Chemical Composition and Potential Properties in Mental Illness (Anxiety, Depression and Insomnia) of Agarwood Essential Oil: A Review
Abstract
:1. Introduction
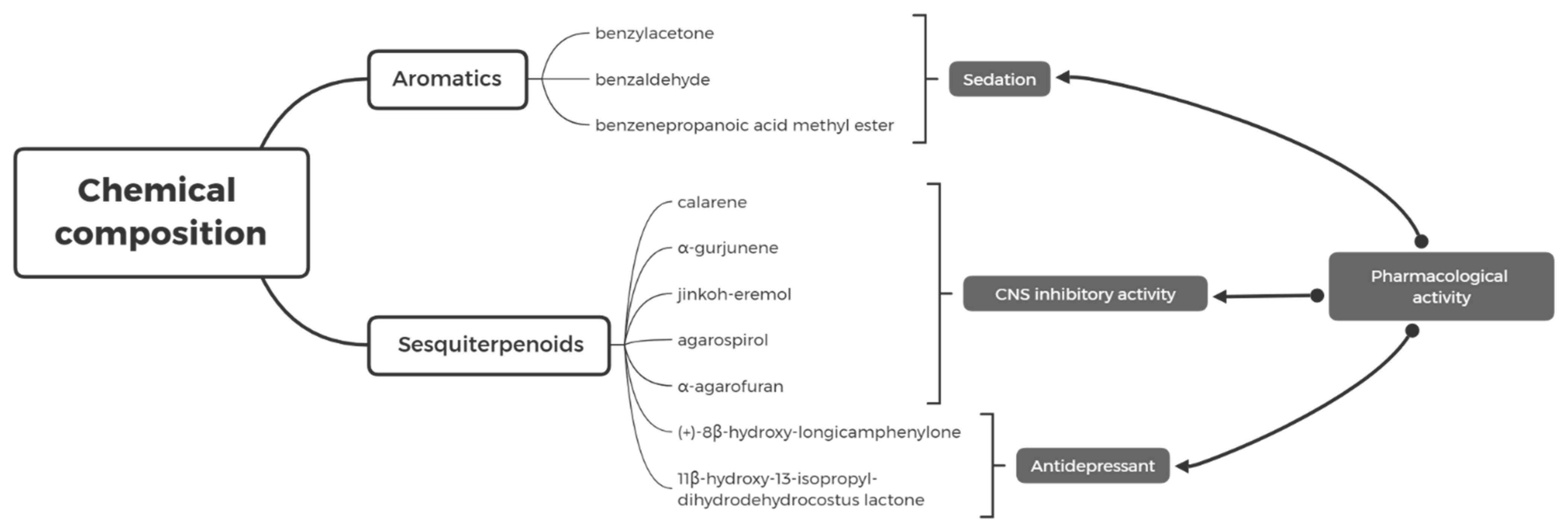
2. The Chemical Composition of AEO
2.1. Sesquiterpenoids
2.2. (2-Phenylethyl)chromones
2.3. Low-Weight Aromatic Compound
2.4. Fatty Acids
3. Factors Affecting the Chemical Composition of AEO
3.1. Plant Species
3.2. Agarwood-Induction Methods
3.3. Extraction Method
4. The Use of AEO to Treat Mental Illness
4.1. Anti-Anxiety Effects
4.2. Anti-Depressant Effects
4.3. Sleep-Promoting Effects
5. Mechanism of Action of AEO in the Treatment of Mental Illness
5.1. Inhibition of HPA Axis Hyperactivity
5.2. Associated Neurotransmitter Changes
5.3. Neuroprotective Effect
5.4. Antioxidant Effect
5.5. Anti-Inflammatory Effect
5.6. Acetylcholinesterase Inhibitory Activity
5.7. Other Pharmacological Actions
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AEO | Agarwood essential oil |
| CNS | central nervous system |
| HD | Hydro-distillation |
| SFE | supercritical fluid carbon dioxide extraction |
| MWE | microwave extraction method |
| PTP | partly-trunk-prunning method |
| BCD | burning-chisel-drilling method |
| FI | fungi-inoculation method |
| Agar-Wit | whole-tree agarwood-inducing technique |
| SE | Soxhlet extraction |
| MAE | Microwave-assisted extraction |
| SSRIs | selective serotonin reuptake inhibitors |
| SNRIs | serotonin-norepinephrine reuptake inhibitors |
| CRS | chronic restraint stress |
| EPM | the elevated plus maze test |
| LDE | the light dark exploration test |
| OF | the open field test |
| TS | tail suspension test |
| FS | forced swimming test |
| HPA | hypothalamic-pituitary-adrenal cortex system |
| BDNF | brain-derived neurotrophic factor |
| PD | Parkinson disease |
| AD | Alzheimer disease |
| ACTH | adrenocorticotropic hormone |
| CRF | corticotropin-releasing factor |
| nNOS | neuronal nitric oxide synthase |
| BBB | the blood-brain barrier |
| Glu | Glutamic Acid |
References
- Gnatta, J.R.; Kurebayashi, L.F.; Turrini, R.N.; Silva, M.J. Aromatherapy and nursing: Historical and theoretical conception. Rev. Esc. Enferm. USP 2016, 50, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Dong, H.; Tang, Y.; Huang, W.; Lu, F. Effects of aromatherapy on anxiety: A meta-analysis of randomized controlled trials. J. Affect. Disord. 2020, 274, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Hines, S.; Steels, E.; Chang, A.; Gibbons, K. Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane. Database Syst. Rev. 2018, 3, Cd007598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, E.; Shin, S. The effects of aromatherapy on sleep improvement: A systematic literature review and meta-analysis. J. Altern. Complement. Med. 2015, 21, 61–68. [Google Scholar] [CrossRef]
