Anti-Aging Effect of Momordica charantia L. on d-Galactose-Induced Subacute Aging in Mice by Activating PI3K/AKT Signaling Pathway
Abstract
1. Introduction
2. Results
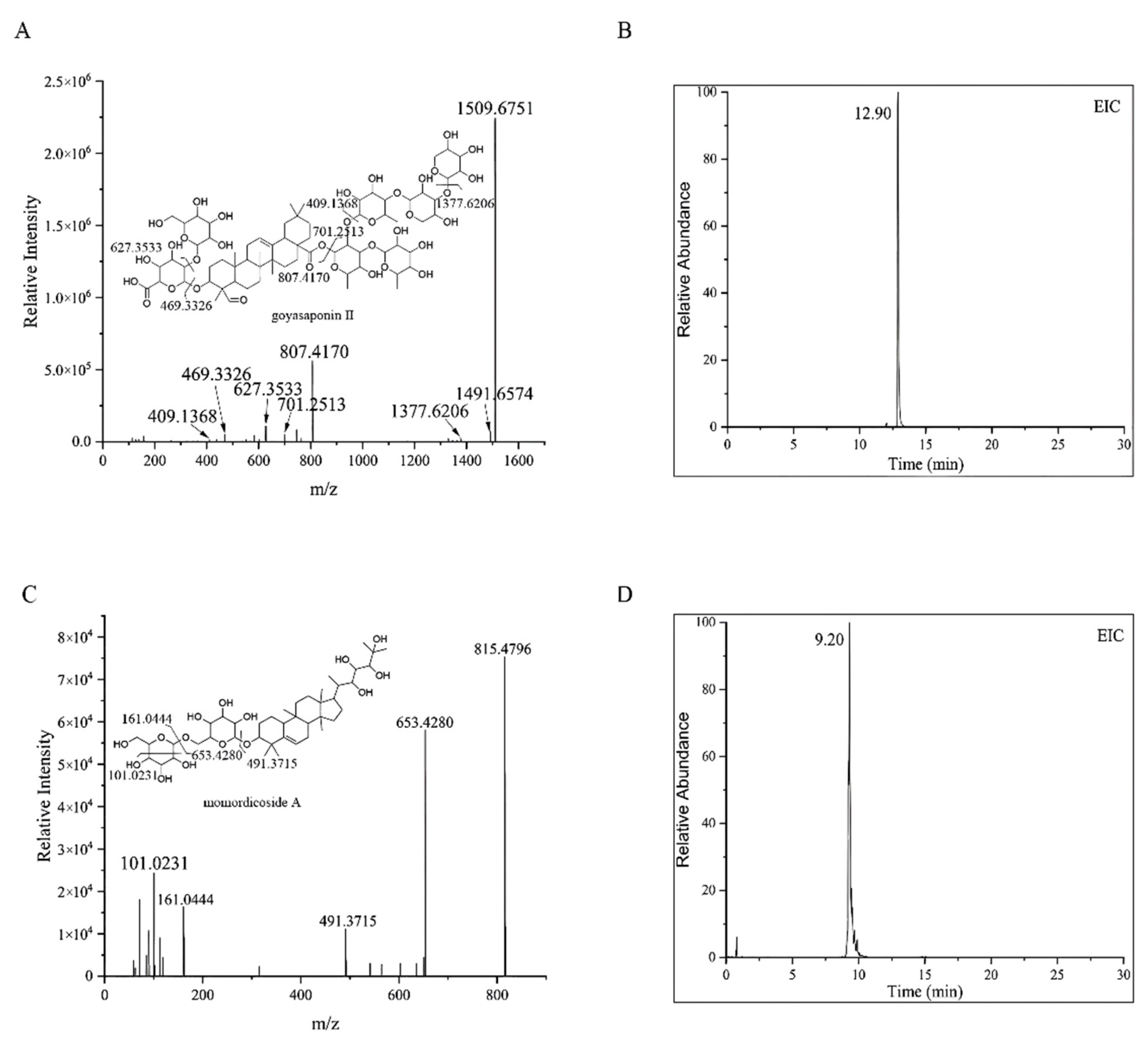
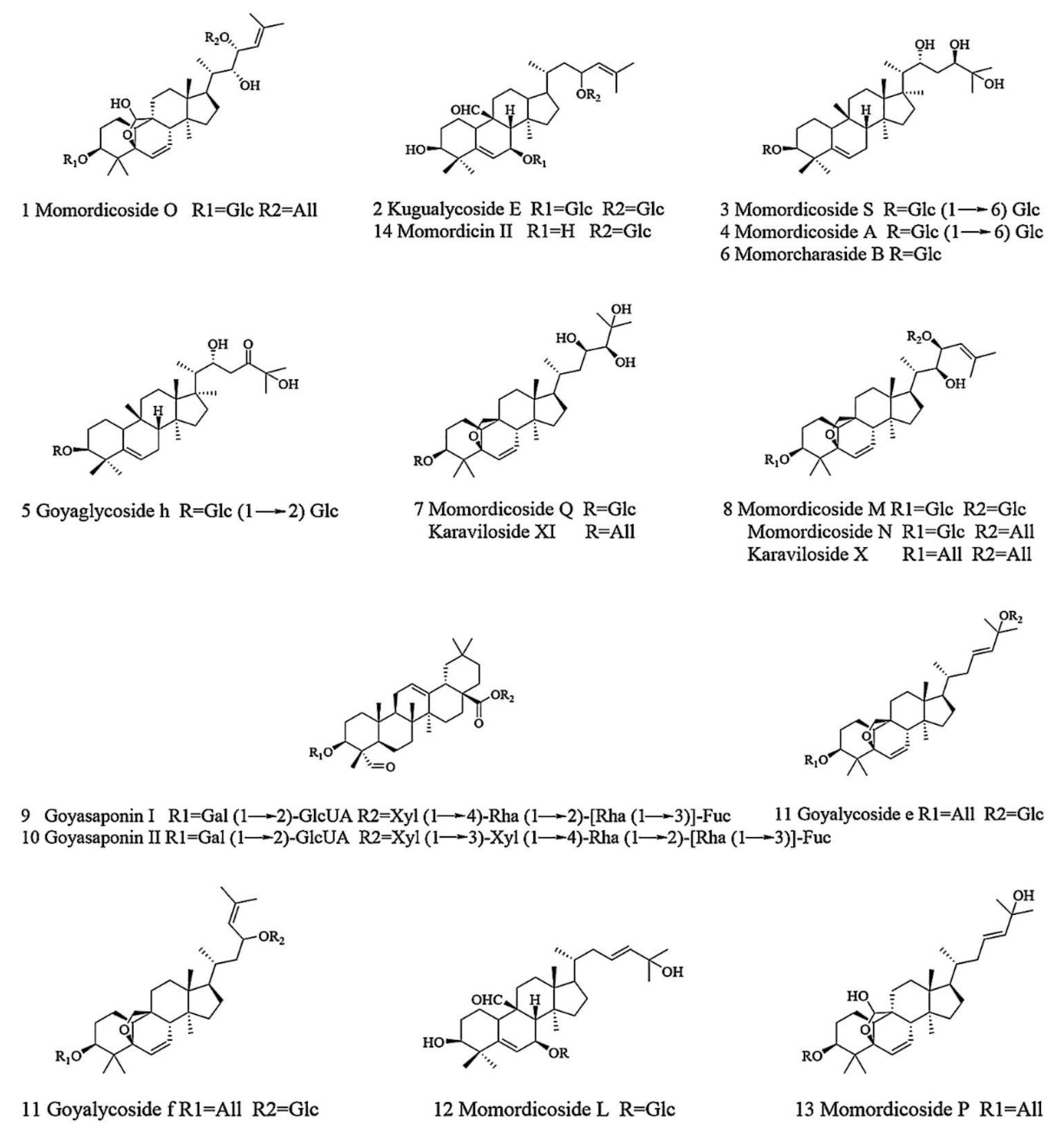
2.1. UHPLC-MS Analysis
2.2. Behavioral Assessments
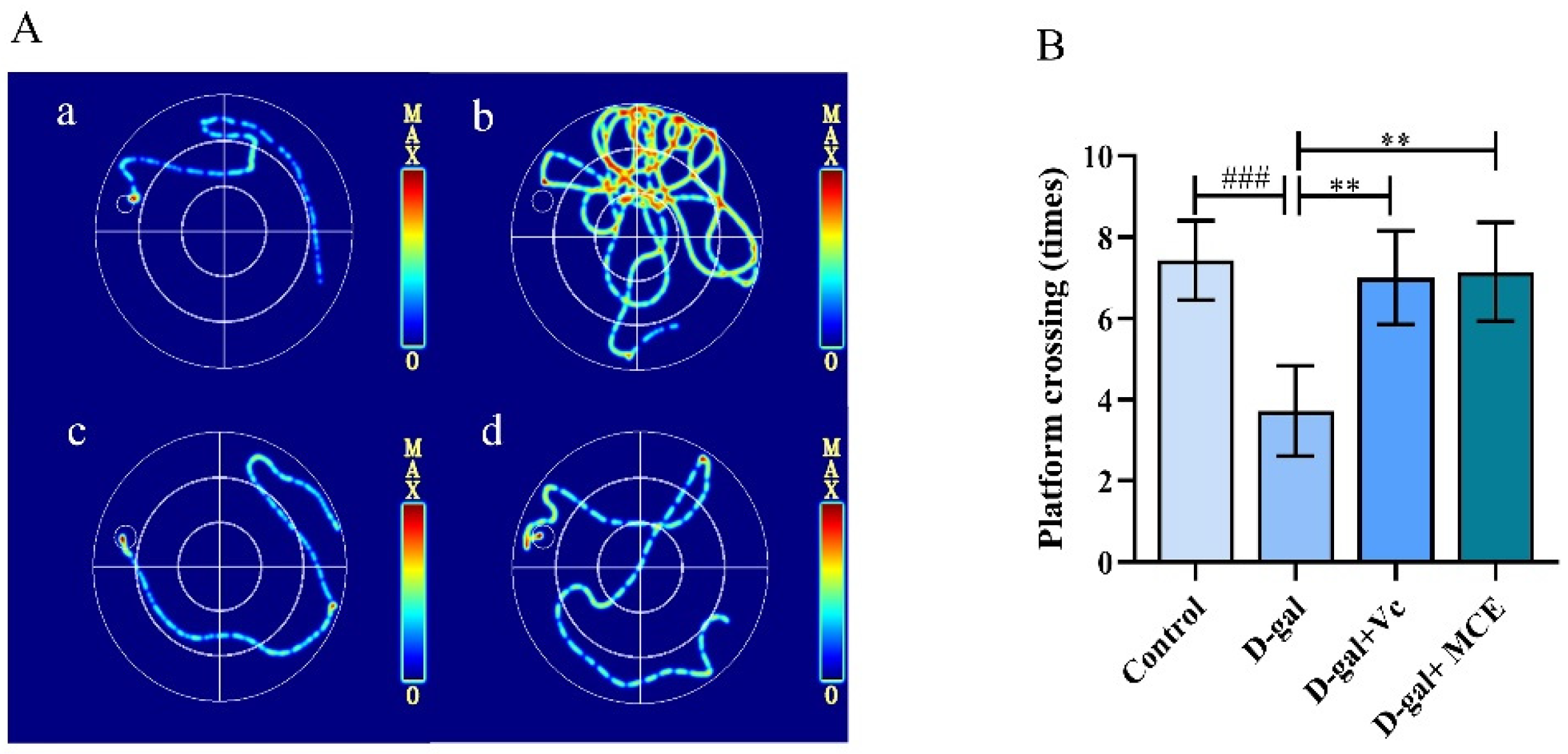
2.2.1. Effects of MCE on Morris Water Maze (MWM) Test
2.2.2. Effects of MCE on Step-Down Test
2.3. Effects of MCE on Oxidant Status In Vivo
2.4. Effects of MCE on Body Weight and Organ Index
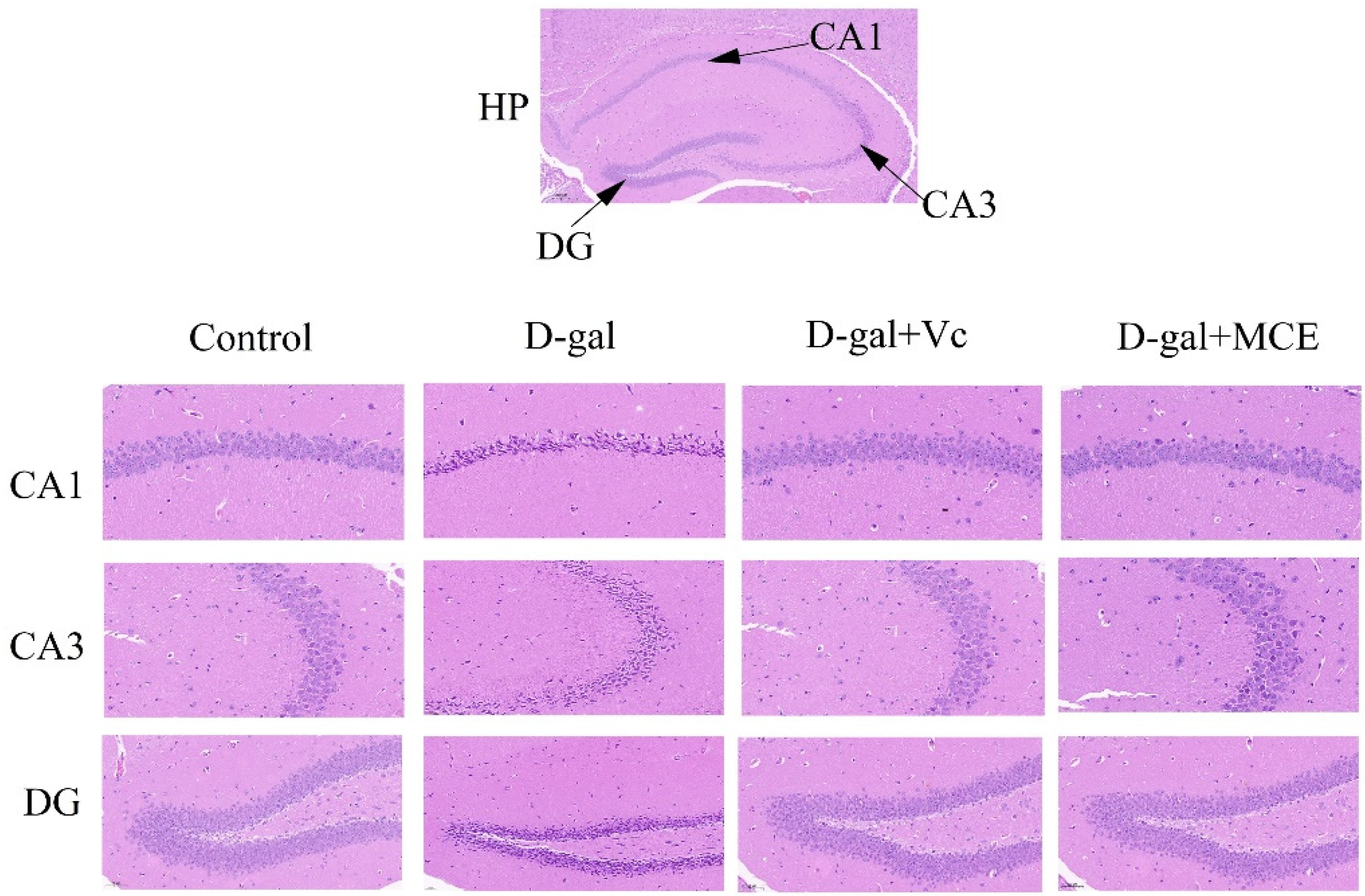
2.5. Effects of MCE on Histopathological Alterations
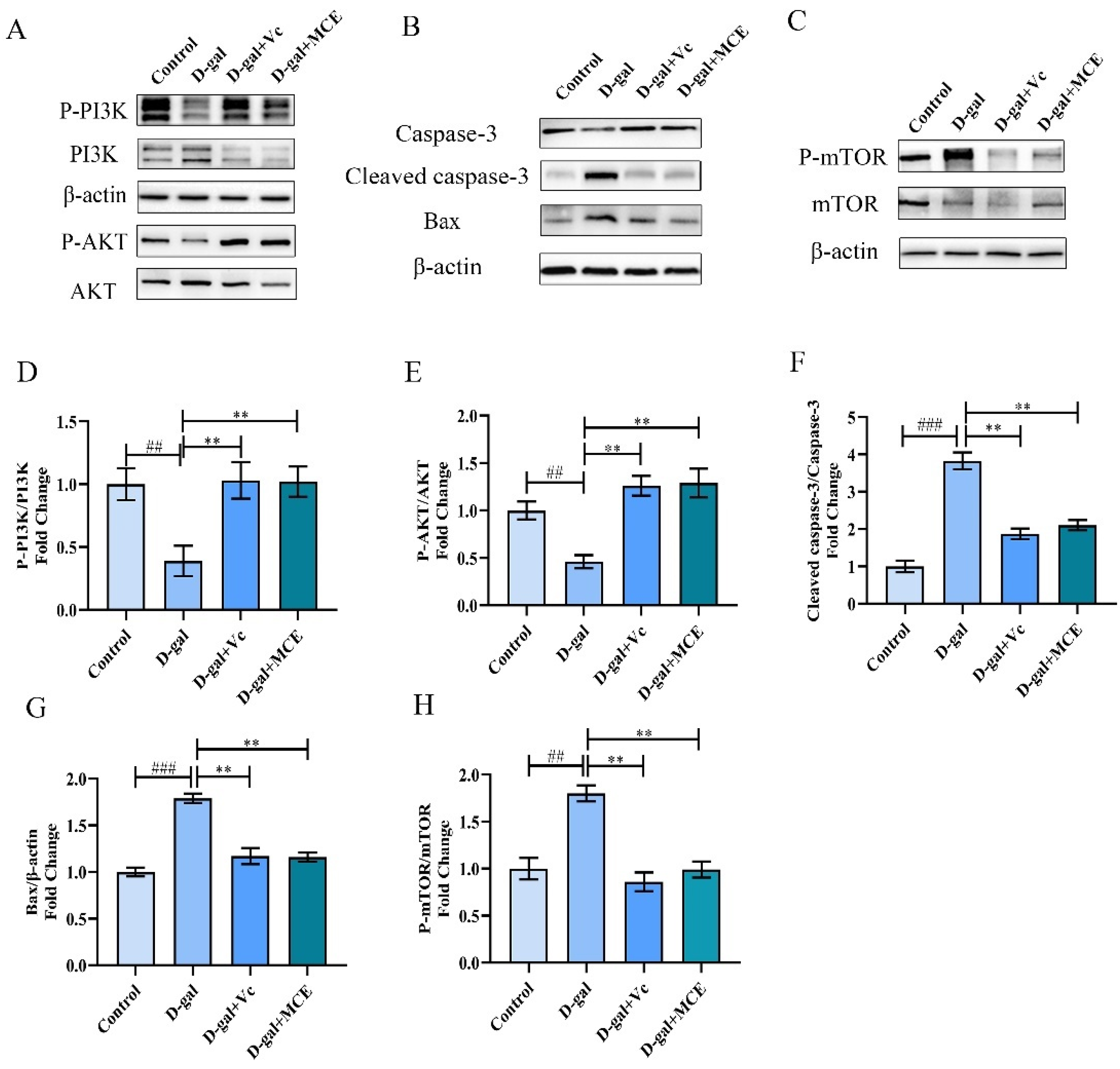
2.6. Effects of MCE on PI3K/AKT Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of MCE
4.3. UHPLC-MS Analysis
4.4. Animal Experiments
4.5. Behavioral Assessments
4.5.1. MWM Test
4.5.2. Step-Down Test
4.6. Biological Sample Collection and Preparation
4.7. Histopathological Examination
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Antiaging Effect of Metformin on Brain in Naturally Aged and Accelerated Senescence Model of Rat. Rejuvenation Res. 2017, 20, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.J.; Wang, S.X. The Role of Rapamycin in Healthspan Extension via the Delay of Organ Aging. Ageing Res. Rev. 2021, 70, 101376. [Google Scholar] [CrossRef] [PubMed]
- Perls, T. The Reappearance of Procaine Hydrochloride (Gerovital H3) for Antiaging. J. Am. Geriatr. Soc. 2013, 61, 1024–1025. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jiang, J.G.; Li, Y.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef]
