Prenylated Flavonoids in Topical Infections and Wound Healing
Abstract
1. Introduction
2. Methods
3. Microenvironment of Skin Wounds
4. Microbiology of Skin Wounds
5. Bacterial Skin Infections in Livestock
Bovine Mastitis
6. Therapeutic Strategies of Skin Infections and Wound Healing
6.1. Wound Dressings Loaded with Natural Compounds
6.1.1. Essential Oils
6.1.2. Polyphenols
Flavonoids
6.2. Therapeutic Strategies for Bovine Mastitis
7. Flavonoids as Effective Anti-Infective and Wound Healing Agents
7.1. Flavonoids as Candidates for Therapy of Skin Lesions
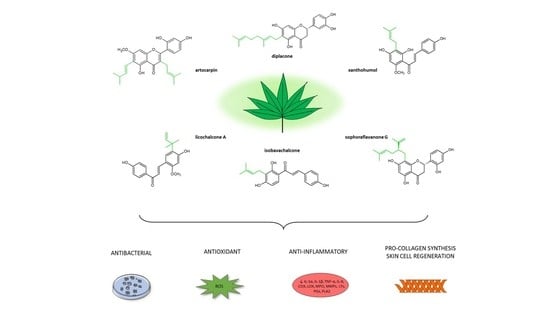
7.2. Prenylated Flavonoids
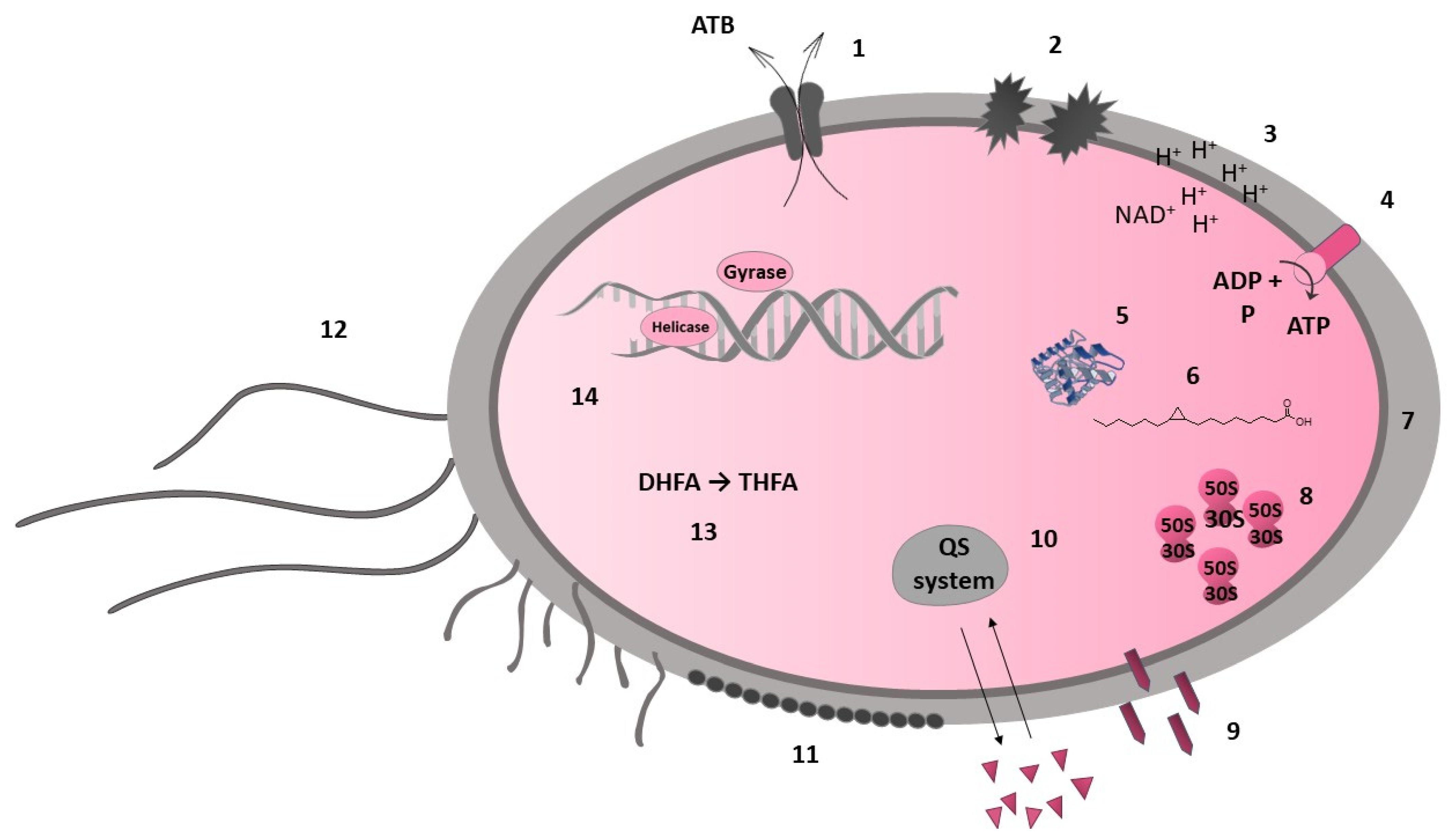
7.3. Mechanisms of Antibacterial Activity
7.3.1. Direct Interaction with Bacterial Cell
7.3.2. Indirect Antimicrobial Activity
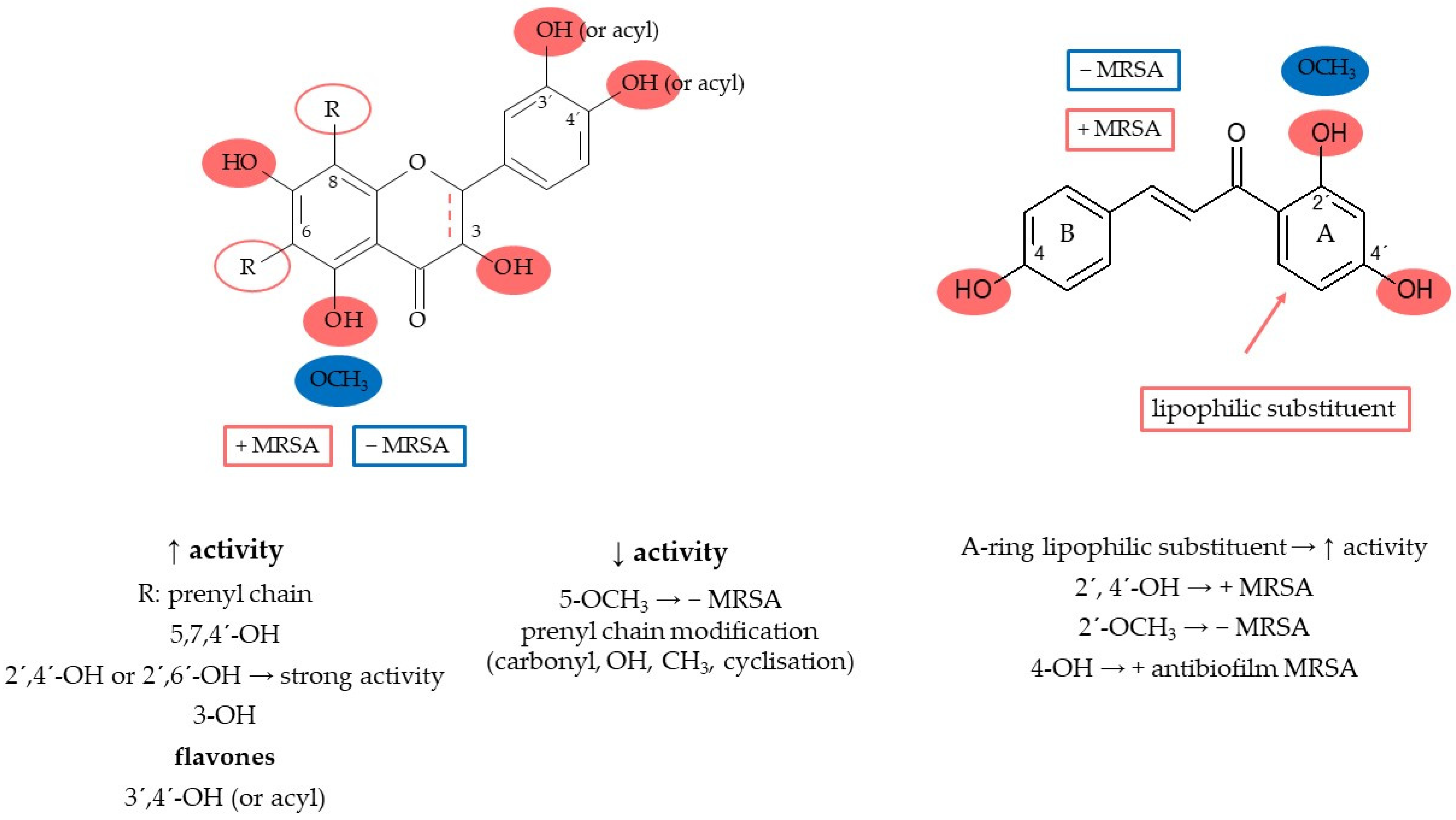
7.4. Structure–Activity Relationship
8. Discussion
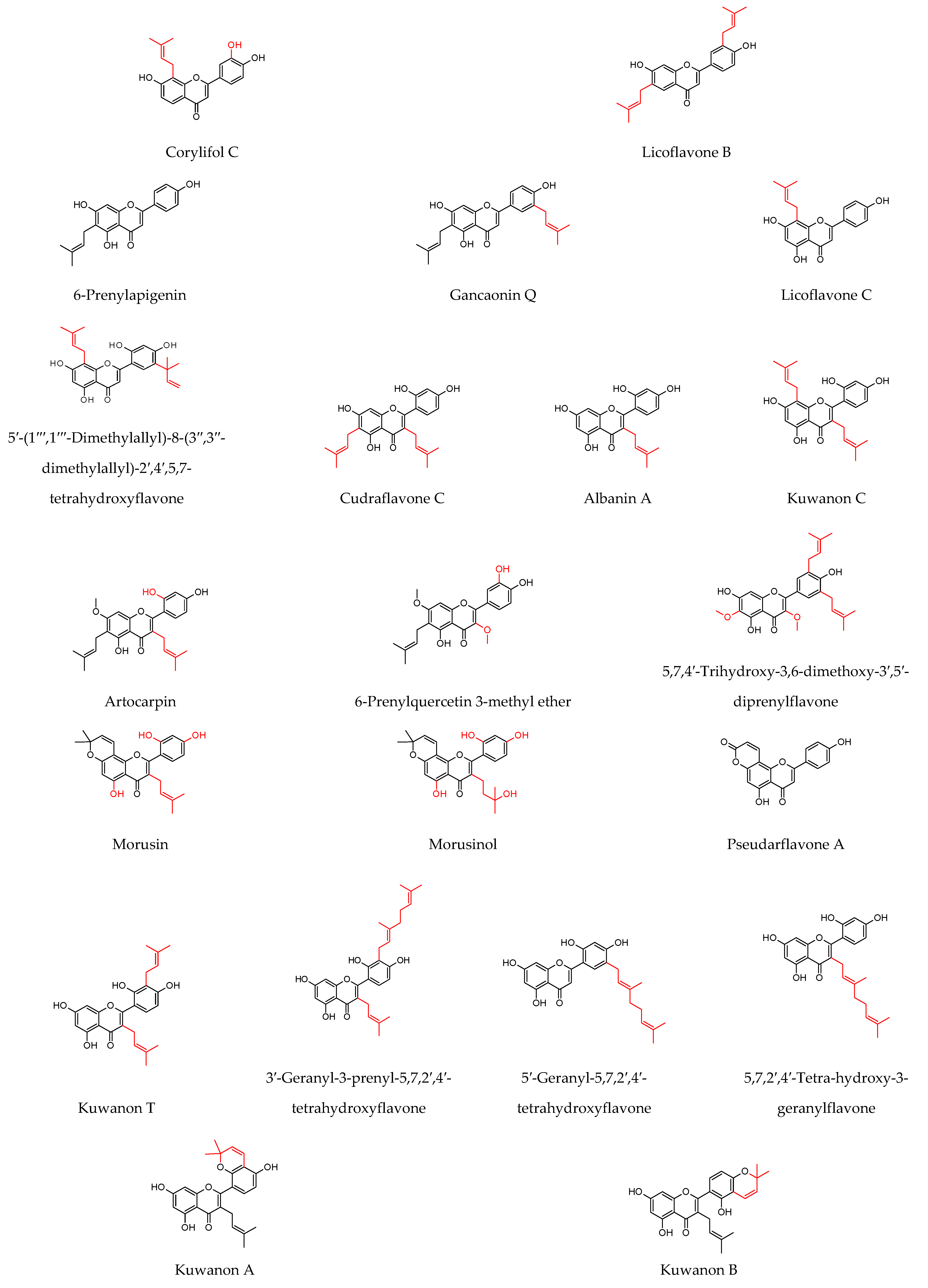
8.1. Prenylated Flavonoids with Potent Antibacterial Activity
8.2. Multiple Active Prenylated Flavonoids as Wound Healing Agents
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| CBC | N-carboxybutylchitosan |
| CFU | colony-forming units |
| CLSI | Clinical and Laboratory Standards Institute |
| COX | cyclooxygenase |
| D-Ala-D-Ala | D-alanine–D-alanine |
| DNA | deoxyribonucleic acid |
| EGCG | epigallocatechin gallate |
| EOs | essential oils |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| H2O2 | hydrogen peroxide |
| IFN-γ | interferon γ |
| IL-1β | interleukin 1β |
| IL-6 | interleukin 6 |
| IL-10 | interleukin 10 |
| LTB-4 | leukotriene B4 |
| MBC | minimum bactericidal concentration |
| MIC | minimum inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| NADH | nicotinamide adenine dinucleotide |
| NF-κB | nuclear factor kappa B |
| PGE2 | prostaglandin E2 |
| PUFA | polyunsaturated fatty acids |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| TNF-α | tumour necrosis factor α |
| UVB | ultraviolet B |
| VEGF | vascular endothelial growth factor |
| VRE | vancomycin-resistant Enterococcus |
References
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini-Rev. Med. Chem. 2017, 18, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Schultz, G.; Sibbald, G.; Falanga, V.; Ayello, E.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.; Teot, L.; Vanscheidt, W. Wound Bed Preparation: A Systematic Approach to Chronic Wounds. Wound Repair Regen 2003, 11 (Suppl. S1), S1–S28. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease, 8th ed.; Elsevier Inc.: Philadelphia, PA, USA, 2010. [Google Scholar]
- Scalise, A.; Bianchi, A.; Tartaglione, C.; Bolletta, E.; Pierangeli, M.; Torresetti, M.; Marazzi, M.; Di Benedetto, G. Microenvironment and Microbiology of Skin Wounds: The Role of Bacterial Biofilms and Related Factors. Semin. Vasc. Surg. 2015, 28, 151–159. [Google Scholar] [CrossRef]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the Skin and the Role of Biofilms in Infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Ayton, M. Wound Care: Wounds That Won’t Heal. Nurs. Times 1985, 81, 16–19. [Google Scholar]
- Lowy, F. The Chromosome, as Well as the Extrachromosomal El- Ements. 6 These Genes Are Transferred between Staphy-Lococcal Strains, Species, or Other Gram-Positive Bacte-Rial Species through the Extrachromosomal Elements. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.F.; Wilson, S.E. Skin and Soft-Tissue Infections: A Critical Review and the Role of Telavancin in Their Treatment. Clin. Infect. Dis. 2015, 61 (Suppl. S2), S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G. Antimicrobial and Antiseptic Strategies in Wound Management. Int. Wound J. 2013, 10 (Suppl. S1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.P. Staphylococcal Skin Disease in Livestock. Vet. Dermatol. 2012, 23, 342–352. [Google Scholar] [CrossRef]
- Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2020. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 3 March 2021).
