; Tikhomirov, F.A.; Shcheglov, A.I.; Kracke, W.; Bunzl, K. Association of Chernobyl-derived 239+240Pu, 241Am, 90Sr and 137Cs with organic matter in the soil solution. J. Environ. Radioact. 1995, 29, 257–269. [Google Scholar] [CrossRef]
- Novikov, A.P. Migration and concentration of artificial radionuclides in environmental objects. Geochem. Int. 2010, 48, 1263–1387. [Google Scholar] [CrossRef]
- Novikov, A.P.; Tkachev, V.V.; Myasoedov, B.F. Speciation methods of actinides in trace concentrations. C. R. Chim. 2004, 7, 1219–1225. [Google Scholar] [CrossRef]
- Altmaier, M.; Gaona, X.; Fanghänel, T. Recent Advances in Aqueous Actinide Chemistry and Thermodynamics. Chem. Rev. 2013, 113, 901–943. [Google Scholar] [CrossRef]
- Guo, H.; Kang, M.L.; Chen, W.L.; Long, J.C.; Zhao, Z. Speciation and solubility of americium in Beishan groundwater. Radiat. Prot. 2016, 36, 40–46. [Google Scholar]
- Ikeda-Ohno, A.; Harrison, J.J.; Thiruvoth, S.; Wilsher, K.; Wong, H.K.Y.; Johansen, M.P.; Waite, T.D.; Payne, T.E. Solution Speciation of Plutonium and Americium at an Australian Legacy Radioactive Waste Disposal Site. Environ. Sci. Technol. 2014, 48, 10045–10053. [Google Scholar] [CrossRef]
- Salbu, B. Speciation of radionuclides—Analytical challenges within environmental impact and risk assessments. J. Environ. Radioact. 2007, 96, 47–53. [Google Scholar] [CrossRef]
- León Vintró, L.; Mitchell, P.I.; Omarova, A.; Burkitbayev, M.; Jiménez Nápoles, H.; Priest, N.D. Americium, plutonium and uranium contamination and speciation in well waters, streams and atomic lakes in the Sarzhal region of the Semipalatinsk Nuclear Test Site, Kazakhstan. J. Environ. Radioact. 2009, 100, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Vangenechten, J.H.D.; Chughtai, N.A.; Bierkens, J.; Vanderborght, O.L.J. Similarity of 241Am and 59Fe specification in selected freshwaters and of their adsorption on crayfish exoskeleton. J. Environ. Radioact. 1987, 5, 275–286. [Google Scholar] [CrossRef]
- Molero, J.; Sanchez-Cabeza, J.A.; Merino, J.; Batlle, J.V.; Mitchell, P.I.; Vidal-Quadras, A. Particulate distribution of plutonium and americium in surface waters from the Spanish Mediterranean coast. J. Environ. Radioact. 1995, 28, 271–283. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Szabó, G.; Guczi, J.; Nisbet, A. Investigation of the solid phase speciation of 90Sr, 137Cs, 239Pu and 241Am in soils determined by extraction and ultra-filtration methods. J. Radioanal. Nucl. Chem. 1997, 226, 255–259. [Google Scholar] [CrossRef]
- Bolsunovsky, A.; Bondareva, L. Actinides and other radionuclides in sediments and submerged plants of the Yenisei River. J. Alloys Compd. 2007, 444, 495–499. [Google Scholar] [CrossRef]
- Alberts, J.J.; Wahlgren, M.A.; Orlandini, K.A.; Durbahn, C.A. The distributions of 239,240Pu, 238Pu, 241Am and 137Cs among chemically-defined components of sediments, settling particulates and net plankton of Lake Michigan. J. Environ. Radioact. 1989, 9, 89–103. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Salazar, W.R. Physicochemical Speciation of Americium in Soils from Rocky Flats, Colorado, USA. J. Radioanal. Nucl. Chem. 2000, 243, 347–351. [Google Scholar] [CrossRef]
- álvarez, A.; Gascó, C.; Navarro, N.; Antón, M.; Sancho, C. Determination of actinides in samples obtained during dismantling activities. J. Radioanal. Nucl. Chem. 2005, 265, 383–387. [Google Scholar] [CrossRef]
- Lujanienė, G.; Aninkevičius, V.; Lujanas, V. Artificial radionuclides in the atmosphere over Lithuania. J. Environ. Radioact. 2009, 100, 108–119. [Google Scholar] [CrossRef]
- Shi, Y.M.; Wang, X.H.; Zhou, G.Q.; Zhang, H.T.; Xie, J.C.; Li, M. Existing Form of 241Am in Sandy Soil. At. Energy Sci. Technol. 2012, 46, 263–267. [Google Scholar]
- Desideri, D.; Meli, M.; Roselli, C.; Testa, C.; Degetto, S. Speciation of natural and antropogenic radionuclides in different sea sediment samples. J. Radioanal. Nucl. Chem. 2001, 248, 727–733. [Google Scholar] [CrossRef]