- Wang, S.Z.; Li, Z.L.; Yang, G.L.; Ho, C.T.; Li, S.M. Momordica charantia: A popular health-promoting vegetable with multifunctionality. Food Funct. 2017, 8, 1749–1762. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Almeida, W.; Wariss, F.; Bezerra, F. Phytochemical profile and biological activities of Momordica Charantia L. (Cucurbitaceae): A review. Afr. J. Biotechnol. 2018, 17, 829–846. [Google Scholar]
- Habtemariam, S. The Chemical and Pharmacological Basis of Bitter Melon (Momordica charantia L.) as a Potential Therapy for Type 2 Diabetes and Obesity—Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Disease, 1st ed.; Andre Gerhard Wolff: London, UK, 2019; pp. 177–249. [Google Scholar]
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef]
- AkyÜz, E.; TÜrkoĞlu, S.; BaŞkan, K.S.; TÜtemet, E.; Apak, M.R. Comparison of antioxidant capacities and antioxidant components of commercial bitter melon (Momordica charantia L.) products. Turk. J. Chem. 2020, 44, 1663–1673. [Google Scholar] [CrossRef]
- Villarreal-La Torre, V.E.; Guarniz, W.S.; Silva-Correa, C.; Cruzado-Razco, L.; Siche, R. Antimicrobial activity and chemical composition of Momordica charantia: A review. Pharmacogn. J. 2020, 12, 213–222. [Google Scholar] [CrossRef]
- Patel, R.; Mahobia, N.; Upwar, N.; Waseem, N.; Talaviya, H.; Patel, Z. Analgesic and antipyretic activities of Momordica charantia Linn. fruits. J. Adv. Pharm. Technol. 2010, 1, 415–418. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Padhye, S.B.; Anant, S. Bitter melon: A panacea for inflammation and cancer. Chin. J. Nat. Med. 2016, 14, 81–100. [Google Scholar] [CrossRef]
- Angamuthu, D.; Purushothaman, I.; Kothandan, S.; Swaminathan, R. Antiviral study on Punica granatum L. Momordica charantia L. Andrographis paniculata Nees, and Melia azedarach L. to Human Herpes Virus-3. Eur. J. Integr. Med. 2019, 28, 415–418. [Google Scholar]