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-Resistant Staphylococcus aureus in Pig Farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Mala, L.; Lalouckova, K.; Skrivanova, E. Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment. Animals 2021, 11, 2473. [Google Scholar] [CrossRef]
- Contreras, G.A.; Rodríguez, J.M. Mastitis: Comparative Etiology and Epidemiology. J. Mammary Gland Biol. Neoplasia 2011, 16, 339–356. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Mushtaq, S.; Shah, A.M.A.; Shah, A.M.A.; Lone, S.A.; Hussain, A.; Hassan, Q.P.; Ali, M.N. Bovine Mastitis: An Appraisal of Its Alternative Herbal Cure. Microb. Pathog. 2018, 114, 357–361. [Google Scholar] [CrossRef]
- Hossain, M.K.; Paul, S.; Hossain, M.M.; Islam, M.R.; Alam, M.G.S. Bovine Mastitis and Its Therapeutic Strategy Doing Antibiotic Sensitivity Test. Austin J. Vet. Sci. Anim. Husb. 2017, 4, 1030. [Google Scholar] [CrossRef]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granié, C.; Rupp, R.; Rainard, P. Differential Response of Bovine Mammary Epithelial Cells to Staphylococcus aureus or Escherichia coli Agonists of the Innate Immune System. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef] [PubMed]
- Amini, B.; Baghchesaraie, H.; Faghihi, M.H.O. Effect of Different Sub MIC Concentrations of Penicillin, Vancomycin and Ceftazidime on Morphology and Some Biochemical Properties of Staphylococcus aureus and Pseudomonas Aeruginosa Isolates. Iran. J. Microbiol. 2009, 1, 43–47. [Google Scholar]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of Plant Extracts and Essential Oils in the Control of Bovine Mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural Wound Healing and Bioactive Natural Products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine—A Review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef]
- Amparo, T.R.; Seibert, J.B.; de Abreu Vieira, P.M.; Teixeira, L.F.M.; dos Santos, O.D.H.; de Souza, G.H.B. Herbal Medicines to the Treatment of Skin and Soft Tissue Infections: Advantages of the Multi-Targets Action. Phyther. Res. 2020, 34, 94–103. [Google Scholar] [CrossRef]
- Thorne, C.H.; Chung, K.C.; Gosain, A.K.; Gurtner, G.C.; Mehrara, B.J.; Rubin, J.P.; Spear, S.L. Grabb and Smith’s Plastic Surgery, 7th ed.; Wolters Kluwer Health Adis (ESP): London, UK, 2013. [Google Scholar]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of Essential Oils to Improve Solubility, Stability and Permeability: A Review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple Biological Activities of Curcumin: A Short Review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- FDA (Food and Drug Administration). Nda 21-902 Veregen. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021902s002lbl.pdf (accessed on 14 June 2022).
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-Based Nanoformulated (-)-Epigallocatechin-3-Gallate (EGCG) Modulates Human Keratinocyte-Induced Responses and Alleviates Imiquimod-Induced Murine Psoriasiform Dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef]
- Silva, J.; Vanat, P.; Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Metal Alginates for Polyphenol Delivery Systems: Studies on Crosslinking Ions and Easy-to-Use Patches for Release of Protective Flavonoids in Skin. Bioact. Mater. 2020, 5, 447–457. [Google Scholar] [CrossRef]
- Amer, S.S.; Mamdouh, W.; Nasr, M.; ElShaer, A.; Polycarpou, E.; Abdel-Aziz, R.T.A.; Sammour, O.A. Quercetin Loaded Cosm-Nutraceutical Electrospun Composite Nanofibers for Acne Alleviation: Preparation, Characterization and Experimental Clinical Appraisal. Int. J. Pharm. 2022, 612, 121309. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; De Sousa, H.C. Development of Natural-Based Wound Dressings Impregnated with Bioactive Compounds and Using Supercritical Carbon Dioxide. Int. J. Pharm. 2011, 408, 9–19. [Google Scholar] [CrossRef]
- Dyja, R.; Jankowski, A. The Effect of Additives on Release and in Vitro Skin Retention of Flavonoids from Emulsion and Gel Semisolid Formulations. Int. J. Cosmet. Sci. 2017, 39, 442–449. [Google Scholar] [CrossRef]
- Roy, P.; Parveen, S.; Ghosh, P.; Ghatak, K.; Dasgupta, S. Flavonoid Loaded Nanoparticles as an Effective Measure to Combat Oxidative Stress in Ribonuclease A. Biochimie 2019, 162, 185–197. [Google Scholar] [CrossRef]
- Pool, H.; Quintanar, D.; Figueroa, J.D.D.; Marinho Mano, C.; Bechara, J.E.H.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.K.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical Apigenin Improves Epidermal Permeability Barrier Homoeostasis in Normal Murine Skin by Divergent Mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Antimicrobial Activities of Green Synthesized Gums-Stabilized Nanoparticles Loaded with Flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Villegas, V.; Clares-Naveros, B.; García-López, M.L.; Calpena-Campmany, A.C.; Bustos-Zagal, P.; Garduño-Ramírez, M.L. Development and Characterization of Two Nano-Structured Systems for Topical Application of Flavanones Isolated from Eysenhardtia Platycarpa. Colloids Surf. B Biointerfaces 2014, 116, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Lister, T.; May-Dracka, T.L. New Natural Products as New Leads for Antibacterial Drug Discovery. Bioorganic Med. Chem. Lett. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F. Prospects for Plant-Derived Antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic Flavonoids with Antimicrobial Activity: A Review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Nagula, R.L.; Wairkar, S. Recent Advances in Topical Delivery of Flavonoids: A Review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A Systematic Review on Biological Activities of Prenylated Flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Barron, D.; Ibrahim, R.K. Isoprenylated Flavonoids—A Survey. Phytochemistry 1996, 43, 921–982. [Google Scholar] [CrossRef]
- Šmejkal, K. Cytotoxic potential of C-prenylated flavonoids. Phytochem. Rev. 2014, 13, 245–275. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Qiu, Y.; Yuan, H.; Khan, S.I.; Hussain, N.; Iqbal Choudhary, M.; Zeng, F.; Guo, D.A.; Khan, I.A.; et al. Prenylated Flavonoids from the Stems and Roots of Tripterygium wilfordii. Fitoterapia 2017, 119, 64–68. [Google Scholar] [CrossRef]
- Al-Rehaily, A.J.; Albishi, O.A.; El-Olemy, M.M.; Mossa, J.S. Flavonoids and Terpenoids from Helichrysum forskahlii. Phytochemistry 2008, 69, 1910–1914. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, D.; Li, F.F.; Yao, G.D.; Li, X.; Li, L.Z.; Huang, X.X.; Song, S.J. Cytotoxic Prenylated Flavones from the Stem and Root Bark of Daphne giraldii. Bioorganic Med. Chem. Lett. 2016, 26, 3968–3972. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Jiang, Y.; Yang, B. An Update of Prenylated Phenolics: Food Sources, Chemistry and Health Benefits. Trends Food Sci. Technol. 2021, 108, 197–213. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation Enhances the Biological Activity of Dietary Flavonoids by Altering Their Bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Shintani, Y.; Aga, Y.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic Constituents of Licorice. VIII. Structures of Glicophenone and Glicoisoflavanone, and Effects of Licorice Phenolics on Methicillin-Resistant Staphylococcus aureus. Chem. Pharm. Bull. 2000, 48, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Yeon, S.C.; Son, K.H.; Hyeun, W.C.; Ju, S.K.; Sam, S.K.; Hyun, P.K. Effects of Sophoraflavanone G, a Prenylated Flavonoid from Sophora flavescens, on Cyclooxygenase-2 and in Vivo Inflammatory Response. Arch. Pharm. Res. 2002, 25, 329–335. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-Alanine Ligase as a New Target for the Flavonoids Quercetin and Apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular Dynamics Study on the Biophysical Interactions of Seven Green Tea Catechins with Lipid Bilayers of Cell Membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef]
- Kusuda, M.; Inada, K.; Ogawa, T.O.; Yoshida, T.; Shiota, S.; Tsuchiya, T.; Hatano, T. Polyphenolic Constituent Structures of Zanthoxylum piperitum Fruit and the Antibacterial Effects of Its Polymeric Procyanidin on Methicillin-Resistant Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2006, 70, 1423–1431. [Google Scholar] [CrossRef]
- Arakawa, H.; Kanemitsu, M.; Tajima, N.; Maeda, M. Chemiluminescence Assay for Catechin Based on Generation of Hydrogen Peroxide in Basic Solution. Anal. Chim. Acta 2002, 472, 75–82. [Google Scholar] [CrossRef]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal Catechins Damage the Lipid Bilayer. BBA-Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Miura, Y.H.; Tomita, I.; Watanabe, T.; Hirayama, T.; Fukui, S. Active Oxygens Generation by Flavonoids. Biol. Pharm. Bull. 1998, 21, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Detection of Galangin-Induced Cytoplasmic Membrane Damage in Staphylococcus aureus by Measuring Potassium Loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial Action of Propolis and Some of Its Components: The Effects on Growth, Membrane Potential and Motility of Bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Iinuma, M. Reduction of Membrane Fluidity by Antibacterial Sophoraflavanone G Isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef]
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary Bioflavonoids Inhibit Escherichia coli ATP Synthase in a Differential Manner. Int. J. Biol. Macromol. 2010, 46, 478–486. [Google Scholar] [CrossRef]
- Bensasson, R.V.; Zoete, V.; Jossang, A.; Bodo, B.; Arimondo, P.B.; Land, E.J. Potency of Inhibition of Human DNA Topoisomerase i by Flavones Assessed through Physicochemical Parameters. Free Radic. Biol. Med. 2011, 51, 1406–1410. [Google Scholar] [CrossRef]
- Ohemeng, K.A.; Schwender, C.F.; Fu, K.P.; Barrett, J.F. DNA Gyrase Inhibitory and Antibacterial Activity of Some Flavones(1). Bioorganic Med. Chem. Lett. 1993, 3, 225–230. [Google Scholar] [CrossRef]