- Cooke, B.; Ernst, E. Aromatherapy: A systematic review. Br. J. Gen. Pract. 2000, 50, 493–496. [Google Scholar]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Forrester, L.T.; Maayan, N.; Orrell, M.; Spector, A.E.; Buchan, L.D.; Soares-Weiser, K. Aromatherapy for dementia. Cochrane. Database Syst. Rev. 2014, 8, Cd003150. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, H.; Ito, M.; Shiraki, T.; Yagura, T.; Honda, G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008, 62, 41–46. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Peng, D.; Liu, X.; Wu, C.; Guo, P.; Wei, J. Agarwood Essential Oil Displays Sedative-Hypnotic Effects through the GABAergic System. Molecules 2017, 22, 2190. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, C.; Yu, Z.; Wu, C.; Peng, D.; Liu, X.; Liu, Y.; Yang, Y.; Guo, P.; Wei, J. Agarwood Essential Oil Ameliorates Restrain Stress-Induced Anxiety and Depression by Inhibiting HPA Axis Hyperactivity. Int. J. Mol. Sci. 2018, 19, 3468. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Yang, S.H.; Wu, S.H.; Huang, Y.Y.; Zhang, W.M.; Chen, X.Y.; Gao, X.X. Optimization of extraction process of volatile oil from Aquilaria malaccensis by microwave combined with fungal fermentation broth immersion and auxiliary steam distillation. Guangdong Yaoke Daxue Xuebao 2019, 35, 740–745. [Google Scholar]
- Abdul Kadir, F.A.; Azizan, K.A.; Othman, R. Datasets of essential oils from naturally formed and synthetically induced Aquilaria malaccensis agarwoods. Data Brief 2020, 28, 104987. [Google Scholar] [CrossRef]
- Chen, H.Q.; Guo, F.J.; Cai, C.H.; Dong, W.H.; Wang, H.; Li, W.; Mei, W.L.; Dai, H.F. Study on sesquiterpenes from agarwood originating from Gyrinops salicifolia. Zhongguo Zhongyao Zazhi 2019, 44, 2274–2277. [Google Scholar]
- Mi, C.N.; Mei, W.L.; Wang, H.; Yang, L.; Dong, W.H.; Gai, C.J.; Yuan, J.Z.; Long, W.X.; Dai, H.F. Four new guaiane sesquiterpenoids from agarwood of Aquilaria filaria. Fitoterapia 2019, 135, 79–84. [Google Scholar] [CrossRef]
- Yu, Z.X.; Wang, C.H.; Chen, D.L.; Liu, Y.Y.; Wei, J.H. Anti-inflammatory sesquiterpenes from agarwood produced via whole-tree agarwood-inducing technique of Aquilaria sinensis. Zhongguo Zhong Yao Za Zhi 2019, 44, 4196–4202. [Google Scholar]
- Ma, C.T.; Ly, T.L.; Le, T.H.V.; Tran, T.V.A.; Kwon, S.W.; Park, J.H. Sesquiterpene derivatives from the agarwood of Aquilaria malaccensis and their anti-inflammatory effects on NO production of macrophage RAW 264.7 cells. Phytochemistry 2021, 183, 112630. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hou, W.C.; Wei, J.H.; Liu, Y.Y. Research progress on sesquiterpenoids in Aquilariae Lignum Resinatum and their biological activities and predictive analysis on quality marker. Zhongcaoyao 2022, 53, 1191–1209. [Google Scholar]
- Takamatsu, S.; Ito, M. Factors affecting 2-(2-phenylethyl)chromones in artificial agarwood. J. Nat. Med. 2021, 76, 321–330. [Google Scholar] [CrossRef]
- Dong, W.H.; Wang, H.; Guo, F.J.; Mei, W.L.; Chen, H.Q.; Kong, F.D.; Li, W.; Zhou, K.B.; Dai, H.F. Three New 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originated from Gyrinops salicifolia. Molecules 2019, 24, 576. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Wang, C.; Zheng, W.; Chen, D.; Liu, Y.; Yang, Y.; Wei, J. Anti-inflammatory 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from agarwood of Aquilaria sinensis. Bioorg. Chem. 2020, 99, 103789. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Mi, C.N.; Yuan, J.Z.; Zhu, M.M.; Yang, L.; Wei, Y.M.; Wang, H.; Long, W.X.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives: Promising α-glucosidase inhibitors in agarwood from Aquilaria filaria. Phytochemistry 2021, 181, 112578. [Google Scholar] [CrossRef]