- Kim, K.B.; Lee, S.; Heo, J.H.; Kim, J.H. Neuroprotective effects of Momordica charantia extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SK-N-MC cells. J. Nutr. Health 2017, 50, 415–425. [Google Scholar] [CrossRef][Green Version]
- Dennam, H. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar]
- Bjorksten, J. The crosslinkage theory of aging. J. Am. Geriatr. Soc. 1968, 16, 408–427. [Google Scholar] [CrossRef]
- Bauer, M.E.; Fuente, M.D.L. Oxidative Stress, Inflammaging, and Immunosenescence—Inflammation, Advancing Age and Nutrition, 1st ed.; Academic Press: London, UK, 2014; pp. 39–47. [Google Scholar]
- Cefalu, C.A. Theories and mechanisms of aging. Clin. Geriatr. Med. 2011, 27, 491–506. [Google Scholar] [CrossRef]
- Lin, C.X.; Chen, Y.; Lin, Y.Z.; Wang, X.B.; Hu, L.Y.; Cao, Y.; Chen, Y.J. Antistress and anti-aging activities of Caenorhabditis elegans were enhanced by Momordica saponin extract. Eur. J. Nutr. 2020, 60, 1819–1832. [Google Scholar] [CrossRef]
- Cao, X.L.; Sun, Y.J.; Lin, Y.F.; Pan, Y.J.; Farooq, U.; Xiang, L.; Qi, J.H. Antiaging of Cucurbitane Glycosides from Fruits of Momordica charantia L. Oxid. Med. Cell. Longev. 2018, 2018, 10. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Kong, S.Z.; Li, J.C.; Li, S.D.; Liao, M.N.; Li, C.P.; Zheng, P.J.; Guo, M.H.; Tan, W.X.; Zheng, Z.H.; Hu, Z. Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice. Mar. Drugs 2018, 16, 181. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Kumsta, C.; Sandri, M.; Ballabio, A.; Hansen, M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 2015, 11, 867–880. [Google Scholar] [CrossRef]