- Bernard, F.X.; Sablé, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated Flavones as Selective Inhibitors of Topoisomerase IV. Antimicrob. Agents Chemother. 1997, 41, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ziegelin, G.; Schröder, W.; Frank, J.; Ayora, S.; Alonso, J.C.; Lanka, E.; Saenger, W. Flavones Inhibit the Hexameric Replicative Helicase RepA. Nucleic Acids Res. 2001, 29, 5058–5066. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial Activity and Mode of Action of Plant Flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Navarro-Martínez, M.D.; Navarro-Perán, E.; Cabezas-Herrera, J.; Ruiz-Gómez, J.; García-Cánovas, F.; Rodríguez-López, J.N. Antifolate Activity of Epigallocatechin Gallate against Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2005, 49, 2914–2920. [Google Scholar] [CrossRef]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium falciparum Fatty Acid Biosynthesis: Evaluation of FabG, FabZ, and FabI as Drug Targets for Flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of Bacterial Cell-Cell Signalling, Biofilm Formation and Type III Secretion System by Citrus Flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157:H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Budzyńska, A.; Rózalski, M.; Karolczak, W.; Wieckowska-Szakiel, M.; Sadowska, B.; Rózalska, B. Synthetic 3-Arylidenefl Avanones as Inhibitors of the Initial Stages of biofilm formation by Staphylococcus aureus and Enterococcus faecalis. Z. Naturforschung. C J. Biosci. 2011, 66, 104–114. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Madan, S.; Singh, G.N.; Kohli, K.; Ali, M.; Kumar, Y.; Singh, R.M.; Prakash, O. Isoflavonoids from Flemingia strobilifera (L.) R. Br. Roots. Acta Pol. Pharm.-Drug Res. 2009, 66, 297–303. [Google Scholar]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Navrátilová, A.; Schneiderová, K.; Veselá, D.; Hanáková, Z.; Fontana, A.; Dall’acqua, S.; Cvačka, J.; Innocenti, G.; Novotná, J.; Urbanová, M.; et al. Minor C-Geranylated Flavanones from Paulownia tomentosa Fruits with MRSA Antibacterial Activity. Phytochemistry 2013, 89, 104–113. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Alcaráz, L.E.; Blanco, S.E.; Puig, O.N.; Tomás, F.; Ferretti, F.H. Antibacterial Activity of Flavonoids against Methicillin-Resistant Staphylococcus aureus Strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Tran, T.D.; Nguyen, T.T.N.; Do, T.H.; Huynh, T.N.P.; Tran, C.D.; Thai, K.M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef]
- Oh, I.; Yang, W.Y.; Chung, S.C.; Kim, T.Y.; Oh, K.B.; Shin, J. In Vitro Sortase A Inhibitory and Antimicrobial Activity of Flavonoids Isolated from the Roots of Sophora flavescens. Arch. Pharm. Res. 2011, 34, 217–222. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative Study on the Antibacterial Activity of Phytochemical Flavanones against Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Idowu, T.O.; Ogundaini, A.O.; Salau, A.O.; Obuotor, E.M.; Bezabih, M.; Abegaz, B.M. Doubly Linked, A-Type Proanthocyanidin Trimer and Other Constituents of Ixora coccinea Leaves and Their Antioxidant and Antibacterial Properties. Phytochemistry 2010, 71, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M.; et al. Proanthocyanidins: Target Compounds as Antibacterial Agents. J. Agric. Food Chem. 2008, 56, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Khadke, S.K.; Yamano, A.; Woo, J.T.; Lee, J. Antimicrobial and Antibiofilm Activities of Prenylated Flavanones from Macaranga tanarius. Phytomedicine 2019, 63, 153033. [Google Scholar] [CrossRef] [PubMed]
- Muharini, R.; Diaz, A.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Rehberg, N.; Kalscheuer, R.; Hartmann, R.; Orfali, R.S.; Lin, W.; et al. Antibacterial and Cytotoxic Phenolic Metabolites from the Fruits of Amorpha fruticosa. J. Nat. Prod. 2017, 80, 169–180. [Google Scholar] [CrossRef]
- Meier, D.; Hernández, M.V.; van Geelen, L.; Muharini, R.; Proksch, P.; Bandow, J.E.; Kalscheuer, R. The Plant-Derived Chalcone Xanthoangelol Targets the Membrane of Gram-Positive Bacteria. Bioorganic Med. Chem. 2019, 27, 115151. [Google Scholar] [CrossRef]
- Nanayakkara, N.P.D.; Burandt, C.L.; Jacob, M.R. Flavonoids with Activity against Methicillin-Resistant Staphylococcus aureus from Dalea scandens var. Paucifolia. Planta Med. 2002, 68, 519–522. [Google Scholar] [CrossRef]
- Belofsky, G.; Percivill, D.; Lewis, K.; Tegos, G.P.; Ekart, J. Phenolic Metabolites of Dalea Versicolor That Enhance Antibiotic Activity against Model Pathogenic Bacteria. J. Nat. Prod. 2004, 67, 481–484. [Google Scholar] [CrossRef]
- Belofsky, G.; Aronica, M.; Foss, E.; Diamond, J.; Santana, F.; Darley, J.; Dowd, P.F.; Coleman, C.M.; Ferreira, D. Antimicrobial and Antiinsectan Phenolic Metabolites of Dalea searlsiae. J. Nat. Prod. 2014, 77, 1140–1149. [Google Scholar] [CrossRef]
- Yusook, K.; Weeranantanapan, O.; Hua, Y.; Kumkrai, P.; Chudapongse, N. Lupinifolin from Derris Reticulata Possesses Bactericidal Activity on Staphylococcus aureus by Disrupting Bacterial Cell Membrane. J. Nat. Med. 2017, 71, 357–366. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and Cytotoxic Activity of 18 Prenylated Flavonoids Isolated from Medicinal Plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Van Heerden, F.R.; Van Staden, J. Antibacterial Activity of Flavonoids from the Stem Bark of Erythrina caffra Thunb. Phyther. Res. 2011, 25, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Oliveira, T.B.; Khumalo, G.P.; van Vuuren, S.F.; van Wyk, B.E. Antimicrobial Isoflavones and Derivatives from Erythrina (Fabaceae): Structure Activity Perspective (SAR & QSAR) on Experimental and Mined Values against Staphylococcus aureus. Antibiotics 2020, 9, 223. [Google Scholar] [CrossRef]
- Salvatore, M.J.; King, A.B.; Graham, A.C.; Onishi, H.R.; Bartizal, K.F.; Abruzzo, G.K.; Gill, C.J.; Ramjit, H.G.; Pitzenberger, S.M.; Witherup, K.M. Antibacterial Activity of Lonchocarpol A. J. Nat. Prod. 1998, 61, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Raksat, A.; Maneerat, W.; Andersen, R.J.; Pyne, S.G.; Laphookhieo, S. Antibacterial Prenylated Isoflavonoids from the Stems of Millettia extensa. J. Nat. Prod. 2018, 81, 1835–1840. [Google Scholar] [CrossRef]
- Raksat, A.; Maneerat, W.; Rujanapun, N.; Andersen, R.J.; Pyne, S.G.; Laphookhieo, S. Antibacterial and Inhibitory Activities against Nitric Oxide Production of Coumaronochromones and Prenylated Isoflavones from Millettia extensa. J. Nat. Prod. 2019, 82, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Tchamgoue, J.; Tchouankeu, J.C.; Kouam, S.F.; Choudhary, M.I.; Bakowsky, U. Antibacterial Activity and Cytotoxicity of Flavonoids Compounds Isolated from Pseudarthria hookeri Wight & Arn. (Fabaceae). S. Afr. J. Bot. 2018, 114, 100–103. [Google Scholar] [CrossRef]
- Yin, S.; Fan, C.Q.; Wang, Y.; Dong, L.; Yue, J.M. Antibacterial Prenylflavone Derivatives from Psoralea corylifolia, and Their Structure-Activity Relationship Study. Bioorganic Med. Chem. 2004, 12, 4387–4392. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, Antifungal and Cytotoxic Activities of Two Flavonoids from Retama Raetam Flowers. Molecules 2012, 17, 7284–7293. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Yu, H.; Wong, C.W.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Paris, J.M.; Morleo, B.; Litaudon, M.; Lau, C.B.S.; et al. Quick Identification of Kuraridin, a Noncytotoxic Anti-MRSA (Methicillin-Resistant Staphylococcus aureus) Agent from Sophora flavescens Using High-Speed Counter-Current Chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 880, 157–162. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, X.; Shi, W.; Lu, Q.; Liang, V.; Heber, D.; Ma, L. Letters to the Editor Inhibition of Growth of Streptococcus mutans, Methicillin-Resistant Staphylococcus. J. Clin. Microbiol. 2005, 43, 3574–3575. [Google Scholar] [CrossRef]
- Cha, J.-D.; Moon, S.-E.; Kim, J.-Y.; Jung, E.-K.; Lee, Y.-S. Antibacterial Activity of Sophoraflavanone G Isolated from the Roots of Sophora flavescens against Methicillin-Resistant Staphylococcus aureus. Phytother. Res. 2009, 23, 1326–1331. [Google Scholar] [CrossRef]
- Lee, G.S.; Kim, E.S.; Cho, S.I.; Kim, J.H.; Choi, G.; Ju, Y.S.; Park, S.H.; Jeong, S.-I.; Kim, H.J. Antibacterial and Synergistic Activity of Prenylated Chalcone Isolated from the Roots of Sophora flavescens. J. Appl. Biol. Chem. 2010, 53, 290–296. [Google Scholar] [CrossRef]
- Daus, M.; Chaithada, P.; Phongpaichit, S.; Watanapokasin, R.; Carroll, A.R.; Mahabusarakam, W. New Prenylated Dihydrochalcones from the Leaves of Artocarpus elasticus. Phytochem. Lett. 2017, 19, 226–230. [Google Scholar] [CrossRef]
- Dej-Adisai, S.; Meechai, I.; Puripattanavong, J.; Kummee, S. Antityrosinase and Antimicrobial Activities from Thai Medicinal Plants. Arch. Pharm. Res. 2014, 37, 473–483. [Google Scholar] [CrossRef]
- Radwan, M.M.; Rodriguez-Guzman, R.; Manly, S.P.; Jacob, M.; Ross, S.A. Sepicanin A-A New Geranyl Flavanone from Artocarpus Sepicanus with Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Phytochem. Lett. 2009, 2, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Simo, I.K.; Ngameni, B.; Bigoga, J.D.; Watchueng, J.; Kapguep, R.N.; Etoa, F.X.; Tchaleu, B.N.; Beng, V.P. Antimicrobial Activity of the Methanolic Extract, Fractions and Four Flavonoids from the Twigs of Dorstenia angusticornis Engl. (Moraceae). J. Ethnopharmacol. 2007, 112, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Hamamoto, H.; Ngameni, B.; Ngadjui, B.T.; Sekimizu, K. Antimicrobial Action Mechanism of Flavonoids from Dorstenia Species. Drug Discov. Ther. 2013, 7, 66–72. [Google Scholar] [CrossRef][Green Version]
- Mbaveng, A.T.; Ngameni, B.; Kuete, V.; Simo, I.K.; Ambassa, P.; Roy, R.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial Activity of the Crude Extracts and Five Flavonoids from the Twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 2008, 116, 483–489. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V.; Ngameni, B.; Beng, V.P.; Ngadjui, B.T.; Meyer, J.J.M.; Lall, N. Antimicrobial Activities of the Methanol Extract and Compounds from the Twigs of Dorstenia mannii (Moraceae). BMC Complement. Altern. Med. 2012, 12, 2–7. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Macluracochinones A-E, Antimicrobial Flavonoids from Maclura cochinchinensis (Lour.) Corner. Phytochemistry 2021, 187, 112773. [Google Scholar] [CrossRef]
- Özçelik, B.; Orhan, I.; Toker, G. Antiviral and Antimicrobial Assessment of Some Selected Flavonoids. Z. Fur Naturforsch.—Sect. C J. Biosci. 2006, 61, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, Z.J.; He, Y.J.; Qin, Y.; Zhou, Y.; Qi, Z.H.; Zhou, Z.S.; Zhu, Y.Y.; Jin, D.N.; Chen, S.S.; et al. Bioguided Isolation, Identification and Bioactivity Evaluation of Anti-MRSA Constituents from Morus alba Linn. J. Ethnopharmacol. 2021, 281, 114542. [Google Scholar] [CrossRef] [PubMed]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple In Vitro Biological Effects of Phenolic Compounds from Morus alba Root Bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Han, F.; Song, M.R.; Chen, S.; Li, Q.; Zhang, Q.; Zhu, K.; Shen, J.Z. Natural Flavones from Morus alba against Methicillin-Resistant Staphylococcus aureus via Targeting the Proton Motive Force and Membrane Permeability. J. Agric. Food Chem. 2019, 67, 10222–10234. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.Y.; Yang, C.X.; Han, J.; Li, Y.Q.; Wang, G.C. Synergism of Prenylflavonoids from Morus alba Root Bark against Clinical MRSA Isolates. Phytomedicine 2018, 39, 93–99. [Google Scholar] [CrossRef]