- Takamatsu, S.; Ito, M. Correction to: Agarotetrol: A source compound for low molecular weight aromatic compounds from agarwood heating. J. Nat. Med. 2019, 73, 685. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, Y.; Xue, J.; Wei, J.; Zhang, Z.; Chen, H. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) gilg trees. Molecules 2011, 16, 4884–4896. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef]
- Tian, P.C.; Song, Y.L.; Xu, H.T.; Niu, S.Q.; Wu, Z.H.; Sheng, L.Q. Composition analysis, antioxidative and antibacterial activities comparison of agarwood oils extracted by supercritical and steam distillation. Zhongguo Zhongyao Zazhi 2019, 44, 4000–4008. [Google Scholar]
- Han, Q.Q.; Yang, S.H.; Chen, X.Y.; Zhong, Z.J.; Zhou, X.; Zhang, W.M.; Gao, X.X. Analysis and comparison of chemical composition of volatile oil from agarwood. Zhongyaocai 2019, 42, 1566–1571. [Google Scholar]
- Thuy, D.; Tuyen, T.T.; Thuy, T.; Minh, P.; Chien, N.Q.J.P. Isolation Process and Compound Identification of Agarwood Essential Oils from Aquilaria crassna Cultivated at Three Different Locations in Vietnam. Processes 2019, 7, 432. [Google Scholar] [CrossRef] [Green Version]
- Wetwitayaklung, P.; Thavanapong, N.; Charoenteeraboon, J. Chemical Constituents and Antimicrobial Activity of Essential Oil and Extracts of Heartwood of Aquilaria crassna Obtained from Water Distillation and Supercritical Fluid Carbon Dioxide Extraction. Silpakorn Univ. Sci. Technol. J. 2009, 3, 25–33. [Google Scholar]
- Geng, T.Y.; Luo, L.Y.; Zeng, L.; Wang, X.H.; Duan, Y.Z. On Effect of Different Extraction Methods on Preparation of Agarwood Essential Oil. Xinan Shifan Daxue Xuebao Ziran Kexueban 2020, 45, 59–67. [Google Scholar]
- Wang, Y.L.; Li, W.; Zeng, J.; Dong, W.H.; Dai, H.F. GC-MS Analysis of the Fragrant Constituents in the Cultivated Aquilaria Malaccensis Lam.Essential Oil. China Trop. Agric. 2021, 1, 90–97. [Google Scholar]
- Chen, P.P.; Chen, R.Z.; Wu, H.H.; Lin, W.Z.; Ma, X.J.; Liu, L.; Zhuang, W.D. Analysis of Components of Essential Oil of Agarwood from Aquilaria sinensis Induced by Different Methods. Redai Shengwu Xuebao 2019, 10, 190–196. [Google Scholar]
- Ninan, P.T. The functional anatomy, neurochemistry, and pharmacology of anxiety. J. Clin. Psychiatry 1999, 60 (Suppl. 22), 12–17. [Google Scholar]
- Bandelow, B.; Sher, L.; Bunevicius, R.; Hollander, E.; Kasper, S.; Zohar, J.; Möller, H.J. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int. J. Psychiatry Clin. Pract. 2012, 16, 77–84. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Buoli, M.; Baldwin, D.S.; Altamura, A.C. Serotonin norepinephrine reuptake inhibitors (SNRIs) in anxiety disorders: A comprehensive review of their clinical efficacy. Hum. Psychopharmacol. 2010, 25, 17–29. [Google Scholar] [CrossRef]