- Yen, P.H.; Dung, D.T.; Nhiem, N.X.; Anh, H.L.T.; Hang, D.T.T.; Yen, D.T.H.; Cuc, N.T.; Ban, N.K.; Minh, C.V.; Kiem, P.V. Cucurbitane-type triterpene glycosides from the fruits of Momordica charantia. Nat. Prod. Commun. 2014, 9, 383–386. [Google Scholar] [CrossRef]
- Perez, J.L.; Jayaprakasha, G.K.; Patil, B.S. Metabolite profiling and in vitro biological activities of two commercial bitter melon (Momordica charantia Linn.) cultivars. Food Chem. 2019, 288, 178–186. [Google Scholar] [CrossRef]
- Kawagishi, H.; Finkel, T. Unraveling the truth about antioxidants: ROS and disease: Finding the right balance. Nat. Med. 2014, 20, 711–713. [Google Scholar] [CrossRef]
- Govindan, S.; Johnson, E.E.R.; Christopher, J.; Shanmugam, J.; Thirumalairaj, V. Antioxidant and anti-aging activities of polysaccharides from Calocybe indica var. APK2. Exp. Toxicol. Pathol. 2016, 68, 329–334. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 14. [Google Scholar] [CrossRef]
- Peng, M.F.; Fang, Y.S.; Miao, M.S.; Wang, T. Effects of Wuweizi Syrup on Brain Aging Mice Model Induced by d-galactose. J. King Saud Univ. Sci. 2020, 32, 2426–2431. [Google Scholar] [CrossRef]
- Akha, A.A.S. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.H.; Xu, Z.Q.; Zhao, H.; Yu, X.Y. Neuroprotective effect and mechanism of daucosterol palmitate in ameliorating learning and memory impairment in a rat model of Alzheimer’s disease. Steroids 2017, 119, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bai, F.; Liu, Y.; Yang, Y.; Yuan, Q.; Zou, D.; Qu, S.; Tian, G.; Song, L.; Zhang, T.; et al. Fibroblast growth factor (FGF21) protects mouse liver against d-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt path-ways. Mol. Cell. Biochem. 