- Šmejkal, K.; Chudík, S.; Klouc, P. Antibacterial C-Geranylflavonoids from Paulownia tomentosa Fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial Activity of Propolins from Taiwanese Green Propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, 2100749. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Z.; Qian, J.; Wang, Y.; Wang, S. Protective Effect of Jie-Geng-Tang against Staphylococcus aureus Induced Acute Lung Injury in Mice and Discovery of Its Effective Constituents. J. Ethnopharmacol. 2019, 243, 112076. [Google Scholar] [CrossRef]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial Effect and Mode of Action of Flavonoids from Licorice against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef]
- De Assis, L.R.; Theodoro, R.D.S.; Costa, M.B.S.; Nascentes, J.A.S.; da Rocha, M.D.; de Souza Bessa, M.A.; de Paula Menezes, R.; Dilarri, G.; Hypolito, G.B.; Dos Santos, V.R.; et al. Antibacterial Activity of Isobavachalcone (IBC) Is Associated with Membrane Disruption. Membranes 2022, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Deng, X.; Qiu, J. Antimicrobial Activity of Licochalcone E against Staphylococcus aureus and Its Impact on the Production of Staphylococcal Alpha-Toxin. J. Microbiol. Biotechnol. 2012, 22, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Rozalski, M.; Micota, B.; Sadowska, B.; Stochmal, A.; Jedrejek, D.; Wieckowska-Szakiel, M.; Rozalska, B. Antiadherent and Antibiofilm Activity of Humulus lupulus L. Derived Products: New Pharmacological Properties. BioMed Res. Int. 2013, 2013, 101089. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, K.; Röderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm Activity of Bioactive Hop Compounds Humulone, Lupulone and Xanthohumol toward Susceptible and Resistant Staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Vu, H.; Pham, N.; Zencak, D.; Forster, P.; Quinn, R.J. Cytotoxic Evaluation of Alkaloids and Isoflavonoids from the Australian Tree Erythrina vespertilio. Planta Med. 2012, 78, 730–736. [Google Scholar] [CrossRef]
- Li, P.Y.; Liang, Y.C.; Sheu, M.J.; Huang, S.S.; Chao, C.Y.; Kuo, Y.H.; Huang, G.J. Alpinumisoflavone Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Regulating the Effects of Anti-Oxidation and Anti-Inflammation Both: In Vitro and in Vivo. RSC Adv. 2018, 8, 31515–31528. [Google Scholar] [CrossRef]
- Fu, G.; Li, W.; Huang, X.; Zhang, R.; Tian, K.; Hou, S.; Li, Y. Antioxidant and Alpha-Glucosidase Inhibitory Activities of Isoflavonoids from the Rhizomes of Ficus tikoua Bur. Nat. Prod. Res. 2018, 32, 399–405. [Google Scholar] [CrossRef]
- Lee, S.; Hoang, G.D.; Kim, D.; Song, H.S.; Choi, S.; Lee, D.; Kang, K.S. Efficacy of Alpinumisoflavone Isolated from Maclura tricuspidata Fruit in Tumor Necrosis Factor-α-Induced Damage of Human Dermal Fibroblasts. Antioxidants 2021, 10, 514. [Google Scholar] [CrossRef]
- Han, A.R.; Kang, Y.J.; Windono, T.; Lee, S.K.; Seo, E.K. Prenylated Flavonoids from the Heartwood of Artocarpus communis with Inhibitory Activity on Lipopolysaccharide-Induced Nitric Oxide Production. J. Nat. Prod. 2006, 69, 719–721. [Google Scholar] [CrossRef]
- Rajendran, M.; Manisankar, P.; Gandhidasan, R.; Murugesan, R. Free Radicals Scavenging Efficiency of a Few Naturally Occurring Flavonoids: A Comparative Study. J. Agric. Food Chem. 2004, 52, 7389–7394. [Google Scholar] [CrossRef]
- Septama, A.W.; Panichayupakaranant, P. Synergistic Effect of Artocarpin on Antibacterial Activity of Some Antibiotics against Methicillin-Resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli. Pharm. Biol. 2016, 54, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Wang, S.; Wang, B.; Liu, Y.; Yuan, H.; Lou, H.; Wang, X. New Phenolic Compounds from the Twigs of Artocarpus heterophyllus. Drug Discov. Ther. 2013, 7, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Hou, A.J.; Chen, L.; Chen, D.F.; Sun, H.D.; Zhao, Q.S.; Bastow, K.F.; Nakanish, Y.; Wang, X.H.; Lee, K.H. New Isoprenylated Flavones, Artochamins A–E, and Cytotoxic Principles from Artocarpus chama. J. Nat. Prod. 2004, 67, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Ko, H.H.; Lin, C.C.; Chai, C.Y.; Chen, W.T.; Yen, F.L. Artocarpin Attenuates Ultraviolet B-Induced Skin Damage in Hairless Mice by Antioxidant and Anti-Inflammatory Effect. Food Chem. Toxicol. 2013, 60, 123–129. [Google Scholar] [CrossRef]
- Yeh, C.J.; Chen, C.C.; Leu, Y.L.; Lin, M.W.; Chiu, M.M.; Wang, S.H. The Effects of Artocarpin on Wound Healing: In Vitro and in Vivo Studies. Sci. Rep. 2017, 7, 15599. [Google Scholar] [CrossRef]
- Boonphong, S.; Baramee, A.; Kittakoop, P. Antitubercular and Antiplasmodial Prenylated Flavones from the Roots of Artocarpus altilis. Chiang Mai J. Sci. 2007, 34, 339–344. [Google Scholar]
- Lee, S.; Yun, B.; Kim, M.; Park, C.; Lee, W.; Oh, H.M.; Rho, M.C. Phenolic Compounds Isolated from Psoralea corylifolia Inhibit IL-6-Induced STAT3 Activation. Planta Med. 2012, 78, 903–906. [Google Scholar] [CrossRef]
- Hung, Y.L.; Wang, S.C.; Suzuki, K.; Fang, S.H.; Chen, C.S.; Cheng, W.C.; Su, C.C.; Yeh, H.C.; Tu, H.P.; Liu, P.L.; et al. Bavachin Attenuates LPS-Induced Inflammatory Response and Inhibits the Activation of NLRP3 Inflammasome in Macrophages. Phytomedicine 2019, 59, 152785. [Google Scholar] [CrossRef]
- Liang, Z.; Luo, Z.; Chen, J.; Li, B.; Li, L.; Shen, C. Bavachin Inhibits IL-4 Expression by Downregulating STAT6 Phosphorylation and GATA-3 Expression and Ameliorates Asthma Inflammation in an Animal Model. Immunobiology 2022, 227, 152182. [Google Scholar] [CrossRef]
- Šmejkal, K.; Grycová, L.; Marek, R.; Lemiere, F.; Jankovská, D.; Forejtníková, H.; Vančo, J.; Suchý, V. C-Geranyl Compounds from Paulownia tomentosa Fruits. J. Nat. Prod. 2007, 70, 1244–1248. [Google Scholar] [CrossRef]
- Hošek, J.; Závalová, V.; Šmejkal, K.; Bartoš, M. Effect of Diplacone on Lps-Induced Inflammatory Gene Expression in Macrophages. Folia Biol. 2010, 56, 124–130. [Google Scholar]
- Asai, T.; Hara, N.; Kobayashi, S.; Kohshima, S.; Fujimoto, Y. Geranylated Flavanones from the Secretion on the Surface of the Immature Fruits of Paulownia tomentosa. Phytochemistry 2008, 69, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Hošek, J.; Toniolo, A.; Neuwirth, O.; Bolego, C. Prenylated and Geranylated Flavonoids Increase Production of Reactive Oxygen Species in Mouse Macrophages but Inhibit the Inflammatory Response. J. Nat. Prod. 2013, 76, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vaněk, T.; Schuster, D.; Dall’Acqua, S.; Cvačka, J.; Polanský, O.; et al. Anti-Inflammatory Activity of Natural Geranylated Flavonoids: Cyclooxygenase and Lipoxygenase Inhibitory Properties and Proteomic Analysis. J. Nat. Prod. 2017, 80, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Molčanová, L.; Kauerová, T.; Dall’Acqua, S.; Maršík, P.; Kollár, P.; Šmejkal, K. Antiproliferative and Cytotoxic Activities of C-Geranylated Flavonoids from Paulownia tomentosa Steud. Fruit. Bioorganic Chem. 2021, 111, 104797. [Google Scholar] [CrossRef]
- Vochyánová, Z.; Bartošová, L.; Bujdáková, V.; Fictum, P.; Husník, R.; Suchý, P.; Šmejkal, K.; Hošek, J. Diplacone and Mimulone Ameliorate Dextran Sulfate Sodium-Induced Colitis in Rats. Fitoterapia 2015, 101, 201–207. [Google Scholar] [CrossRef]
- Zima, A.; Hošek, J.; Treml, J.; Muselík, J.; Suchý, P.; Pražanová, G.; Lopes, A.; Žemlička, M. Antiradical and Cytoprotective Activities of Several C-Geranyl-Substituted Flavanones from Paulownia tomentosa Fruit. Molecules 2010, 15, 6035–6049. [Google Scholar] [CrossRef]
- Šmejkal, K.; Svačinová, J.; Šlapetová, T.; Schneiderová, K.; Dall’Acqua, S.; Innocenti, G.; Závalová, V.; Kollár, P.; Chudík, S.; Marek, R.; et al. Cytotoxic Activities of Several Geranyl-Substituted Flavanones. J. Nat. Prod. 2010, 73, 568–572. [Google Scholar] [CrossRef]
- Jin, Q.; Lee, C.; Lee, J.W.; Lee, D.; Kim, Y.; Hong, J.T.; Kim, J.S.; Kim, J.H.; Lee, M.K.; Hwang, B.Y. Geranylated Flavanones from Paulownia coreana and Their Inhibitory Effects on Nitric Oxide Production. Chem. Pharm. Bull. 2015, 63, 384–387. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Taira, N.; Ishii, T.; Halim, M.A.; Hossain, M.A.; Tawata, S. Anti-Inflammatory, Anti-Diabetic, and Anti-Alzheimer’s Effects of Prenylated Flavonoids from Okinawa Propolis: An Investigation by Experimental and Computational Studies. Molecules 2018, 23, 2479. [Google Scholar] [CrossRef]
- Kumazawa, S.; Ueda, R.; Hamasaka, T.; Fukumoto, S.; Fujimoto, T.; Nakayama, T. Antioxidant Prenylated Flavonoids from Propolis Collected in Okinawa, Japan. J. Agric. Food Chem. 2007, 55, 7722–7725. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ji, S.; Song, W.; Kuang, Y.; Lin, Y.; Tang, S.; Cui, Z.; Qiao, X.; Yu, S.; Ye, M. Glycybridins A-K, Bioactive Phenolic Compounds from Glycyrrhiza glabra. J. Nat. Prod. 2017, 80, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Mbaveng, A.T.; Zeino, M.; Fozing, C.D.; Ngameni, B.; Kapche, G.D.W.F.; Ngadjui, B.T.; Efferth, T. Cytotoxicity of Three Naturally Occurring Flavonoid Derived Compounds (Artocarpesin, Cycloartocarpesin and Isobavachalcone) towards Multi-Factorial Drug-Resistant Cancer Cells. Phytomedicine 2015, 22, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Shon, D.H.; Youn, H.S. Isobavachalcone Suppresses Expression of Inducible Nitric Oxide Synthase Induced by Toll-like Receptor Agonists. Int. Immunopharmacol. 2013, 15, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.M.A.; Lee, H.W.; Lee, S.H.; Lim, C.H.; Jang, H.D.; Kim, Y.H. Anti-Osteoporotic and Antioxidant Activities of Chemical Constituents of the Aerial Parts of Ducrosia ismaelis. Bioorg. Med. Chem. Lett. 2014, 24, 3434–3439. [Google Scholar] [CrossRef]
- Wang, M.; Lin, L.; Lu, J.J.; Chen, X. Pharmacological Review of Isobavachalcone, a Naturally Occurring Chalcone. Pharmacol. Res. 2021, 165, 105483. [Google Scholar] [CrossRef]
- Matsuda, H.; Kiyohara, S.; Sugimoto, S.; Ando, S.; Nakamura, S.; Yoshikawa, M. Bioactive Constituents from Chinese Natural Medicines. XXXIII. Inhibitors from the Seeds of Psoralea corylifolia on Production of Nitric Oxide in Lipopolysaccharide-Activated Macrophages. Biol. Pharm. Bull. 2009, 32, 147–149. [Google Scholar] [CrossRef]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Antioxidative Components of Psoralea corylifolia (Leguminosae). Phyther. Res. 2002, 16, 539–544. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Nkuete, A.H.L.; Ngameni, B.; Eloff, J.N. Anti-Inflammatory and Anticholinesterase Activity of Six Flavonoids Isolated from Polygonum and Dorstenia Species. Arch. Pharm. Res. 2017, 40, 1129–1134. [Google Scholar] [CrossRef]
- Gao, D.; Liu, F.; Li, Z.; Guan, Y. Isobavachalcone Attenuates Sephadex-Induced Lung Injury via Activation of A20 and NRF2/HO-1 in Rats. Eur. J. Pharmacol. 2019, 848, 49–54. [Google Scholar] [CrossRef]