- Errington-Evans, N. Acupuncture for anxiety. CNS Neurosci. Ther. 2012, 18, 277–284. [Google Scholar] [CrossRef]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, M.; Kitayama, T.; Isomori, S.; Yamashita, F. Sedative effects of inhaled benzylacetone and structural features contributing to its activity. Biol. Pharm. Bull. 2013, 36, 1474–1481. [Google Scholar] [CrossRef] [Green Version]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, A. Effects of agarwood extracts on the central nervous system in mice. Planta Med. 1993, 59, 32–36. [Google Scholar] [CrossRef]
- Liu, Q.D.; Wang, H.; Li, C.; Lv, D.; Wang, W.J.; Guo, J.Y. Synthesis and CNS activities of α-agarofuran derivatives. Zhongguo Yaowu Huaxue Zazhi 2003, 13, 125–130. [Google Scholar]
- Ruberto, V.L.; Jha, M.K.; Murrough, J.W. Pharmacological Treatments for Patients with Treatment-Resistant Depression. Pharmaceuticals 2020, 13, 116. [Google Scholar] [CrossRef]
- Wang, F.H.; Li, D.L.; Wang, R.K. Effects of Chenxiang pellets on behavior and hippocampal 5-HT system in desperate mice. Zhongchengyao 2019, 41, 3029–3032. [Google Scholar]
- Huang, G.Y. The Clinical Research of tambac incense improvingdepressivestate. Ph.D. Thesis, Shandong University of Traditional Chinese Medicine, Jinan, China, 2016. [Google Scholar]
- Li, S.G. Study on the mechanism of the effect of agarwood extract on soothing and helping sleep thesis. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2019. [Google Scholar]
- Riemann, D.; Nissen, C.; Palagini, L.; Otte, A.; Perlis, M.L.; Spiegelhalder, K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet. Neurol. 2015, 14, 547–558. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Ma, F.C.; Zhang, Q.Y.; Liu, Y.Y.; Gong, B.; Guo, P.; Wei, J.H. Effect of agarwood produced by whole-tree agarwood-inducing technique on hypnotic and spontaneous activity inhibition of mice. Guoji Yaoxue Yanjiu Zazhi 2016, 43, 1082–1087. [Google Scholar]
- Hou, J.L.; Zhang, Y.Y.; Zhang, H.; Geng, X.W.; Li, Z.F.; Qi, D.M.; Deng, H.L.; Wei, S. Experimental Study on Antianxiety and Hypnotic Effects of Aquilariae Lignum Resinatum Tablet on Mice by Oral and Nasal Inhalation. Shandong Zhongyiyao Xuebao 2021, 45, 113–119. [Google Scholar]
- Liang, L.; Kong, D.W.; Zhou, Q.M.; Zhao, X.Y.; Yu, Z.R.; Wang, J.R.; Li, G.Y.; Du, G.H. Effect of Agarwood Gas on the Sleep of Mice by Regulating Neurotransmitters. Zhongyao Yaoli Yu Linchuang 2019, 35, 71–77. [Google Scholar]
- Lei, L.; Zhang, T.; Gao, D.; Huang, Q.L. The clinical effect of aromatherapy of Best Agarwood Production in patients with insomnia. Zhongfeng Yu Shenjing Jibing Zazhi 2019, 36, 609–612. [Google Scholar]
- Masuo, Y.; Satou, T.; Takemoto, H.; Koike, K. Smell and Stress Response in the Brain: Review of the Connection between Chemistry and Neuropharmacology. Molecules 2021, 26, 2571. [Google Scholar] [CrossRef]
- Lv, X.N.; Liu, Z.J.; Zhang, H.J.; Tzeng, C.M. Aromatherapy and the central nerve system (CNS): Therapeutic mechanism and its associated genes. Curr. Drug Targets 2013, 14, 872–879. [Google Scholar] [CrossRef]
- Ströhle, A.; Holsboer, F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry 2003, 36 (Suppl. 3), S207–S214. [Google Scholar]