2015, 403, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. The Mitochondrial Pathway of Apoptosis Part II: The BCL-2 Protein Family. Csh. Perspect. Biol. 2022, 14, a041046. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Huang, C.Y. Swimming exercise stimulates IGF1/PI3K/Akt and AMPK/SIRT1/PGC1α survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar]
- Caglayan, C.; Kandemir, F.M.; Darendelioğlu, E.; Küçükler, S.; Ayna, A. Hesperidin protects liver and kidney against sodium fluoride-induced toxicity through anti-apoptotic and anti-autophagic mechanisms. Life Sci. 2021, 281, 119730. [Google Scholar] [CrossRef]
- Phu, H.T.; Thuan, D.T.B.; Nguyen, T.H.D.; Posadino, A.M.; Eid, A.H.; Pintus, G. Herbal Medicine for Slowing Aging and Aging-associated Conditions: Efficacy, Mechanisms and Safety. Curr. Vasc. Pharmacol. 2020, 18, 369–393. [Google Scholar] [CrossRef]
- Tong, Q.; Zhang, M.; Cao, X.; Xu, S.; Wang, D.; Zhao, Y. Expression and activation of Daphnia pulex Caspase-3 are involved in regulation of aging. Gene 2017, 15, 37–46. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell. Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Hurez, V.; Dao, V.; Liu, A.; Pandeswara, S.; Gelfond, J.; Sun, L.; Bergman, M.; Orihuela, C.J.; Galvan, V.; Padrón, Á.; et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell 2016, 14, 945–956. [Google Scholar] [CrossRef]
- Chang, K.; Kang, P.; Liu, Y.; Huang, K.; Miao, T.; Sagona, A.P.; Nezis, I.P.; Bodmer, R.; Ocorr, K.; Bai, H. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy 2019, 16, 1807–1822. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Li, Q.; Zhou, B. Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates d-Galactose-Induced Brain Aging in Mice. Neurotherapeutics 2019, 16, 1269–1282. [Google Scholar] [CrossRef]