- Jing, H.; Zhou, X.; Dong, X.; Cao, J.; Zhu, H.; Lou, J.; Hu, Y.; He, Q.; Yang, B. Abrogation of Akt Signaling by Isobavachalcone Contributes to Its Anti-Proliferative Effects towards Human Cancer Cells. Cancer Lett. 2010, 294, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a Chalcone Constituent of Angelica keiskei, Induces Apoptosis in Neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Shirataki, Y.; Wakae, M.; Yamamoto, Y.; Hashimoto, K.; Satoh, K.; Ishihara, M.; Kikuchi, H.; Nishikawa, H.; Minagawa, K.; Motohashi, N.; et al. Cytotoxicty and Radical Modulating Activity of Isoflavones and Isoflavanones from Sophora Species. Anticancer Res. 2004, 24, 1481–1488. [Google Scholar] [PubMed]
- Sun, Q.; Yao, G.D.; Song, X.Y.; Qi, X.L.; Xi, Y.F.; Li, L.Z.; Huang, X.X.; Song, S.J. Autophagy Antagonizes Apoptosis Induced by Flavan Enantiomers from Daphne giraldii in Hepatic Carcinoma Cells in Vitro. Eur. J. Med. Chem. 2017, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Ahn, H.; Lee, H.J. Inhibition of Nitric Oxide Production on LPS-Activated Macrophages by Kazinol B from Broussonetia kazinoki. Fitoterapia 2003, 74, 350–354. [Google Scholar] [CrossRef]
- Kim, A.Y.; Lee, C.G.; Lee, D.Y.; Li, H.; Jeon, R.; Ryu, J.H.; Kim, S.G. Enhanced Antioxidant Effect of Prenylated Polyphenols as Fyn Inhibitor. Free Radic. Biol. Med. 2012, 53, 1198–1208. [Google Scholar] [CrossRef]
- Chi, Y.S.; Jong, H.G.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of Naturally Occurring Prenylated Flavonoids on Enzymes Metabolizing Arachidonic Acid: Cyclooxygenases and Lipoxygenases. Biochem. Pharmacol. 2001, 62, 1185–1191. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, X.; Huang, Y.; Cheng, N.; Ma, Y. Hepatotoxicity Induced by Sophora flavescens and Hepatic Accumulation of Kurarinone, a Major Hepatotoxic Constituent of Sophora flavescens in Rats. Molecules 2017, 22, 1809. [Google Scholar] [CrossRef]
- Nishikawa, S.; Inoue, Y.; Hori, Y.; Miyajima, C.; Morishita, D.; Ohoka, N.; Hida, S.; Makino, T.; Hayashi, H. Anti-Inflammatory Activity of Kurarinone Involves Induction of Ho-1 via the Keap1/Nrf2 Pathway. Antioxidants 2020, 9, 842. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Wang, Q.; Deng, S.; Huang, M.; Ma, X.; Song, P.; Du, J.; Huang, Y.; Wen, Y.; et al. Inhibitory Effect of Kurarinone on Growth of Human Non-Small Cell Lung Cancer: An Experimental Study Both in Vitro and in Vivo Studies. Front. Pharmacol. 2018, 9, 252. [Google Scholar] [CrossRef]
- Kumar, S.; Prajapati, K.S.; Shuaib, M.; Kushwaha, P.P.; Tuli, H.S.; Singh, A.K. Five-Decade Update on Chemopreventive and Other Pharmacological Potential of Kurarinone: A Natural Flavanone. Front. Pharmacol. 2021, 12, 737137. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Na, K.M.; Oh, I.; Song, I.H.; Lee, Y.S.; Shin, J.; Kim, T.Y. Kurarinone Regulates Immune Responses through Regulation of the JAK/STAT and TCR-Mediated Signaling Pathways. Biochem. Pharmacol. 2013, 85, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dong, Q.; Zhong, Q.; Xiu, W.; Chen, Q.; Wang, J.; Zhou, Z. The Flavonoid Kurarinone Regulates Macrophage Functions via Aryl Hydrocarbon Receptor and Alleviates Intestinal Inflammation in Irritable Bowel Syndrome. J. Inflamm. Res. 2021, 14, 4347–4359. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Matsuzaki, K.; Takamatsu, S.; Kitanaka, S. Inhibitory Effects of Constituents from Morus alba Var. Multicaulis on Differentiation of 3T3-L1 Cells and Nitric Oxide Production in RAW264.7 Cells. Molecules 2011, 16, 6010–6022. [Google Scholar] [CrossRef]
- Baek, S.H.; Hwang, S.; Park, T.; Kwon, Y.J.; Cho, M.; Park, D. Evaluation of Selective Cox-2 Inhibition and in Silico Study of Kuwanon Derivatives Isolated from Morus alba. Int. J. Mol. Sci. 2021, 22, 3659. [Google Scholar] [CrossRef]
- Arung, E.T.; Yoshikawa, K.; Shimizu, K.; Kondo, R. Isoprenoid-Substituted Flavonoids from Wood of Artocarpus heterophyllus on B16 Melanoma Cells: Cytotoxicity and Structural Criteria. Fitoterapia 2010, 81, 120–123. [Google Scholar] [CrossRef]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ Acqua, S.; Babula, P.; Cvačka, J.; Hošek, J. Evaluation of Anti-Inflammatory Activity of Prenylated Substances Isolated from Morus alba and Morus nigra. J. Nat. Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Abbas, G.M.; Gohar, A.A.; Lahloub, M.F.I. Antiproliferative Activity of Stilbene Derivatives and Other Constituents from the Stem Bark of Morus nigra L. Nat. Prod. Res. 2020, 34, 3506–3513. [Google Scholar] [CrossRef]
- Ko, W.; Yoon, C.S.; Kim, K.W.; Lee, H.; Kim, N.; Woo, E.R.; Kim, Y.C.; Kang, D.G.; Lee, H.S.; Oh, H.; et al. Neuroprotective and Anti-Inflammatory Effects of Kuwanon c from Cudrania tricuspidata Are Mediated by Heme Oxygenase-1 in Ht22 Hippocampal Cells, Raw264.7 Macrophage, and Bv2 Microglia. Int. J. Mol. Sci. 2020, 21, 4839. [Google Scholar] [CrossRef]
- Lim, H.J.; Jin, H.G.; Woo, E.R.; Lee, S.K.; Kim, H.P. The Root Barks of Morus alba and the Flavonoid Constituents Inhibit Airway Inflammation. J. Ethnopharmacol. 2013, 149, 169–175. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhang, X.W.; Wang, K.; Wang, X.Y.; Ma, W.L.; Cao, W.; Mo, D.; Sun, Y.; Li, X.Q. Kuwanon G Attenuates Atherosclerosis by Upregulation of LXRα-ABCA1/ABCG1 and Inhibition of NFκB Activity in Macrophages. Toxicol. Appl. Pharmacol. 2018, 341, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Ha, H.; Shin, H.K.; Seo, C.S. Anti-Allergic and Anti-Inflammatory Effects of Kuwanon G and Morusin on MC/9 Mast Cells and HaCaT Keratinocytes. Molecules 2019, 24, 265. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kuang, Y.; Li, K.; Wang, S.; Song, W.; Qiao, X.; Sabir, G.; Ye, M. Screening for Bioactive Natural Products from a 67-Compound Library of Glycyrrhiza inflata. Bioorganic Med. Chem. 2017, 25, 3706–3713. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Meng, R.; Shi, C.; Guo, N. Antioxidative and Anticancer Properties of Licochalcone A from Licorice. J. Ethnopharmacol. 2017, 198, 331–337. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, H.; Xiang, H.; Wang, D.; Xia, L.; Jiang, Y.; Song, K.; Lu, J.; Yu, L.; Deng, X. Influence of Subinhibitory Concentrations of Licochalcone A on the Secretion of Enterotoxins A and B by Staphylococcus aureus. FEMS Microbiol. Lett. 2010, 307, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, H.E.; Yeon, S.H.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Zouboulis, C.C.; Han, S.H.; Lee, J.H.; Lee, J.Y. Licochalcone A Attenuates Acne Symptoms Mediated by Suppression of NLRP3 Inflammasome. Phyther. Res. 2018, 32, 2551–2559. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Bok, Y.K.; Seung, H.C. Topoisomerase I Inhibition and Cytotoxicity of Licochalcones A and E from Glycyrrhiza inflata. Arch. Pharm. Res. 2007, 30, 313–316. [Google Scholar] [CrossRef]
- Phan, H.T.L.; Kim, H.J.; Jo, S.; Kim, W.K.; Namkung, W.; Nam, J.H. Anti-Inflammatory Effect of Licochalcone a via Regulation of ORAI1 and K+ Channels in T-Lymphocytes. Int. J. Mol. Sci. 2021, 22, 10847. [Google Scholar] [CrossRef]
- Furusawa, J.I.; Funakoshi-Tago, M.; Tago, K.; Mashino, T.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A Significantly Suppresses LPS Signaling Pathway through the Inhibition of NF-ΚB P65 Phosphorylation at Serine 276. Cell. Signal. 2009, 21, 778–785. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J. Licochalcone A Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting NF-ΚB Activation. Inflammation 2016, 39, 569–574. [Google Scholar] [CrossRef]
- Jia, T.; Qiao, J.; Guan, D.; Chen, T. Anti-Inflammatory Effects of Licochalcone A on IL-1β-Stimulated Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1894–1902. [Google Scholar] [CrossRef]
- Song, N.R.; Kim, J.E.; Park, J.S.; Kim, J.R.; Kang, H.; Lee, E.; Kang, Y.G.; Son, J.E.; Seo, S.G.; Heo, Y.S.; et al. Licochalcone A, a Polyphenol Present in Licorice, Suppresses UV-Induced COX-2 Expression by Targeting PI3K, MEK1, and B-Raf. Int. J. Mol. Sci. 2015, 16, 4453–4470. [Google Scholar] [CrossRef]
- Furusawa, J.I.; Funakoshi-Tago, M.; Mashino, T.; Tago, K.; Inoue, H.; Sonoda, Y.; Kasahara, T. Glycyrrhiza inflata-Derived Chalcones, Licochalcone A, Licochalcone B and Licochalcone D, Inhibit Phosphorylation of NF-ΚB P65 in LPS Signaling Pathway. Int. Immunopharmacol. 2009, 9, 499–507. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Zhang, B.; Zhang, L. Licochalcone B Attenuates Neuronal Injury through Anti-Oxidant Effect and Enhancement of Nrf2 Pathway in MCAO Rat Model of Stroke. Int. Immunopharmacol. 2021, 100, 108073. [Google Scholar] [CrossRef]
- Li, Q.; Feng, H.; Wang, H.; Wang, Y.; Mou, W.; Xu, G.; Zhang, P.; Li, R.; Shi, W.; Wang, Z.; et al. Licochalcone B Specifically Inhibits the NLRP3 Inflammasome by Disrupting NEK7-NLRP3 Interaction. EMBO Rep. 2022, 23, e53499. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.P.; Patruno, A.; De Lutiis, M.A.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological Effect of Licochalcone C on the Regulation of PI3K/Akt/ENOS and NF-ΚB/INOS/NO Signaling Pathways in H9c2 Cells in Response to LPS Stimulation. Int. J. Mol. Sci. 2017, 18, 690. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Vinciguerra, I.; Ferrone, A.; Riccioni, G.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Licocalchone-C Extracted from Glycyrrhiza glabra Inhibits Lipopolysaccharide-Interferon-γ Inflammation by Improving Antioxidant Conditions and Regulating Inducible Nitric Oxide Synthase Expression. Molecules 2011, 16, 5720–5734. [Google Scholar] [CrossRef]
- Cho, Y.C.; Lee, S.H.; Yoon, G.; Kim, H.S.; Na, J.Y.; Choi, H.J.; Cho, C.W.; Cheon, S.H.; Kang, B.Y. Licochalcone E Reduces Chronic Allergic Contact Dermatitis and Inhibits IL-12p40 Production through down-Regulation of NF-ΚB. Int. Immunopharmacol. 2010, 10, 1119–1126. [Google Scholar] [CrossRef]
- Lee, H.N.; Cho, H.J.; Lim, D.Y.; Kang, Y.H.; Lee, K.W.; Park, J.H.Y. Mechanisms by Which Licochalcone e Exhibits Potent Anti-Inflammatory Properties: Studies with Phorbol Ester-Treated Mouse Skin and Lipopolysaccharide-Stimulated Murine Macrophages. Int. J. Mol. Sci. 2013, 14, 10926–10943. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (Licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-KB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Anti-Inflammatory and Vascularprotective Properties of 8-Prenylapigenin. Eur. J. Pharmacol. 2009, 620, 120–130. [Google Scholar] [CrossRef]
- Wätjen, W.; Weber, N.; Lou, Y.J.; Wang, Z.Q.; Chovolou, Y.; Kampkötter, A.; Kahl, R.; Proksch, P. Prenylation Enhances Cytotoxicity of Apigenin and Liquiritigenin in Rat H4IIE Hepatoma and C6 Glioma Cells. Food Chem. Toxicol. 2007, 45, 119–124. [Google Scholar] [CrossRef]
- Jang, D.S.; Cuendet, M.; Hawthorne, M.E.; Kardono, L.B.S.; Kawanishi, K.; Fonga, H.H.S.; Mehta, R.G.; Pezzuto, J.M.; Kinghorn, A.D. Prenylated Flavonoids of the Leaves of Macaranga conifera with Inhibitory Activity against Cyclooxygenase-2. Phytochemistry 2002, 61, 867–872. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Li, C.H.; Shen, Y.C.; Wu, T.S. Anti-Inflammatory Principles from the Stem and Root Barks of Citrus medica. Chem. Pharm. Bull. 2010, 58, 61–65. [Google Scholar] [CrossRef]