- Li, H.; Li, Y.; Zhang, X.; Ren, G.; Wang, L.; Li, J.; Wang, M.; Ren, T.; Zhao, Y.; Yang, M.; et al. The Combination of Aquilaria sinensis (Lour.) Gilg and Aucklandia costus Falc. Volatile Oils Exerts Antidepressant Effects in a CUMS-Induced Rat Model by Regulating the HPA Axis and Levels of Neurotransmitters. Front. Pharmacol. 2020, 11, 614413. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Wang, Y.; Wang, P.; Li, Y.; Li, B. Herbal Medicine for Anxiety, Depression and Insomnia. Curr. Neuropharmacol 2015, 13, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.A.; Weinshenker, D. Good night and good luck: Norepinephrine in sleep pharmacology. Biochem. Pharmacol. 2010, 79, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Graeff, F.G.; Guimarães, F.S.; De Andrade, T.G.; Deakin, J.F. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996, 54, 129–141. [Google Scholar] [CrossRef]
- Wang, H.N. Effect of Agarwood Volatile Oil on GABA and GABAA Receptor Gene Expression in Oxidative Damaged Neurons. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2020. [Google Scholar]
- Kao, W.Y.; Hsiang, C.Y.; Ho, S.C.; Ho, T.Y.; Lee, K.T. Novel serotonin-boosting effect of incense smoke from Kynam agarwood in mice: The involvement of multiple neuroactive pathways. J. Ethnopharmacol. 2021, 275, 114069. [Google Scholar] [CrossRef]
- He, Q.; Hu, D.B.; Zhang, L.; Xia, M.Y.; Yan, H.; Li, X.N.; Luo, J.F.; Wang, Y.S.; Yang, J.H.; Wang, Y.H. Neuroprotective compounds from the resinous heartwood of Aquilaria sinensis. Phytochemistry 2021, 181, 112554. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, L.; Xie, D.; Yuan, Y.; Chen, N.; Dai, J.; Guo, S. 2-(2-phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef]
- Ueda, J.Y.; Imamura, L.; Tezuka, Y.; Tran, Q.L.; Tsuda, M.; Kadota, S. New sesquiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. Bioorg. Med. Chem. 2006, 14, 3571–3574. [Google Scholar] [CrossRef]
- Xiong, L.Y.; Li, L.Y.; Lin, L.; Chen, D.L.; Wu, J.S.; Zheng, M.; Liao, F.Y. Protective Effects of Lignum Aquilariae Resinatum Essent ial Oil on H2O2-induced Oxidative Damage of Pc12 Cells. Zhongyao Xinyao Yu Linchuang Yaoli 2014, 25, 28–32. [Google Scholar]
- Lee, H.Y.; Lee, J.S.; Kim, H.G.; Kim, W.Y.; Lee, S.B.; Choi, Y.H.; Son, C.G. The ethanol extract of Aquilariae Lignum ameliorates hippocampal oxidative stress in a repeated restraint stress mouse model. BMC Complement Altern. Med. 2017, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Gao, Z.Y.; Lin, M.; Zhao, X.; Sun, Y.; Pu, X.P. Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants 2021, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Peng, D.; Liu, Y.; Yu, Z.; Guo, P.; Wei, J. Agarwood Alcohol Extract Ameliorates Isoproterenol-Induced Myocardial Ischemia by Inhibiting Oxidation and Apoptosis. Cardiol. Res. Pract. 2020, 2020, 3640815. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Van Eeden, W.A.; El Filali, E.; van Hemert, A.M.; Carlier, I.V.E.; Penninx, B.; Lamers, F.; Schoevers, R.; Giltay, E.J. Basal and LPS-stimulated inflammatory markers and the course of anxiety symptoms. Brain Behav. Immun. 2021, 98, 378–387. [Google Scholar] [CrossRef]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, E.M. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Li, H.N. Comparison of the Pharmacological Effects between Leaves of Aquilaria sinensis(Lour.) Gilg and the Lignum of Aquilaria sinensis(Lour.) Gilg Thesis. Master’s Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2013. [Google Scholar]