- Jiang, R.; Gao, J.; Shen, J.; Zhu, X.; Wang, H.; Feng, S.; Huang, C.; Shen, H.; Liu, H. Glycyrrhizic Acid Improves Cognitive Levels of Aging Mice by Regulating T/B Cell Proliferation. Front. Aging Neurosci. 2020, 12, 570116. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, Y.; Han, J.; Cui, C.; Lu, C.; Zhou, J.; Cheong, L.; Li, Y.; Sun, T.; et al. Sex-Based Differences in Gut Microbiota Composition in Response to Tuna Oil and Algae Oil Supplementation in a d-Galactose-Induced Aging Mouse Model. Front. Aging Neurosci. 2018, 10, 187. [Google Scholar] [CrossRef]
- Fu, C.X.; Dai, L.; Yuan, X.Y.; Xu, Y.J. Effects of Fish Oil Combined with Selenium and Zinc on Learning and Memory Impairment in Aging Mice and Amyloid Precursor Protein Processing. Biol. Trace Elem. Res. 2021, 199, 1855–1863. [Google Scholar] [CrossRef]
- Gong, P.; Wang, D.; Cui, D.; Yang, Q.; Wang, P.; Yang, W.; Chen, F. Anti-aging function and molecular mechanism of Radix Astragali and Radix Astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine 2021, 84, 153509. [Google Scholar] [CrossRef]
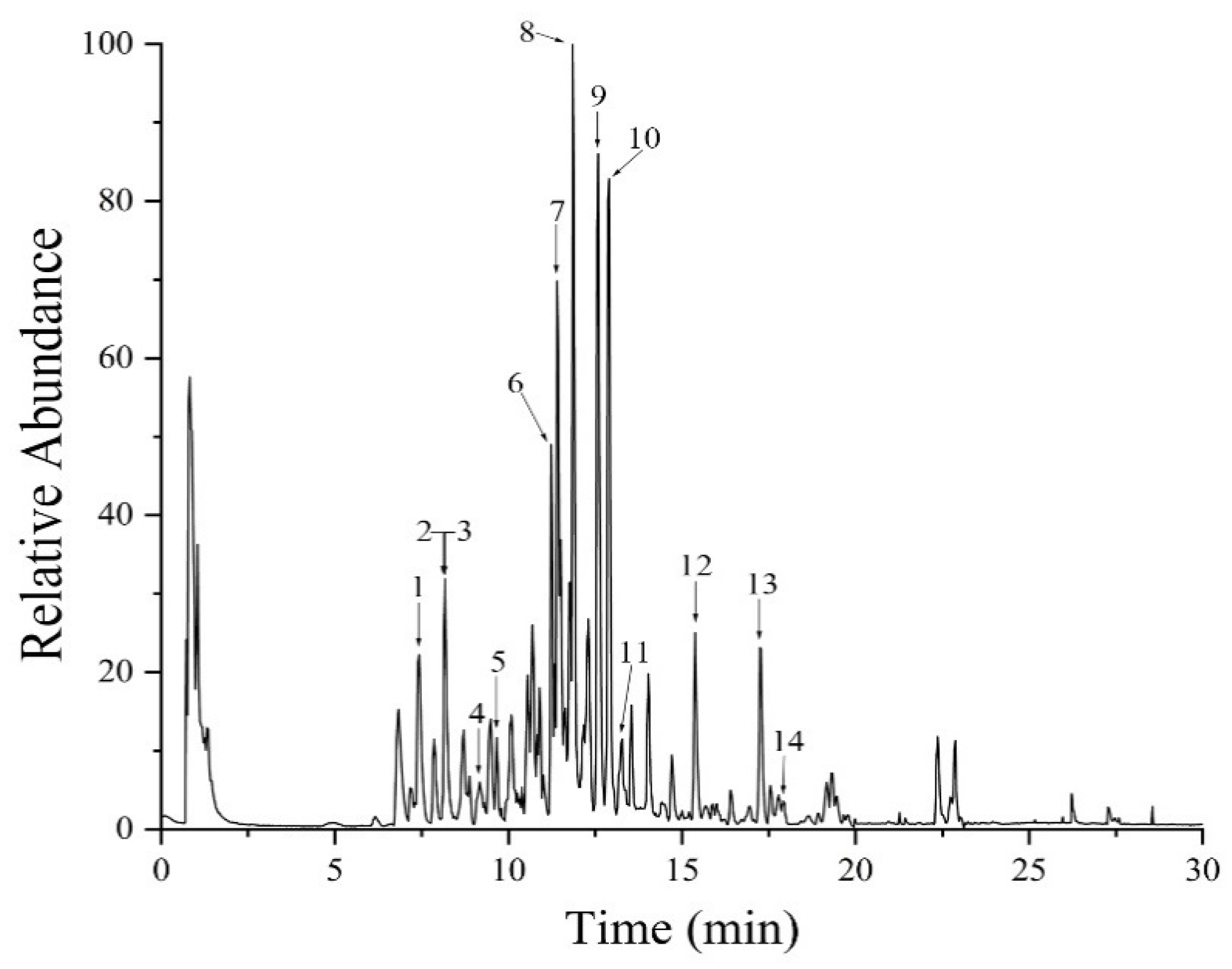

| Peak | tR min | Measured m/z | Mass Error (Δppm) | Molecular Formula | Adduct | MS/MS | Compound |
|---|---|---|---|---|---|---|---|
| 1 | 7.43 | 857.4567 | 3.1 | C42H68O15 | [M + HCOO]− | 179.0548, 631.3800 161.0444, 101.0230 | Momordicoside O |
| 2 | 8.17 | 843.4779 | 3.7 | C42H70O14 | [M + HCOO]− | 797.4688, 635.4132 617.4055, 161.0444 | Kugualycoside E |
| 3 | 8.18 | 977.5341 | 1.4 | C48H82O20 | [M − H]− | 815.4693, 797.4680 161.0444, 179.0550 | Momordicoside S |