- Tedasen, A.; Sukrong, S.; Sritularak, B.; Srisawat, T.; Graidist, P. 5,7,4′-Trihydroxy-6,8-Diprenylisoflavone and Lupalbigenin, Active Components of Derris Scandens, Induce Cell Death on Breast Cancer Cell Lines. Biomed. Pharmacother. 2016, 81, 235–241. [Google Scholar] [CrossRef]
- Sriklung, K.; Apiratikul, N.; Samosorn, S.; Narkwichean, A.; Watanapokasin, R. Lupalbigenin Inhibiting NF-ΚB Translocation Associated with Anti-Inflammatory Responses in Lipopolysaccharide Stimulated RAW 264.7 Macrophages. J. Med. Assoc. Thail. 2022, 105, S32–S38. [Google Scholar] [CrossRef]
- Navrátilová, A.; Nešuta, O.; Vančatová, I.; Čížek, A.; Varela-M, R.E.; López-Abán, J.; Villa-Pulgarin, J.A.; Mollinedo, F.; Muro, A.; Žemličková, H.; et al. C-Geranylated Flavonoids from Paulownia tomentosa Fruits with Antimicrobial Potential and Synergistic Activity with Antibiotics. Pharm. Biol. 2016, 54, 1398–1407. [Google Scholar] [CrossRef]
- Hanáková, Z.; Hošek, J.; Babula, P.; Dall’Acqua, S.; Václavík, J.; Šmejkal, K. C-Geranylated Flavanones from Paulownia tomentosa Fruits as Potential Anti-Inflammatory Compounds Acting via Inhibition of TNF-α Production. J. Nat. Prod. 2015, 78, 850–863. [Google Scholar] [CrossRef]
- Dat, N.T.; Binh, P.T.X.; Quynh, L.T.P.; Van Minh, C.; Huong, H.T.; Lee, J.J. Cytotoxic Prenylated Flavonoids from Morus alba. Fitoterapia 2010, 81, 1224–1227. [Google Scholar] [CrossRef]
- Kwak, W.J.; Moon, T.C.; Lin, C.X.; Rhyn, H.G.; Jung, H.; Lee, E.; Kwon, D.Y.; Son, K.H.; Kim, H.P.; Kang, S.S.; et al. Papyriflavonol A from Broussonetia Papyrifera Inhibits the Passive Cutaneous Anaphylaxis Reaction and Has a Secretory Phospholipase A 2-Inhibitory Activity. Biol. Pharm. Bull. 2003, 26, 299–302. [Google Scholar] [CrossRef]
- Mao, L.; Liu, H.; Zhang, R.; Deng, Y.; Hao, Y.; Liao, W.; Yuan, M.; Sun, S. Pink1/Parkin-Mediated Mitophagy Inhibits Warangalone-Induced Mitochondrial Apoptosis in Breast Cancer Cells. Aging 2021, 13, 12955–12972. [Google Scholar] [CrossRef] [PubMed]
- Kupeli, E.; Orhan, I.; Toker, G.; Yesilada, E. Anti-Inflammatory and Antinociceptive Potential of Maclura pomifera (Rafin.) Schneider Fruit Extracts and Its Major Isoflavonoids, Scandenone and Auriculasin. J. Ethnopharmacol. 2006, 107, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Wun, Z.Y.; Lin, C.F.; Huang, W.C.; Huang, Y.L.; Xu, P.Y.; Chang, W.T.; Wu, S.J.; Liou, C.J. Anti-Inflammatory Effect of Sophoraflavanone G Isolated from Sophora flavescens in Lipopolysaccharide-Stimulated Mouse Macrophages. Food Chem. Toxicol. 2013, 62, 253–361. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Cao, H.; Sun, L.; Dong, S.; Bian, Y.; Han, J.; Zhang, L.; Ren, S.; Hu, Y.; Liu, C.; et al. Antitumor Activities of Kushen: Literature Review. Evid.-Based Complement. Altern. Med. 2012, 2012, 373219. [Google Scholar] [CrossRef]
- Kang, T.H.; Jeong, S.J.; Ko, W.G.; Kim, N.Y.; Lee, B.H.; Inagaki, M.; Miyamoto, T.; Higuchi, R.; Kim, Y.C. Cytotoxic Lavandulyl Flavanones from Sophora flavescens. J. Nat. Prod. 2000, 63, 680–681. [Google Scholar] [CrossRef]
- Cha, S.M.; Cha, J.D.; Jang, E.J.; Kim, G.U.; Lee, K.Y. Sophoraflavanone G Prevents Streptococcus mutans Surface Antigen I/II-Induced Production of NO and PGE2 by Inhibiting MAPK-Mediated Pathways in RAW 264.7 Macrophages. Arch. Oral Biol. 2016, 68, 97–104. [Google Scholar] [CrossRef]
- Ko, W.G.; Kang, T.H.; Kim, N.Y.; Lee, S.J.; Kim, Y.C.; Ko, G.I.; Ryu, S.Y.; Lee, B.H. Lavandulylflavonoids: A New Class of in Vitro Apoptogenic Agents from Sophora flavescens. Toxicol. Vitr. 2000, 14, 429–433. [Google Scholar] [CrossRef]
- Shirataki, Y.; Motohashi, N.; Tani, S.; Sakagami, H.; Satoh, K.; Nakashima, H.; Mahapatra, S.K.; Ganguly, K.; Dastidar, S.G.; Chakrabarty, A.N. In Vitro Biological Activity of Prenylflavanones. Anticancer Res. 2001, 21, 275–280. [Google Scholar]
- Yasuda, M.; Kawabata, K.; Miyashita, M.; Okumura, M.; Yamamoto, N.; Takahashi, M.; Ashida, H.; Ohigashi, H. Inhibitory Effects of 4-Hydroxyderricin and Xanthoangelol on Lipopolysaccharide-Induced Inflammatory Responses in RAW264 Macrophages. J. Agric. Food Chem. 2014, 62, 462–467. [Google Scholar] [CrossRef]
- Hartkorn, A.; Hoffmann, F.; Ajamieh, H.; Vogel, S.; Heilmann, J.; Gerbes, A.L.; Vollmar, A.M.; Zahler, S. Antioxidant Effects of Xanthohumol and Functional Impact on Hepatic Ischemia-Reperfusion Injury. J. Nat. Prod. 2009, 72, 1741–1747. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Y.D.; Wang, T.; Guo, H.Y.; Liu, Q.M.; Su, H.X. Evaluation on Antioxidant Effect of Xanthohumol by Different Antioxidant Capacity Analytical Methods. J. Chem. 2014, 2014, 249485. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and Antiproliferative Activity of Glycosides Obtained by Biotransformation of Xanthohumol. Bioorganic Med. Chem. Lett. 2013, 23, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Nozawa, H.; Daikonnya, A.; Kondo, K.; Kitanaka, S. Inhibitors of Nitric Oxide Production from Hops (Humulus lupulus L.). Biol. Pharm. Bull. 2003, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Logan, I.E.; Miranda, C.L.; Lowry, M.B.; Maier, C.S.; Stevens, J.F.; Gombart, A.F. Antiproliferative and Cytotoxic Activity of Xanthohumol and Its Non-Estrogenic Derivatives in Colon and Hepatocellular Carcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 1203. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.C.; Kim, H.J.; Kim, Y.J.; Lee, K.Y.; Choi, H.J.; Lee, I.S.; Kang, B.Y. Differential Anti-Inflammatory Pathway by Xanthohumol in IFN-γ and LPS-Activated Macrophages. Int. Immunopharmacol. 2008, 8, 567–573. [Google Scholar] [CrossRef]
- Kontek, B.; Jedrejek, D.; Oleszek, W.; Olas, B. Antiradical and Antioxidant Activity in Vitro of Hops-Derived Extracts Rich in Bitter Acids and Xanthohumol. Ind. Crops Prod. 2021, 161, 113208. [Google Scholar] [CrossRef]
- Pan, L.; Becker, H.; Gerhäuser, C. Xanthohumol Induces Apoptosis in Cultured 40-16 Human Colon Cancer Cells by Activation of the Death Receptor- and Mitochondrial Pathway. Mol. Nutr. Food Res. 2005, 49, 837–843. [Google Scholar] [CrossRef]
- Lupinacci, E.; Meijerink, J.; Vincken, J.P.; Gabriele, B.; Gruppen, H.; Witkamp, R.F. Xanthohumol from Hop (Humulus lupulus L.) Is an Efficient Inhibitor of Monocyte Chemoattractant Protein-1 and Tumor Necrosis Factor-α Release in LPS-Stimulated RAW 264.7 Mouse Macrophages and U937 Human Monocytes. J. Agric. Food Chem. 2009, 57, 7274–7281. [Google Scholar] [CrossRef]
- Koo, J.H.; Hyoung, T.K.; Yoon, H.Y.; Kwon, K.B.; Choi, I.W.; Sung, H.J.; Kim, H.U.; Park, B.H.; Park, J.W. Effect of Xanthohumol on Melanogenesis in B16 Melanoma Cells. Exp. Mol. Med. 2008, 40, 313–319. [Google Scholar] [CrossRef]
- Cho, Y.C.; You, S.K.; Kim, H.J.; Cho, C.W.; Lee, I.S.; Kang, B.Y. Xanthohumol Inhibits IL-12 Production and Reduces Chronic Allergic Contact Dermatitis. Int. Immunopharmacol. 2010, 10, 556–561. [Google Scholar] [CrossRef]
- Negrão, R.; Costa, R.; Duarte, D.; Gomes, T.T.; Coelho, P.; Guimarães, J.T.; Guardão, L.; Azevedo, I.; Soares, R. Xanthohumol-Supplemented Beer Modulates Angiogenesis and Inflammation in a Skin Wound Healing Model. Involvement of Local Adipocytes. J. Cell. Biochem. 2012, 113, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Xie, C.; Wu, W.; Xiang, M.; Liu, Z.; Li, Y.; Tang, M.; Li, S.; Yang, J.; Tang, H.; et al. Millettia pachycarpa Exhibits Anti-Inflammatory Activity through the Suppression of LPS-Induced NO/INOS Expression. Am. J. Chin. Med. 2014, 42, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Chan, H.T. Chemistry and Pharmacology of Artocarpin: An Isoprenyl Flavone from Artocarpus Species. Syst. Rev. Pharm. 2018, 9, 58–63. [Google Scholar] [CrossRef]
- Wang, X.L.; Di, X.X.; Shen, T.; Wang, S.Q.; Wang, X.N. New Phenolic Compounds from the Leaves of Artocarpus heterophyllus. Chin. Chem. Lett. 2017, 28, 37–40. [Google Scholar] [CrossRef]
- Wang, Y.A.; Guo, X.; Jia, X.H.; Xue, J.; Du, H.F.; Du, C.L.; Tang, W.Z.; Wang, X.J.; Zhao, Y.X. Undescribed C-Geranylflavonoids Isolated from the Fruit Peel of Paulownia catalpifolia T. Gong Ex D.Y. Hong with Their Protection on Human Umbilical Vein Endothelial Cells Injury Induced by Hydrogen Peroxide. Phytochemistry 2019, 158, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P. Isobavachalcone: An Overview. Chin. J. Integr. Med. 2012, 18, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Trakanwittayarak, S.; Tuchinda, P.; Chularojanamontri, L.; Limphoka, P.; Varothai, S. A Double-Blinded, Randomized, Vehicle-Controlled Study of the Efficacy of Moisturizer Containing Licochalcone A, Decanediol, L-Carnitine, and Salicylic Acid for Prevention of Acne Relapse in Asian Population. BioMed Res. Int. 2020, 2020, 2857812. [Google Scholar] [CrossRef]
- Kolbe, L.; Immeyer, J.; Batzer, J.; Wensorra, U.; Dieck, K.T.; Mundt, C.; Wolber, R.; Stäb, F.; Schönrock, U.; Ceilley, R.I.; et al. Anti-Inflammatory Efficacy of Licochalcone A: Correlation of Clinical Potency and in Vitro Effects. Arch. Dermatol. Res. 2006, 298, 23–30. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Angabini, N.; Ehlen, S.; Mokos, Z.B.; Subotic, M.; Neufang, G. Efficacy and Tolerability of a Cosmetic Skin Care Product with Trans-4-t-Butylcyclohexanol and Licochalcone A in Subjects with Sensitive Skin Prone to Redness and Rosacea. J. Drugs Dermatol. 2017, 16, 605–610. [Google Scholar]
- Schoelermann, A.M.; Weber, T.M.; Arrowitz, C.; Rizer, R.L.; Qian, K.; Babcock, M. Skin Compatibility and Efficacy of a Cosmetic Skin Care Regimen with Licochalcone A and 4-t-Butylcyclohexanol in Patients with Rosacea Subtype I. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 21–27. [Google Scholar] [CrossRef]
- Sulzberger, M.; Worthmann, A.C.; Holtzmann, U.; Buck, B.; Jung, K.A.; Schoelermann, A.M.; Rippke, F.; Stäb, F.; Wenck, H.; Neufang, G.; et al. Effective Treatment for Sensitive Skin: 4-t-Butylcyclohexanol and Licochalcone A. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.; Marconato, D.G.; Nascimento Da Silva, M.P.; Barbosa Raposo, N.R.; De Faria Silva Facchini, G.; MacEdo, G.C.; Teixeira, F.D.S.; Barbosa Da Silveira Salvadori, M.C.; De Faria Pinto, P.; De Moraes, J.; et al. Licochalcone A-Loaded Solid Lipid Nanoparticles Improve Antischistosomal Activity in Vitro and in Vivo. Nanomedicine 2021, 16, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, Y.; Chen, T.; Du, Q.; Zhu, Z.; Wang, Y.; Wu, Y.; Zeng, Q.; Shen, C.; Jiang, C.; et al. Glycyrrhiza Acid Micelles Loaded with Licochalcone A for Topical Delivery: Co-Penetration and Anti-Melanogenic Effect. Eur. J. Pharm. Sci. 2021, 167, 106029. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.J.; Conte, J.; Natarajan, P.; Haas, G.; Gonzales, S. Direct Inhibition of Elastase and Matrixmetalloproteinases and Stimulation of Biosynthesis of Fibrillar Collagens, Elastin, and Fibrillins by Xanthohumol. J. Cosmet. Sci. 2010, 61, 485. [Google Scholar] [CrossRef]