- Lee, J.S.; Jeon, Y.J.; Kang, J.Y.; Lee, S.K.; Lee, H.D.; Son, C.G. Aquilariae Lignum Methylene Chloride Fraction Attenuates IL-1β-Driven Neuroinflammation in BV2 Microglial Cells. Int. J. Mol. Sci. 2020, 21, 5465. [Google Scholar] [CrossRef]
- Wang, S.L.; Tsai, Y.C.; Fu, S.L.; Cheng, M.J.; Chung, M.I.; Chen, J.J. 2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis with Anti-Inflammatory Effects in LPS-Induced Macrophages. Molecules 2018, 23, 289. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sun, J.; Ma, A.Q. Research progress of depression. Zhonghua Xiandai Neikexue Zazhi 2005, 002, 323–325. [Google Scholar]
- Fitzgerald, P.J.; Hale, P.J.; Ghimire, A.; Watson, B.O. Multiple cholinesterase inhibitors have antidepressant-like properties in the mouse forced swim test. Behav. Brain Res. 2021, 409, 113323. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.E.; Zueva, I.V.; Mukhamedyarov, M.A.; Lushchekina, S.V.; Petukhova, E.O.; Gubaidullina, L.M.; Krylova, E.S.; Saifina, L.F.; Lenina, O.A.; Petrov, K.A. Novel Acetylcholinesterase Inhibitors Based on Uracil Moiety for Possible Treatment of Alzheimer Disease. Molecules 2020, 25, 4191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.H.; Chang, G.P.; Yang, Y.F.; Du, X.P.; Jiang, Z.D.; Ni, H.; Li, Q.B. Based on Acetylcholinesterase and Oxidative Stress to St udy the Mechanism of Echinenone on Alzheimer’s Disease. Shipin Kexue 2021, 43, 105–112. [Google Scholar]
- Wang, H.N.; Dong, W.H.; Huang, S.Z.; Li, W.; Kong, F.D.; Wang, H.; Wang, J.; Mei, W.L.; Dai, H.F. Three new sesquiterpenoids from agarwood of Aquilaria crassna. Fitoterapia 2016, 114, 7–11. [Google Scholar] [CrossRef]
- Li, W.; Liao, G.; Dong, W.H.; Kong, F.D.; Wang, P.; Wang, H.; Mei, W.L.; Dai, H.F. Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing. Molecules 2016, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Isolation and Identification of 2-(2-phenylethyl) Chromone Polymers from Agarwood of Aquilaria crassna as Well as Their Bioactivities. Master’s Thesis, Heilongjiang Bayi Agricultural University, Daqing, China, 2017. [Google Scholar]
- Dong, M.; Du, H.; Li, X.; Zhang, L.; Wang, X.; Wang, Z.; Jiang, H. Discovery of Biomarkers and Potential Mechanisms of Agarwood Incense Smoke Intervention by Untargeted Metabolomics and Network Pharmacology. Drug Des. Devel. Ther. 2022, 16, 265–278. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, S.; Peng, D.Q.; Yu, Z.X.; Liu, Y.Y.; Yang, Y.; Guo, P.; Wei, J.H. Prediction and Analysis of the Components and Therapeutic Targets of Agarwood Essential Oil. Chin. Pharm. J. 2019, 54, 1958–1964. [Google Scholar]
| Agarwood Species | Origin | Agarwood-Induction Methods | Extraction Method | Chemical Composition | Ref. |
|---|---|---|---|---|---|
| A. sinensis | China | Agar-Wit (6 month) | HD | Aromatics: 6.44~10.07% Sesquiterpenes: 47.43~52.78% Fatty acids: 0.50~0.98% | [25] |
| A. sinensis | China | FI (12 month) | HD | Aromatics: 2.70% Sesquiterpenes: 34.84% Fatty acids: 0.16% | [25] |