- Lujanienė, G.; Beneš, P.; Atamberg, K.; Jokšas, K.; Kulakauskaitė, I. Pu and Am sorption to the Baltic Sea bottom sediments. J. Radioanal. Nucl. Chem. 2013, 295, 1957–1967. [Google Scholar] [CrossRef]
- Ketterer, M.E.; Hafer, K.M.; Jones, V.J.; Appleby, P.G. Rapid dating of recent sediments in Loch Ness: Inductively coupled plasma mass spectrometric measurements of global fallout plutonium. Sci. Total Environ. 2004, 322, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, F.; Magand, O.; Chapron, E.; Bertrand, S.; Boës, X.; Charlet, F.; Mélières, M.A. Radionuclide dating (210Pb, 137Cs, 241Am) of recent lake sediments in a highly active geodynamic setting (Lakes Puyehue and Icalma—Chilean Lake District). Sci. Total Environ. 2006, 366, 837–850. [Google Scholar] [CrossRef]
- Appleby, P.G. Radiometric dating of sediment records in European mountain lakes. J. Limnol. 2000, 59, 1–14. [Google Scholar] [CrossRef]
- Kuzmenkova, N.V.; Ivanov, M.M.; Alexandrin, M.Y.; Grachev, A.M.; Rozhkova, A.K.; Zhizhin, K.D.; Grabenko, E.A.; Golosov, V.N. Use of natural and artificial radionuclides to determine the sedimentation rates in two North Caucasus lakes. Environ. Pollut. 2020, 262, 114269. [Google Scholar] [CrossRef]
- Appleby, P.G.; Richardson, N.; Nolan, P.J. 241Am dating of lake sediments. Hydrobiologia 1991, 214, 35–42. [Google Scholar] [CrossRef]
- Mayer, K.; Wallenius, M.; Varga, Z. Nuclear Forensic Science: Correlating Measurable Material Parameters to the History of Nuclear Material. Chem. Rev. 2013, 113, 884–900. [Google Scholar] [CrossRef]
- Keegan, R.P.; Gehrke, R.J. A method to determine the time since last purification of weapons grade plutonium. Appl. Radiat. Isot. 2003, 59, 137–143. [Google Scholar] [CrossRef]
- Archer, D.E.; Luke, S.J.; Parker, W. Pu300: A Tool for Measurement of Plutonium Age for Arms Control Transparency via Gamma-Ray Spectroscopy[R]; UCRL-JC-136626; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2000. [Google Scholar]
- Wallenius, M.; Mayer, K. Age determination of plutonium material in nuclear forensics by thermal ionisation mass spectrometry. Fresenius’ J. Anal. Chem. 2000, 366, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, F.R.; Xu, J.; Dai, Y.H.; Li, D.M.; Yi, X.W.; Zhang, L.X.; Zhao, Y.G. Age determination of plutonium material by alpha spectrometry and thermal ionization mass spectrometry. Radiochim. Acta 2008, 96, 327–331. [Google Scholar] [CrossRef]
- Chen, Y.; Chang, Z.; Zhao, Y.; Zhang, J.; Li, J.; Shu, F. Studies on the age determination of trace plutonium. J. Radioanal. Nucl. Chem. 2009, 281, 675–678. [Google Scholar] [CrossRef]
- Eriksson, M.; Lindahl, P.; Roos, P.; Dahlgaard, H.; Holm, E. U, Pu, and Am Nuclear Signatures of the Thule Hydrogen Bomb Debris. Environ. Sci. Technol. 2008, 42, 4717–4722. [Google Scholar] [CrossRef]
- Aarkrog, A.; Dahlgaard, H.; Nilsson, K.; Holm, E. Further studies of plutonium and americium at Thule, Greenland. Health Phys. 1984, 46, 29–44. [Google Scholar] [CrossRef]
- Varga, Z.; Nicholl, A.; Zsigrai, J.; Wallenius, M.; Mayer, K. Methodology for the Preparation and Validation of Plutonium Age Dating Materials. Anal. Chem. 2018, 90, 4019–4024. [Google Scholar] [CrossRef]
- Pittet, P.; Josset, M.; Boilley, D.; Bernollin, A.; Rougier, G.; Froidevaux, P. Origin and age of an ongoing radioactive contamination of soils near La hague reprocessing plant based on 239 + 240Pu/238Pu and 241Am/241Pu current ratios and 90Sr and Ln(III) soil contents. Chemosphere 2021, 270, 129332. [Google Scholar] [CrossRef]
- Bouisset, P.; Nohl, M.; Cossonnet, C.; Boulet, B.; Thomas, S.; Cariou, N.; Salaun, G. Contribution of close-in fallout from the French atmospheric tests in inventories of 137Cs, 241Am and plutonium (238, 239, 240) in Gambier Islands (French Polynesia)—Signatures of stratospheric fallout in the Southern Hemisphere. J. Environ. Radioact. 2021, 235–236, 106624. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).