- Jiang, C.H.; Sun, T.L.; Xiang, D.X.; Wei, S.S.; Li, W.Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid from Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]







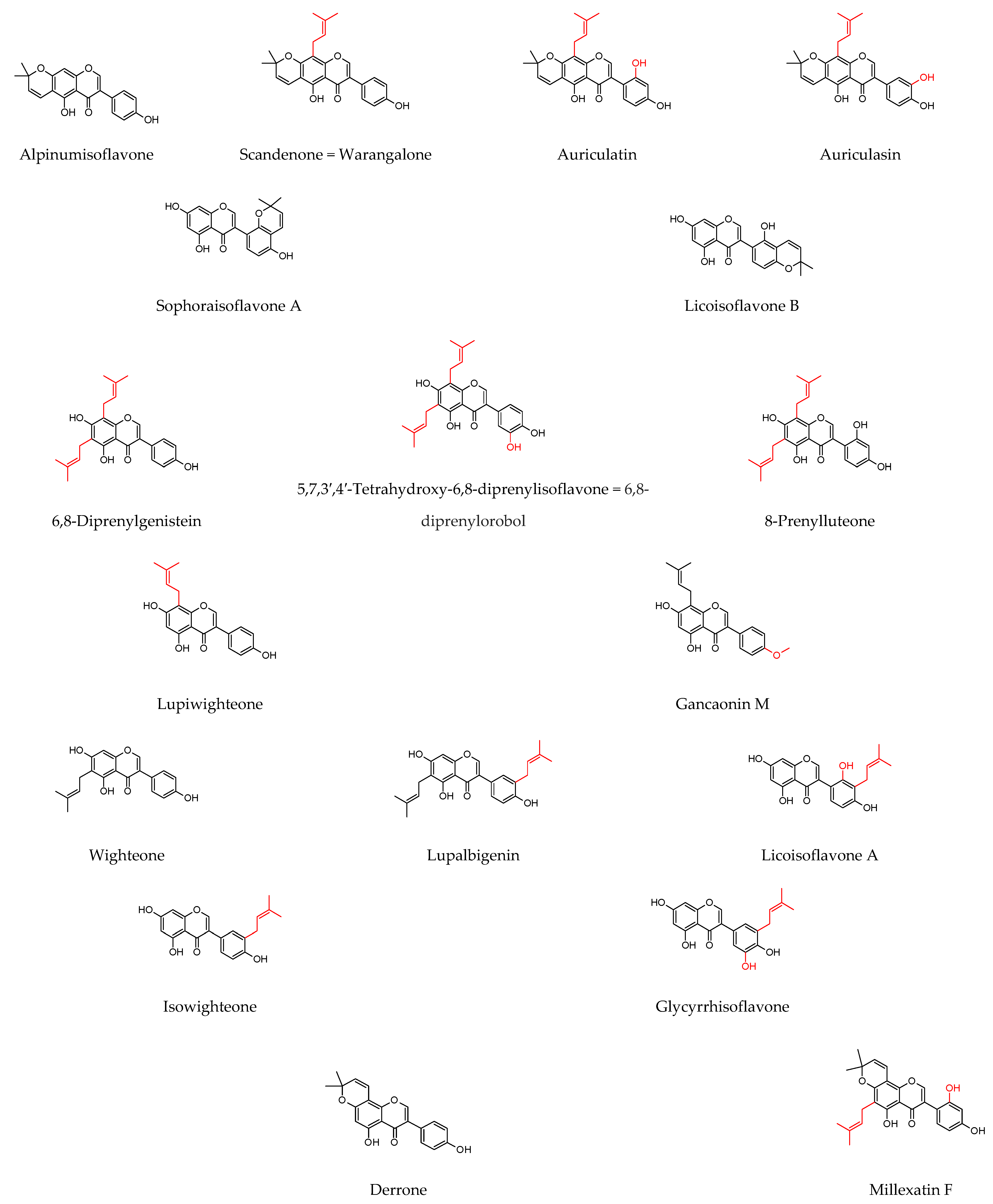
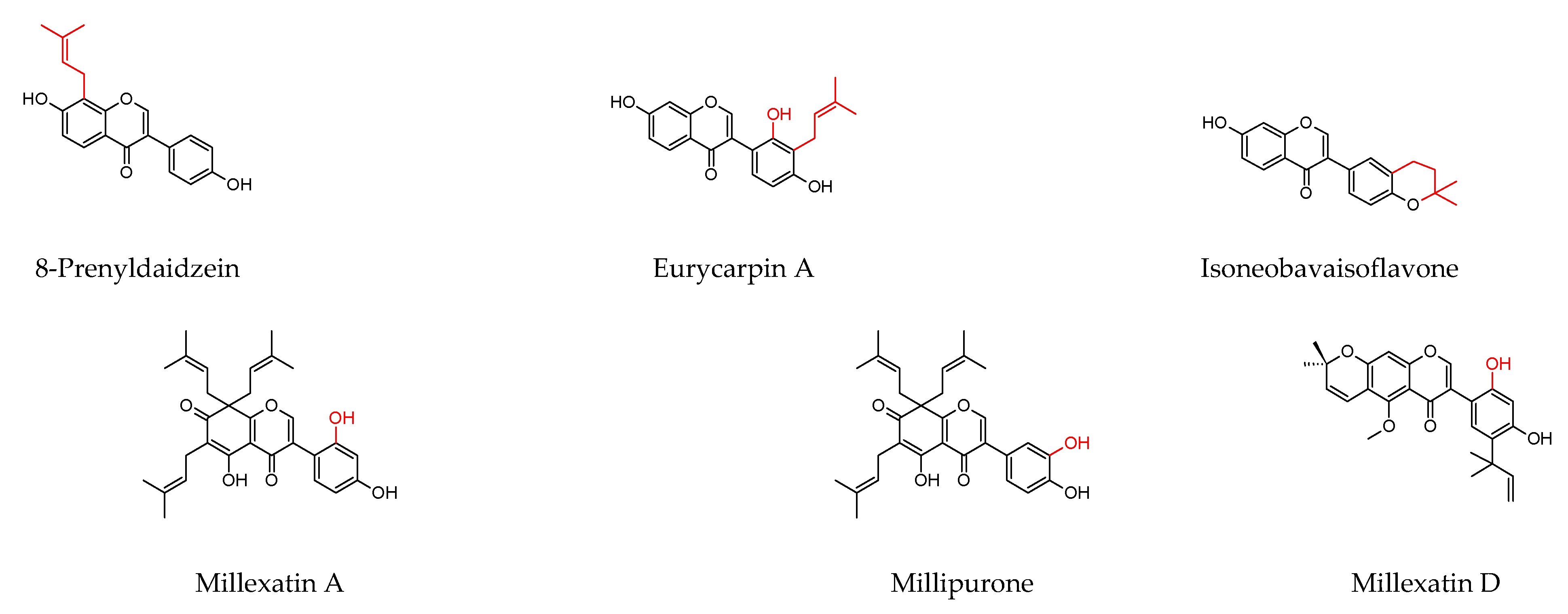
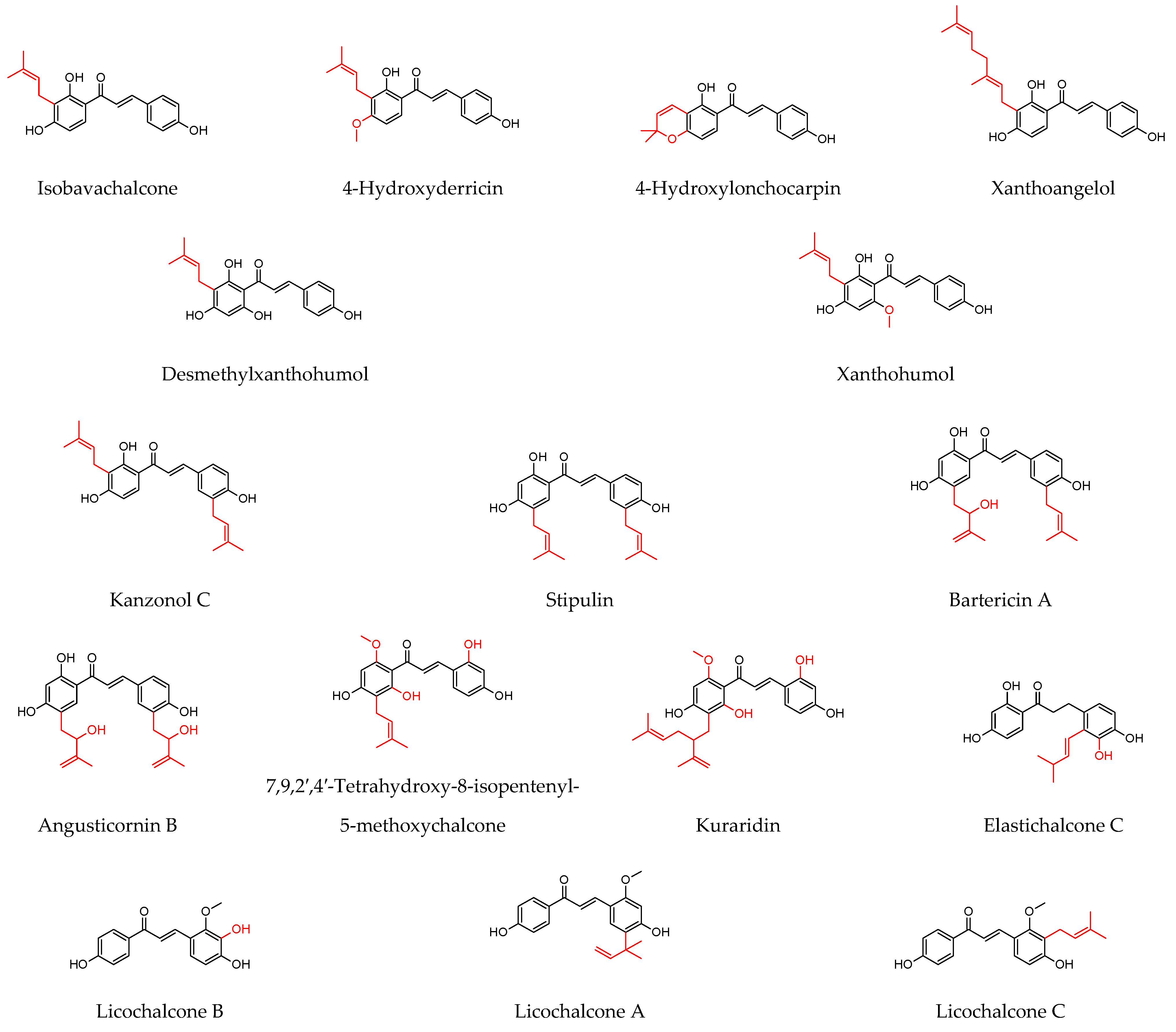

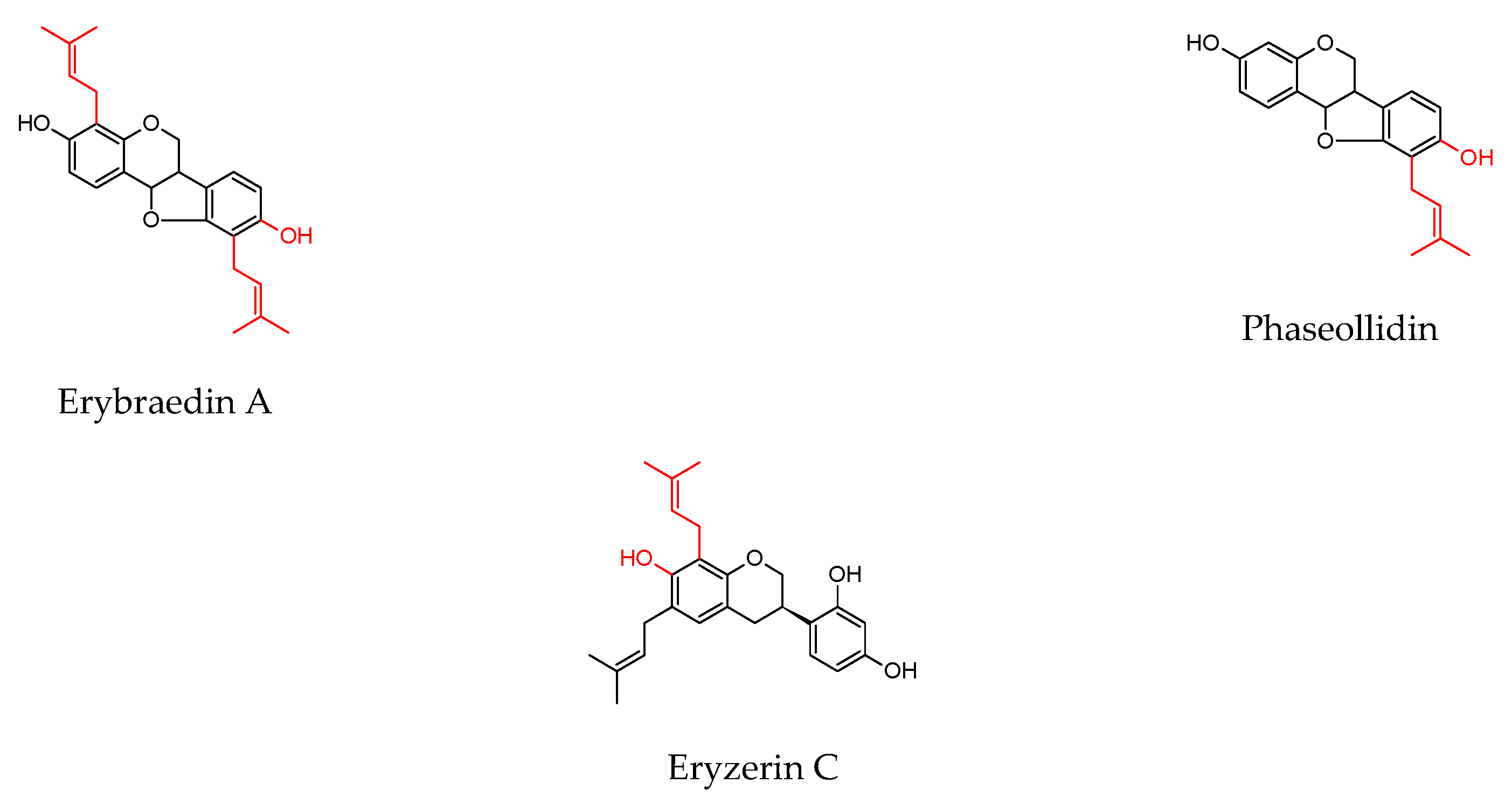
| Plant Source | Compound | Bacteria | IC50 (µg/mL) * or MIC (µg/mL) | PC (µg/mL) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Asteraceae | ||||||||
| Helichrysum forskahlii(J.F. Gmel.) Hilliard and Burtt (aerial parts) | Glabranin | B. subtilis S. aureus | 3 6 | [64] | ||||
| Celastraceae | ||||||||
| AB | CI | VA | [63] | |||||
| Tripterygium wilfordiiHook.f. (stems and roots) | Tripteryol B | C. neoformans P. aeruginosa VRE MRSA | 3.0 * 8.6 * 4.3 * 4.5 * | 0.8 - - - | - 0.1 - 0.2 | - - 3.3 - | ||
| (±)-5,4′-Dihydroxy-2′-methoxy-6′,6″-dimethypyraro-(2″,3″:7,8)-6-methyflavanone | MRSA S. aureus | 2.1 * 2.6 * | - - | 0.2 0.1 | - - | |||
| ((2S)-5,7,4′-Trihydroxy-2′-methoxy-8,5′-di(3-methyl-2-butenyl)-6-methylflavanone | C. neoformans MRSA S. aureus | 1.1 * 2.0 * 2.2 * | 0.8 - - | - 0.2 0.10 | - - - | |||
| Euphorbiaceae | ||||||||
| Macaranga tanariusL. (fruit) | Propolin D | MSSA (n = 2) MRSA S. epidermidis | 10 * 10 * 10 * | [107] | ||||
| Fabaceae | ||||||||
| MO | [108] | |||||||
| Amorpha fruticosaL. (fruit) | Xanthoangelol | S. aureus MRSA | 25 µM 3.1 µM | <0.9 µM 3.8 µM | ||||
| S. aureus MRSA E. faecalis VRE E. faecium VRE. faecium B. subtilis | 6.3 3.1 12.5 6.3 12.5 3.1 3.1 | [109] | ||||||
| CI | [110] | |||||||
| Dalea scandens(Miller) R. Clausen var. paucifolia (roots) | 2(S)-5′-(-1‴,1‴-Dimethylallyl)-8-(3″,3″-dimethylallyl)-2′,4′,5,7-tetrahydroxyflavanone | S. aureus MRSA | 1.6 1.6 | 0.2 0.2 | ||||
| 2(S)-5′-(-1‴,1‴-Dimethylallyl)-8-(3″,3″-dimethylallyl)-2′-methoxy-4′,5,7-trihydroxyflavanone | 3.1 3.1 | |||||||
| 5′-(1‴,1‴-Dimethylallyl)-8-(3″,3″-dimethylallyl)-2′,4′,5,7-tetrahydroxyflavone | 3.1 3.1 | |||||||
| Dalea versicolorZucc. var. sessilis (A. Gray) Barneby (whole plants) | 2(S)-5′-(1‴,1‴-Dimethylallyl)-8-(3″,3″-dimethylallyl)-2′,4′,5,7-tetrahydroxyflavanone | S. aureus | 7.8 | [111] | ||||
| OX | [112] | |||||||
| Dalea searlsiae(Gray) Barneby (roots and aerial parts) | Malheuran A | OSSA ORSA B. cereus | 4.3 3.7 3.7 | 0.4 21.3 106.7 | ||||
| Malheuran B | 3.4 3.7 2.7 | |||||||
| Malheuran C | 4.6 4.3 3 | |||||||
| Malheuran D | 6.1 6.5 5.0 | |||||||
| (2S)-5′-(2-Methylbut-3-en-2-yl)-8-(3-methylbut-2-en-1-yl)-5,7,2′,4′-tetrahydroxyflavanone | 3.1 3.4 2.3 | |||||||
| Prostratol F | 6.8 6.4 8 | |||||||
| AP | [113] | |||||||
| Derris reticulataCraib. (stem) | Lupinifolin | S. aureus | 8 | 0.3 | ||||
| AP | ER | [114] | ||||||
| Echinosophora koreensisNakai (roots) | Kenusanone C | S. epidermidis S. aureus | 20 20 | 20 1.3 | 1.3 1.3 | |||
| Isosophoranone | 20 20 | |||||||
| Sophoraisoflavanone A | E. coli S. epidermidis S. aureus | 20 20 20 | 1.3 20 1.3 | 1.3 1.3 1.3 | ||||
| Kenusanone A | E. coli S. epidermidis S. aureus | 20 20 20 | 1.3 20 1.3 | 1.3 1.3 1.3 | ||||
| Sophoraflavanone D | 20 20 20 | |||||||
| NE | [115] | |||||||
| Erythrina caffraThunb. (stem bark) | Abyssinone V 4′-O-methyl ether | E. coli B. subtilis | 3.9 15.6 | 1.6 0.8 | ||||
| 6,8-Diprenylgenistein | S. aureus E. coli B. subtilis | 7.8 7.8 15.6 | 0.8 1.6 0.8 | |||||
| Alpinumisoflavone | 3.9 3.9 7.8 | |||||||
| CI | [116] | |||||||
| Erythrina lysistemonHutch. (stem bark) | Erybraedin A | B. cereus S. aureus S. epidermidis E. coli | 1 2 2 2 | 0.02 0.1 0.1 0.1 | ||||
| Phaseollidin | B. cereus S. aureus S. epidermidis | 10 10 5 | 0.02 0.1 0.1 | |||||
| Abyssinone V 4′-O-methyl ether | B. cereus | 26 | 0.02 | |||||
| Eryzerin C | B. cereus S. aureus S. epidermidis E. coli P. aeruginosa | 10 5 2 5 5 | 0.02 0.1 0.1 0.1 0.1 | |||||
| Alpinumisoflavone | B. cereus S. aureus P. aeruginosa | 31 31 20 | 0.02 0.1 0.1 | |||||
| Lysisteisoflavone | B. cereus S. epidermidis E. coli P. aeruginosa | 2 26 6 31 | 0.02 0.1 0.1 0.1 | |||||
| Larval stage of Melipotis perpendicularis (Noctuidae) feeding on the leaves of Lonchocarpus minimiflorus Donn. Sm. | Lonchocarpol A | MRSA VRE. faecium B. megaterium | 0.8–1.6 0.8–1.6 1.0–2.0 | [117] | ||||
| VA | [118] | |||||||
| Millettia extensa(Benth.) Baker (stem) | Millexatin A | S. aureus S. epidermidis B. subtilis | 2 2 2 | 0.3 0.3 0.3 | ||||
| Millexatin F | 2 2 2 | |||||||
| Auriculatin | 2 2 2 | |||||||
| Scandenone | 2 4 2 | |||||||
| Auriculasin | 4 4 8 | |||||||
| Millettia extensa(Benth.) Baker (leaves and roots) | Millipurone | S. epidermidis B. cereus S. aureus | 4 32 4 | 0.3 0.1 0.3 | [119] | |||
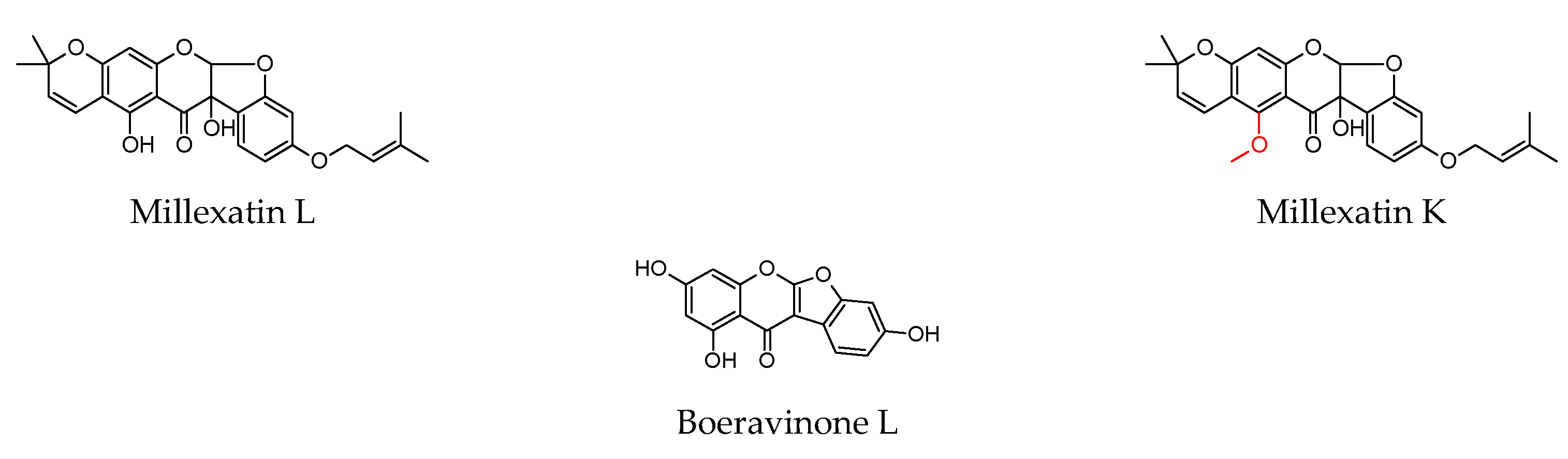
| Millexatin K | B. cereus S. aureus | 32 32 | 0.1 0.3 | |||||
| Millexatin L | 32 32 | |||||||
| Millexatin D | 8 8 | |||||||
| 5,7,3′,4′-Tetrahydroxy-6,8-diprenylisoflavone = 6,8-diprenylorobol | 16 32 | |||||||
| CI | [120] | |||||||
| Pseudarthria hookeriWight and Arn. (whole plant) | Pseudarflavone A | E. coli P. aeruginosa S. aureus | 4 16 8 | 0.3 0.1 0.3 | ||||
| 6-Prenylpinocembrin | E. coli E. faecalis S. aureus | 4 16 8 | 0.3 8 0.3 | |||||
| Boeravinone L | E. coli | 16 | 0.3 | |||||
| Psoralea corylifoliaL. (seeds) | Bavachin | S. aureus S. epidermidis | 37 µM 37 µM | [121] | ||||
| GE | OF | AB | [122] | |||||
| Retama raetam Forssk. Webb (flowers) | Licoflavone C | E. coli P. aeruginosa C. glabrata C. albicans C. parapsilosis C. krusei | 7.8 15.6 15.6 15.6 15.6 15.6 | - 0.5 - - - - | 0.1 1 - - - - | - - 0.5 0.5 0.5 0.5 | ||
| Derrone | 7.8 15.6 7.8 7.8 7.8 7.8 | |||||||
| ATB (n = 8) | [123] | |||||||
| Sophora flavescensAiton (roots) | Kuraridin | S. aureus MRSA (n = 6) | 8 8–16 | 0.1→128 | ||||
| AP | ER | [114] | ||||||
| E. coli S. epidermidis S. aureus | 20 20 20 | 1.3 20 1.3 | 1.3 1.3 1.3 | |||||
| Kurarinone | E. coli S. epidermidis S. aureus | 20 20 20 | 1.3 20 1.3 | 1.3 1.3 1.3 | ||||
| AP | VA | [124] | ||||||
| MRSA VRE | 2 2 | 250 250 | 2.5 150 | |||||
| Sophoraflavanone G | AP | ER | [114] | |||||
| E. coli S. epidermidis S. aureus | 20 20 20 | 1.3 20 1.3 | 1.3 1.3 1.3 | |||||
| ATB (n = 8) | [123] | |||||||
| S. aureus MRSA (n = 6) | 2 2–4 | 0.1→128 | ||||||
| AP | OX | [125] | ||||||
| MSSA MRSA (n = 11) | 4 0.5–8 | 2 64–1024 | 0.3 256–1024 | |||||
| 7,9,2′,4′-Tetrahydroxy-8-isopentenyl-5-methoxychalcone | S. aureus MRSA (n = 5) VRE (n = 5) | 1.0 1.0–15.6 7.8–15.6 | [126] | |||||
| Moraceae | ||||||||
| VA | [127] | |||||||
| Artocarpus elasticus(leaves) | Elastichalcone C | S. aureus MRSA | 8 4 | 1 1 | ||||
| OX | [128] | |||||||
| Artocarpus integer (Thunb.) Merr. (roots) | Artocarpin | P. acnes S. aureus S. epidermidis | 2 2 4 | 0.1 0.5 0.5 | ||||
| Cudraflavone C | 2 2 4 | |||||||
| CI | [129] | |||||||
| Artocarpus sepicanusDiels (leaves) | Sepicanin A | MRSA | 2.9 µM | 0.8 | ||||
| AP | ER | AB | MI | [114] | ||||
| Broussonetia papyrifera(L.) Vent. (root bark) | Papyriflavonol A | E. coli S. epidermidis S. aureus S. cerevisiae | 20 10 15 12.5 | 1.3 20 1.3 - | 1.3 1.3 1.3 - | - - - 1.3 | - - - 1.3 | |
| Kazinol B | S. epidermidis S. aureus | 20 20 | 20 1.3 | 1.3 1.3 | - - | |||
| GE | NY | [130] | ||||||
| Dorstenia angusticornisEngl. (twigs) | Gancaonin Q | B. cereus B. stearothermophilus B. subtilis E. faecalis | 0.6 9.8 9.8 0.6 | 1.2 4.9 1.2 2.4 | - - - - | |||
| Stipulin | P. aeruginosa B. cereus E. faecalis | 19.5 4.9 2.4 | 4.9 1.2 2.4 | - - - | ||||
| Angusticornin B | E. coli P. aeruginosa B. cereus B. megaterium B. stearothermophilus B. subtilis S. aureus E. faecalis C. albicans C. krusei C. glabrata | 1.2 9.8 1.2 2.4 2.4 1.2 2.4 1.2 0.6 1.2 0.6 | 1.2 4.9 1.2 2.4 4.9 1.2 4.9 2.4 - - - | - - - - - - - - 2.4 2.4 9.8 | ||||
| Bartericin A | E. coli P. aeruginosa B. cereus B. megaterium B. stearothermophilus B. subtilis S. aureus E. faecalis C. albicans C. krusei C. glabrata | 0.6 <0.3 0.6 1.2 2.4 1.2 0.6 0.6 0.6 1.2 0.6 | 1.2 4.9 1.2 2.4 4.9 1.2 4.9 2.4 - - - | - - - - - - - - 2.4 2.4 9.8 | ||||
| ATB (n = 7) | [131] | |||||||
| Dorstenia barteriBureau var. multiradiata (stems) | Isobavachalcone | MSSA (n = 2) MRSA (n = 6) | 2–16 4–8 | 0.02→128 | ||||
| GE | NY | [132] | ||||||
| E. faecalis S. aureus B. cereus B. megaterium B. stearothermophilus B. subtilis C. albicans C. glabrata T. rubrum | 0.3 0.3 0.6 0.6 0.3 0.6 0.3 0.3 1.2 | 4.9 2.4 1.2 2.4 4.9 1.2 - - - | - - - - - 2.4 2.4 4.9 1.2 | |||||
| 4-Hydroxylonchocarpin | E. faecalis S. aureus B. cereus B. megaterium B. stearothermophilus B. subtilis C. albicans C. glabrata T. rubrum | 4.9 4.9 4.9 1.2 1.2 4.9 4.9 4.9 4.9 | 4.9 2.4 1.2 2.4 4.9 1.2 - - - | - - - - - - 2.4 2.4 1.2 | ||||
| Kanzonol C | E. faecalis B. cereus B. megaterium B. stearothermophilus B. subtilis C. albicans C. glabrata | 4.9 9.8 4.9 4.9 9.8 4.9 4.9 | 4.9 1.2 2.4 4.9 1.2 - - | - - - - - 2.4 2.4 | ||||
| CH | NY | [133] | ||||||
| Dorstenia manniiHook. f. (stems) | Dorsmanin C | P. aeruginosa | 4 | 64 | - | |||
| Dorsmanin E | C. albicans | 8 | - | 16 | ||||
| Dorsmanin F | E. coli C. albicans | 4 16 | 2 - | - 16 | ||||
| Dorsmanin G | E. coli P. aeruginosa | 16 8 | 2 64 | - - | ||||
| VA | AP | [134] | ||||||
| Maclura cochinchinensis(Lour.) Corner (fruit and leaves) | Gancaonin M | E. faecalis S. aureus MRSA C. albicans | 8 2 2 4 | 2 0.5 1 0.5 | 0.5 0.3 0.5 0.3 | |||
| Lupiwighteone | 8 4 8 8 | |||||||
| Lupalbigenin | 2 1 1 4 | |||||||
| Scandenone | 4 2 2 8 | |||||||
| Auriculatin | 2 2 2 2 | |||||||
| Millexatin F | 4 1 2 4 | |||||||
| Derrone | S. aureus MRSA C. albicans | 4 4 32 | 0.5 1 0.5 | 0.3 0.5 0.3 | ||||
| Macluracochinone E | 8 32 32 | |||||||
| AP | OF | KE | [135] | |||||
| Maclura pomifera(Rafin.) Schneider (fruit) | Scandenone | E. coli S. aureus B. subtilis E. faecalis C. albicans | 2 0.5 8 0.5 1 | 2 0.1 0.5 0.5 - | 0.1 0.5 1 1 - | - - - - 1 | ||
| Morus albaL. (root bark) | KS | AP | ME | [136] | ||||
| Kuwanon C | S. aureus MRSA (n = 3) | 2 2–4 | 2 4 | 4 8–16 | 32 128–256 | |||
| AP | CI | VA | [137] | |||||
| MRSA (n = 3) E. faecalis VRE (n = 3) | 2 4 4–8 | >16 8 8 | 8–128 <8 NT | - <16 512→>1024 | ||||
| VA | [138] | |||||||
| MSSA MRSA B. subtilis E. faecalis | 2 2 4 4 | 2 2 ≤0.3 >128 | ||||||
| AP | CI | VA | [137] | |||||
| Kuwanon T | MRSA (n = 3) E. faecalis | 4 4 | >16 8 | 8–128 <8 | - <16 | |||
| Morusinol | MRSA MRSA (n = 2) | 16 | >16 | 16 | - | |||
| Kuwanon U | 4–8 | >16 | 16–128 | - | ||||
| KS | AP | ME | [136] | |||||
| S. aureus MRSA (n = 3) | 4 4–8 | 2 4 | 4 8–16 | 32 128–256 | ||||
| AP | CI | VA | [137] | |||||
| Kuwanon E | MRSA (n = 3) | 4–8 | >16 | 8–128 | - | |||
| KS | AP | ME | [136] | |||||
| S. aureus MRSA (n = 3) | 4 4 | 2 4 | 4 8–16 | 32 128–256 | ||||
| ATB (n = 6) | [139] | |||||||
| MSSA MRSA (n = 10) | 4 4–16 | 4–128 4–256 | ||||||
| AP | CI | VA | [137] | |||||