| A. sinensis | China | BCD (20 month) | HD | Aromatics: 2.10% Sesquiterpenes: 57.58% Fatty acids: 0.36% | [25] |
| A. sinensis | China | PTP (28 month) | HD | Aromatics: 1.51% Sesquiterpenes: 52.03% Fatty acids: 0.69% | [25] |
| A. sinensis | China | Wild (unkown) | HD | Aromatics: 2.34~2.39% Sesquiterpenes: 62.35~71.48% Fatty acids: 0.06~0.35% | [25] |
| A. sinensis | China | BCD (unkown) | HD | Aromatics: 10.63% Sesquiterpenes: 68.68% | [26] |
| A. sinensis | China | BCD (unkown) | SFE | Aromatics: 12.46% Sesquiterpenes: 23.78% chromones: 29.42% | [26] |
| A. sinensis | China | unkown | SFE | Aromatics: 0.22~1.15% Sesquiterpenes: 8.93~26.10% chromones: 23.95~53.27% Fatty acids: 0~0.38% | [27] |
| A. sinensis | China | unkown | HD | Sesquiterpenes: 54.97~57.53% Fatty acids: 1.79~2.42% | [27] |
| A. malaccensis | Malaysia | unkown | HD | Aromatics: 2.81~6.26% Sesquiterpenes: 37.66~75.43% chromones: 0~0.38% Fatty acids: 0~16.376% | [27] |
| A. crassna | Vietnam | FI (3 year) | HD | Aromatics: 2.54~12.47% Sesquiterpenes: 61.06~72.37% Fatty acids: 6.57~8.46% | [28] |
| A. crassna | Thailand | hammering nails into its trunk | HD | Aromatics: 1.79% Sesquiterpenes: 92.86% Fatty acids: 2.84% | [29] |
| A. crassna | Thailand | hammering nails into its trunk | SFE | Aromatics: 0.8% Sesquiterpenes: 34.52% Fatty acids: 5.91% | [29] |
| A. sinensis | China | insect infestation | HD (95% ethanol) | Aromatics: 5.12% Sesquiterpenes: 12.34% chromones: 31.62% Fatty acids: 23.83% | [30] |
| A. sinensis | China | insect infestation | SE (95% ethanol) | Aromatics: 11.6% Sesquiterpenes: 7.72% chromones: 21.68% Fatty acids: 19.72% | [30] |
| A. sinensis | China | insect infestation | SFE (95% ethanol) | Aromatics: 9.64% Sesquiterpenes: 13.58% chromones: 34.48% Fatty acids: 28% | [30] |
| A. sinensis | China | insect infestation | MAE (95% ethanol) | Aromatics: 11.02% Sesquiterpenes: 21.6% chromones: 30.81% Fatty acids: 25.77% | [30] |
| A. sinensis (Chi-Nan) | China | hole-drilling (18 month) | HD | Aromatics: 0.98% Sesquiterpenes: 90.28% chromones: 0.93% | [31] |
| Pharmacological Activity | Extracts/Compounds (Administration Route) | Function/Mechanism | Ref. |
|---|---|---|---|
| anti-anxiety (restraint stress-induced anxiety) | AEO: 10, 20 and 40 mg/kg Positive drug: diazepam 2.5 mg/kg i.p. | In EPM, LDE and OF test, the AEO (40 mg/kg) dose was equivalent to the positive drug diazepam (2.5 mg/kg). Mechanism: inhibition of HPA axis hyperactivity. | [10] |
| anti-depressant (restraint stress-induced depression) | AEO: 10, 20 and 40 mg/kg Positive drug: paroxetine 10 mg/kg i.p. | 40 mg/kg AEO showed the best efficiency among the tested dosages with a comparable effect to that of paroxetine (10 mg/kg). | |
| sedative effect | AEO: 400 μL Optimal dose: benzylacetone 0.1%, calarene 0.17%, α-gurjunene 1.5% inh | On mice’s spontaneous motor activity, both AEO and monomers had dose-dependent benefits. | [8] |
| sedative effect | Benzylacetone: 4 × 10−4, 4 × 10−3, 4 × 10−2, 4 × 10−1, or 4 mg. inh | Significantly reduced autonomic motor activity (4 × 10−4 mg had the strongest sedative effect). | [38] |