| 4 | 9.20 | 815.4794 | −0.5 | C42H72O15 | [M − H]− | 653.4269, 491.3715 161.0444 | Momordicoside A |
| 5 | 9.65 | 859.4731 | 4.0 | C42H70O15 | [M + HCOO]− | 813.4646, 651.4112 633.3995, 489.3572 | Goyaglycoside h |
| 6 | 11.26 | 699.4344 | 2.7 | C36H62O10 | [M + HCOO]− | 653.4269, 491.3735 161.0444 | Momorcharaside B |
| 7 | 11.40 | 697.4191 | 3.2 | C36H60O10 | [M + HCOO]− | 651.4116, 633.3973 489.3567, 161.0442 | Momordicoside Q/ Karaviloside XI |
| 8 | 12.00 | 841.4617 | 3.1 | C42H68O14 | [M + HCOO]− | 795.4536, 633,4033 615.3884, 161.0442 | Momorcharaside M/N Karaviloside Ⅹ |
| 9 | 12.58 | 1377.6312 | −1.5 | C65H102O31 | [M − H]− | 1377.6312, 807.4169 627.3515, 469.3347 | Goyasaponin I |
| 10 | 12.90 | 1509.6751 | −0.3 | C70H110O35 | [M − H]− | 1509.6751, 627.3533 807.4170, 409.1368 | Goyasaponin II |
| 11 | 13.02 | 825.4681 | 4.7 | C42H68O13 | [M + HCOO]− | 779.4583, 617.4055 161.0443, 101.0230 | Goyalycoside e/f |
| 12 | 15.38 | 679.4094 | 4.6 | C36H58O9 | [M + HCOO]− | 633.4007, 471.3479 161.0443, 101.0230 | Momordicoside L |
| 13 | 17.28 | 679.4091 | 4.1 | C36H58O9 | [M + HCOO]− | 633.4006, 471.3443 161.0443, 101.0230 | Momord icoside P |
| 14 | 17.87 | 679.4088 | 3.7 | C36H58O9 | [M + HCOO]− | 633.4030, 455.3177 161.0444 | Momordicin II |
| Groups | Escape Latency (s) | Target Quadrant Crossing Times | Dwell Time (s) |
|---|---|---|---|
| Control | 10.39 ± 1.91 | 12.00 ± 0.82 | 49.05 ± 6.86 |
| d-gal | 45.17 ± 5.13 ### | 5.00 ± 0.82 ### | 19.50 ± 3.31 ### |
| d-gal+ Vc | 12.5 ± 1.65 *** | 10.50± 1.29 ** | 40.4 ± 4.32 ** |
| d-gal+ MCE | 13.07 ± 2.57 *** | 11.75 ± 0.96 *** | 39.13 ± 5.47 ** |
| Groups | Escape Latency (s) | Error Times | Dwell Time (s) |
|---|---|---|---|
| Control | 277.89 ± 19.77 | 1.17 ± 0.75 | 49.05 ± 6.86 |