| CNS inhibitory activity | agarwood benzene extract: 400 mg/kg Positive drug: trazepam 10 mg/kg i.p. | It could reduce spontaneous exercise and increase sleep time induced by hexabarbituric acid, and the intensity of its effect (400 mg/kg) was greater than that of trazepam (10 mg/kg). | [39] |
| anti-depressant | Chenxiang pellets: 1.0 g/kg Positive drug: Fluoxetine 0.01 g/kg i.g. | Chenxiang pellets could significantly reduce the immobility time of mice during tail suspension and forced swimming. Mechanism: It could be related to increasing 5-HT content in the mouse hippocampus, inhibiting the expression of s1c6a4 and HTR1A mRNA in the mouse hippocampus, and improving the function and excitability of the central 5-HT system. | [42] |
| anti-depressant | agarwood incense (twice a day, 1 month) inh | Agarwood aromatherapy had a positive clinical effect on CES-D and HAMD scores in patients with depression. | [43] |
| sedative-hypnotic effects | AEO: 50 μL and 100μL (80 °C, 30 min) inh | AEO could significantly increase the sleep rate, sleep time, and sleep latency in mice. Mechanism: It actedas a sedative and hypnotic by modulating GABA receptor gene expression and improving GABA receptor function. | [9,46] |
| anti-anxiety promoting sleep | Chenxiang pellets: 0.4 g, 1.2 g, 3.6 g (150 °C 20 min) inh | ① In EPM, LDE, and OF tests, AEO demonstrated significant anti-anxiety activity. ② Three doses of pentobarbital sodium could significantly reduce sleep latency while increasing sleep time. | [47] |
| promoting sleep (induced by PCPA) | agarwood incense: 0.3 g for 6 mice and 0.5 g for 6 mice inh | It could improve the insomnia of mice. Mechanism: it was mainly related to the regulatory effect of aloe gas inhalation on Glu/GABA. | [48] |
| promoting sleep | Agarwood: 0.5 g, 3 h inh | Agarwood treatment could significantly improve the quality of sleep and the severity of insomnia in patients with insomnia, as well as anxiety, depression, and other emotional disorders. | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, C.; He, Q.; Feng, J.; Chen, D.; Wei, J.; Liu, Y. Chemical Composition and Potential Properties in Mental Illness (Anxiety, Depression and Insomnia) of Agarwood Essential Oil: A Review. Molecules 2022, 27, 4528. https://doi.org/10.3390/molecules27144528
Chen X, Wang C, He Q, Feng J, Chen D, Wei J, Liu Y. Chemical Composition and Potential Properties in Mental Illness (Anxiety, Depression and Insomnia) of Agarwood Essential Oil: A Review. Molecules. 2022; 27(14):4528. https://doi.org/10.3390/molecules27144528
Chicago/Turabian StyleChen, Xiqin, Canhong Wang, Qingqin He, Jian Feng, Deli Chen, Jianhe Wei, and Yangyang Liu. 2022. "Chemical Composition and Potential Properties in Mental Illness (Anxiety, Depression and Insomnia) of Agarwood Essential Oil: A Review" Molecules 27, no. 14: 4528. https://doi.org/10.3390/molecules27144528
APA StyleChen, X., Wang, C., He, Q., Feng, J., Chen, D., Wei, J., & Liu, Y. (2022). Chemical Composition and Potential Properties in Mental Illness (Anxiety, Depression and Insomnia) of Agarwood Essential Oil: A Review. Molecules, 27(14), 4528. https://doi.org/10.3390/molecules27144528