| d-gal | 84.56 ± 20.24 ### | 3.33 ± 1.03 ## | 19.50 ± 3.31 ### |
| d-gal+ Vc | 262.63 ± 32.58 *** | 1.00 ± 063 ** | 40.4 ± 4.32 ** |
| d-gal+ MCE | 262.15 ± 26.41 *** | 1.17 ± 0.75 ** | 39.13 ± 5.47 ** |
| Groups | Body Weight (g) | Organ Index | |
|---|---|---|---|
| Spleen (mg/g) | Thymus (mg/g) | ||
| Control | 47.86 ± 3.15 | 3.65 ± 0.04 | 1.50 ± 0.07 |
| d-gal | 41.25 ± 2.46 ## | 2.00 ± 0.08 ### | 0.84 ± 0.17 ## |
| d-gal + Vc | 45.68 ± 2.32 ** | 3.43 ± 0.15 ** | 1.42 ± 0.10 ** |
| d-gal + FS | 45.88 ± 2.65 ** | 3.49 ± 0.11 *** | 1.45 ± 0.04 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, E.; Li, Y.; Teng, Y.; Li, H.; Jiao, L.; Wu, W. Anti-Aging Effect of Momordica charantia L. on d-Galactose-Induced Subacute Aging in Mice by Activating PI3K/AKT Signaling Pathway. Molecules 2022, 27, 4502. https://doi.org/10.3390/molecules27144502
Wang D, Wang E, Li Y, Teng Y, Li H, Jiao L, Wu W. Anti-Aging Effect of Momordica charantia L. on d-Galactose-Induced Subacute Aging in Mice by Activating PI3K/AKT Signaling Pathway. Molecules. 2022; 27(14):4502. https://doi.org/10.3390/molecules27144502
Chicago/Turabian StyleWang, Dongxue, Enhui Wang, Ying Li, Yaran Teng, Hui Li, Lili Jiao, and Wei Wu. 2022. "Anti-Aging Effect of Momordica charantia L. on d-Galactose-Induced Subacute Aging in Mice by Activating PI3K/AKT Signaling Pathway" Molecules 27, no. 14: 4502. https://doi.org/10.3390/molecules27144502
APA StyleWang, D., Wang, E., Li, Y., Teng, Y., Li, H., Jiao, L., & Wu, W. (2022). Anti-Aging Effect of Momordica charantia L. on d-Galactose-Induced Subacute Aging in Mice by Activating PI3K/AKT Signaling Pathway. Molecules, 27(14), 4502. https://doi.org/10.3390/molecules27144502