| Morusin | MRSA (n = 3) E. faecalis VRE (n = 3) | 2–4 8 4–8 | >16 8 8 | 8–128 <8 - | - <16 512→>1024 | |||
| KS | AP | ME | [136] | |||||
| S. aureus MRSA (n = 3) | 4 2–8 | 2 4 | 4 8–16 | 32 128–256 | ||||
| VA | [138] | |||||||
| MSSA MRSA B. subtilis E. faecalis | 8 8 4 8 | 2 2 ≤0.3 >128 | ||||||
| ATB (n = 6) | [139] | |||||||
| MSSA MRSA (n = 10) | 16 8–32 | 4–128 4–256 | ||||||
| KS | AP | ME | [136] | |||||
| 5′-Geranyl-5, 7, 2′, 4′ -tetrahydroxyflavone | S. aureus MRSA (n = 3) | 2 2–4 | 2 4 | 4 8–16 | 32 128–256 | |||
| Kuwanon B | 4 4 | |||||||
| Morus mongolicaSchneider (root bark) | AP | ER | [114] | |||||
| Morusin | S. epidermidis | 20 | 20 | 1.3 | ||||
| Kuwanon C | E. coli S. epidermidis S. aureus | 10 6.3 6.3 | 1.3 20 1.3 | 1.3 1.3 1.3 | ||||
| Paulowniaceae | ||||||||
| Paulownia tomentosa(Thunb.) Steud (fruit) | Tomentodiplacone B | ATB (n = 8) | [98] | |||||
| S. aureus MRSA (n = 5) | 8 8–16 | 0.1→32 | ||||||
| Mimulone | 2 2–4 | |||||||
| CI | [140] | |||||||
| B. cereus B. subtilis E. faecalis S. aureus | 4 4 4 8 | 1 2 1 0.5 | ||||||
| Diplacone | 4 4 4 4 | |||||||
| ATB (n = 8) | [98] | |||||||
| S. aureus MRSA (n = 5) | 8 8–16 | 0.1→32 | ||||||
| 3′-O-methyl-5′-hydroxydiplacone | 4 4–8 | |||||||
| CI | [140] | |||||||
| B. cereus B. subtilis E. faecalis S. aureus | 4 4 4 2 4 | 1 2 1 0.5 | ||||||
| 3′-O-methyl-5′-O-methyldiplacone | 4 4 4 4 | |||||||
| ATB (n = 8) | [98] | |||||||
| S. aureus MRSA (n = 5) | 2 4–8 | 0.1→32 | ||||||
| 3′-O-methyldiplacol | 4 4–8 | |||||||
| CI | [140] | |||||||
| B. cereus B. subtilis E. faecalis S. aureus | 2 4 4 2 | 1 2 1 0.5 | ||||||
| 3′-O-methyldiplacone | 4 8 8 8 | |||||||
| Tomentodiplacone | B. cereus S. aureus | 16 16 | 1 0.5 | |||||
| Propolis | ||||||||
| Taiwanese green propolis | Propolin C | B. subtilis S. aureus (n = 3) | 2.5 1.3–5 | [141] | ||||
| Propolin D | 5 10–20 | |||||||
| Propolin F | 10 10–20 | |||||||
| Propolin G | 10 10–20 | |||||||
| Prenylated flavonoids obtained from indefinite source | ||||||||
| FLAVONES | ||||||||
| Albanin A | MSSA MRSA | 32 32 | [142] | |||||
| Artocarpin | 1 2 | |||||||
| Broussoflavonol F | 16 16 | |||||||
| Corylifol C | MSSA | 16 | ||||||
| Glyasperin A | OX | VA | CH | ST | [143] | |||
| S. aureus | 16–32 | 0.3–0.5 | 0.5–1 | 4 | 8–16 | |||
| Kuwanon A | MSSA MRSA | 4 8 | [142] | |||||
| Kuwanon C | 1 1 | |||||||
| Licoflavone B | 32 32 | |||||||
| 16 32 | [138] | |||||||
| Licoflavone C | MSSA | 32 32 | [142,144] | |||||
| Licoflavonol | MSSA MRSA | 8 8 | [142] | |||||
| Morusin | 8 8 | |||||||
| 3′-Geranyl -3-prenyl-5,7,2′,4′-tetrahydroxyflavone | 4 4 | |||||||
| 5,7,2′,4′-Tetra-hydroxy-3-geranylflavone | 8 8 | |||||||
| 5,7,4′-Trihydroxy-3,6-dimethoxy-3′,5′-diprenylflavone | MRSA | 32 | ||||||
| 5′-Geranyl-5,7,2′,4′-tetrahydroxyflavone | MSSA MRSA | 2 2 | ||||||
| 6-Prenylapigenin | 32 32 | |||||||
| 6-Prenylquercetin 3-methyl ether | MSSA | 32 | ||||||
| 8-Prenylkaempferol | MSSA MRSA | 32 32 | ||||||
| ISOFLAVONES | ||||||||
| Eurycarpin A | MSSA MRSA | 8 8 | [142] | |||||
| Gancaonin M | 8 8 | |||||||
| Glycyrrhisoflavone | 32 32 | [144] | ||||||
| Isoneobavaisoflavone | 4 4 | [142] | ||||||
| Isowighteone | 16 16 | |||||||
| Licoisoflavone A | 32 32 | [142,144] | ||||||
| Licoisoflavone B | OX | VA | CH | ST | [143] | |||
| S. aureus | 4–8 | 0.3–0.5 | 0.5–1 | 4 | 8–16 | |||
| Lupalbigenin | MSSA MRSA | 1 1 | [142] | |||||
| Lupiwighteone | MSSA | 32 | ||||||
| Scandenone, syn. Warangalone | MSSA MRSA | 8 2 | ||||||
| Sophoraisoflavone A | 32 32 | |||||||
| Wighteone | 8 8 | |||||||
| 6,8-Diprenylgenistein | 4 2 | |||||||
| 6,8-Diprenylorobol | 16 8 | |||||||
| 8-Prenyldaidzein | 32 32 | |||||||
| 8-Prenylluteone | 8 8 | |||||||
| FLAVANONES | ||||||||
| Bavachin | MSSA | 32 | [142] | |||||
| Bavachinin A | MSSA MRSA | 4 8 | ||||||
| Euchrestaflavanone A | 2 2 | |||||||
| Glabrol | 1 1 | |||||||
| 2 2 | [144] | |||||||
| VA | ||||||||
| MRSA (n = 20) | 1–4 | 2–16 | ||||||
| Leachianone G | MSSA MRSA | 32 32 | [142] | |||||
| Licoflavanone | 32 32 | |||||||
| Sophoraflavanone C | 2 2 | |||||||
| 6-Prenylnaringenin | 8 8 | |||||||
| CHALCONES | ||||||||
| Desmethylxanthohumol | MSSA MRSA | 16 16 | ||||||
| Isobavachalcone | MSSA MRSA | 4 4 | ||||||
| TE | [145] | |||||||
| 1.6 3.1 | 0.2 >5.9 | |||||||
| Kanzonol C | 4 4 | [142] | ||||||
| Licochalcone A | 4 4 | |||||||
| 2 4 | [144] | |||||||
| VA | [144] | |||||||
| MRSA (n = 20) | 1–8 | 2–16 | ||||||
| OX | VA | CH | ST | [143] | ||||
| S. aureus | 4 | 0.3–0.5 | 0.5–1 | 4 | 8–16 | |||
| Licochalcone B | MRSA | 16 | [142,144] | |||||
| Licochalcone C | MSSA MRSA | 4 4 | ||||||
| VA | [144] | |||||||
| MRSA (n = 20) | 1–16 | 2–16 | ||||||
| Licochalcone D | MSSA MRSA | 16 32 | [142] | |||||
| 32 16 | [144] | |||||||
| Licochalcone E | 4 4 | [142] | ||||||
| 4 4 | [144] | |||||||
| VA | [144] | |||||||
| MRSA (n = 20) | 0.5–16 | 2–16 | ||||||
| OX | [146] | |||||||
| S. aureus MRSA (n = 6) | 2 1–4 | 0.3 0.1–256 | ||||||
| Xanthoangelol | MSSA | 32 | [142] | |||||
| Xanthohumol | S. aureus (n = 3) | 15.6–62.5 | [147] | |||||
| MSSA MRSA | 4 4 | [142] | ||||||
| S. epidermidis (n = 2) S. capitis ssp. ureolyticus S. aureus MRSA | 2 2 2 4 | [148] | ||||||
| 4-Hydroxyderricin | MSSA MRSA | 2 2 | [142] | |||||
| ISOFLAVEN | ||||||||
| Glabrene | MSSA MRSA | 16 16 | [144] | |||||
| Compound | Cytotoxic Activity | Anti-Inflammatory Activity | Antioxidant Activity | ↓ Bacterial Pathogenicity |
|---|---|---|---|---|
| Alpinumisoflavone | Weak cytotoxicity in PC-3 cells [149] | 5, 10 µg/mL → ↓ TNF-α, IL-6, IL-1β, IL-17, ICAM-1, NO in LPS-stimulated RAW 264.7 cells [150]. | DPPH scavenging activity, IC50 = 54.0 µg/mL [151]. | |
| 1, 5, 10 mg/kg i.p 1 h before → protective effect against pulmonary inflammation in LPS-stimulated acute lung injury in mice [150]. | 5, 10 µg/mL → ↑ the levels of CAT, HO-1, GPx, SOD in LPS-stimulated RAW 264.7 cells [150]. | |||
| 25 and 50 µM → inhibition of TNF-α-induced ↑ in MMP-1, ↓: procollagen I α1, NOS, COX-2, IL-1β, IL-6, IL-8, NF-κB, MAPKs [152]. | ↓ ROS and NO in TNF-α-treated HDFs [152]. | |||
| Artocarpin | IC50 = 45.3 μM in RAW 264.7 cells [153]. | Inhibition of LPS-induced NO production in RAW 264.7 cells, IC50 = 18.7 µM [153]. | TEACABTS = 0.9 mM [154]. | Synergy with norfloxacin against MRSA, P. aeruginosa, and E. coli. Synergy with tetracycline against MRSA and P. aeruginosa. Synergy with ampicillin against MRSA [155]. |
| IC50 = 7.9 µM in PC-3 cells. IC50 = 8.3 µM in NCI-H460 cells [156]. | ||||
| ED50 = 3.3 µg/mL in MCF-7 cells. ED50 = 3.8 µg/mL in MDA-MB-231 cells. ED50 = 3.3 µg/mL in A549 cells. ED50 = 3.4 µg/mL in 1A9 cells. ED50 = 3.8 µg/mL in HCT-8 cells. ED50 = 4.9 µg/mL in CAKI-1 cells. ED50 = 5.4 µg/mL in SK-MEL-2 cells. ED50 = 3.7 µg/mL in U87-MG cells. ED50 = 4.1 µg/mL in PC-3 cells. ED50 = 3.2 µg/mL in KB cells. ED50 = 3.6 µg/mL in KB-VIN cells. [157]. | Topical dose 0.05–0.1% ↓ TNF-α levels, COX-2 and cPLA2 protein expressions in the skin homogenate. Photoprotective effect on ultraviolet B (UVB)-induced skin damage in hairless mice [158]. | 0.05% artocarpin treatment prevents UVB-induced oxidative stress by affecting antioxidant activity [158]. | ||
| IC50 = 5.1 μmol/L in PC-3 cells. IC50 = 10.2 μmol/L in NCI-H460 cells. IC50 = 8.1 μmol/L in A-549 cells [159]. | ||||
| IC50 = 5.1 µg/mL in KB cells. IC50 = 3.3 µg/mL in BC cells. IC50 = 5.6 µg/mL in Vero cells [160]. | ||||
| Bavachin | CC50 = 20.2 µM in Hep3B cells [161]. | Inhibitory effect on IL-6-induced STAT3 promoter activity in Hep3B cells, IC50 = 4.9 µM [161]. | ||
| Suppression of LPS-induced NO and PGE2 production, and ↓ iNOS and mPGES-1 expression. ↓ of LPS-induced IL-6 and IL-12p40 production and ↓ the activation of MAPKs and NF-κB. Suppression of NLRP3 inflammasome-derived IL-1β secretion, ↓ caspase-1 activation, repression of mature IL-1β expression, and inhibition of inflammasome complex formation [162]. | ||||
| Downregulation of IL-4 in the spleen of T cells from 4get IL-4-GFP mice. ↓ the IL-4 levels by downregulating the level of Gata-3 expression and STAT6 phosphorylation. 50 mg/kg dissolved in the solution by daily lavage administration [163]. | ||||
| Diplacone = propolin C = nymphaeol A | IC50 = 14.3 µM in WB-F344 cells [164]. | 10 µM ↓ the expression of TNF-α and MCP-1 and ↑ the expression of ZFP36 [165]. | DPPH scavenging by SC50 = 3.2 µg/mL) [166]. | Dose-dependent inhibition of S. aureus biofilm formation [107]. |
| Inhibition of IκB-α degradation, ↓ of COX-2 expression [167]. | ||||
| COX-1 inhibitor IC50 = 1.8 μM COX-2 inhibitor IC50 = 4.2 μM 5-LOX inhibitor IC50 = 0.1 μM [168]. | ||||
| Antiproliferative (IC50 = 9.3 μM) and cytotoxic (LC50 = 18.0 µM) effect in THP-1 cells [169]. | 25 mg/kg prior and after induction of colitis ameliorates its symptoms and delays the onset. ↓ of the levels of COX-2 and ↑ the ratio of pro-MMP2/MMP2 activity, ↓ of SOD2 and CAT [170]. | TEACABTS 3.2, TEACDPPH = 1.1, TEACFRAP = 0.5, TEACInhibition of. peroxynitrite induced tyrosine nitration = 0.8. Superoxide scavenging activity-enzymatic = 45.2%, nonenzymatic = 25.9% at 50 μM [171]. | ||
| EC50 =< 10 µM in MCF-7 cells. EC50 = 3.2 µM in CEM cells. EC50 =< 10 µM in RPMI8226 cells. EC50 = 2.4 µM in U266 cells. EC50 =< 10 µM in HeLa cells. EC50 = 5.9 µM in BJ cells. EC50 =< 10 µM in THP-1 cells [172]. | Inhibition of LPS-induced NO production in RAW 264.7 cells, IC50 = 5.0 µM [173]. | DPPH quenching activity TEAC 5.2 at 10 µM [164]. | ||
| At tested concentrations did not inhibit cell proliferation; it induced cell proliferation to some extent in RAW 264.7 cells [174]. | Inhibition of albumin denaturation, IC50 = 0.3 µM. Inhibition of nitrite production stimulated by LPS in RAW 264.7 cells, IC50 = 3.2 µM. COX-2 inhibitor = 11.7 µM [174]. | DPPH radical scavenging activity, IC50 = 6.5 µg/mL [175]. | ||
| Glabrene | Cytotoxic activity for: HepG2 cells (10 μM) = 25.9%. SW480 cells (10 μM) = 30.7%. A549 cells (10 μM) = 0%. MCF7 cells (10 μM) = 17.6% [176]. | 10 μM inhibited LPS-induced NO production in RAW 264.7 cells by 57.5%, IC50 = 9.5 μM. 10 μM inhibited LPS-induced NF-κB activation by 41.7% [176]. | 10 μM treated HepG2 cells transfected with the ARE luciferase reporter gene (HepG2C8 cells) to evaluate Nrf2 activation. 2.7-fold of control for Nrf2 activation activity [176]. | |
| Isobavachalcone | IC50 = 2.90 μM (CCRF-CEM cells) to >123. 46 μM (AML12 cells) [177]. | 20 μg/mL and 50 μg/mL → suppression of iNOS expression induced by TLR agonists in murine macrophages [178]. | Peroxyl radical scavenging activity with an ORAC value of 24.8 μM [179]. | Antibiofilm activity with MBIC = 0.8 µg/mL against MSSA and MRSA → 75% inhibition of biofilm formation [145]. |
| Cell viability = 98.2% at 50 μM, 64.8% at 100 μM in RAW 264.7 cells [178]. | Inhibition of NO production in LPS-activated RAW 264.7 cells, IC50 = 6.4 µM [153]. | |||
| IC50 = 16.4 µM in RAW 264.7 cells [153]. | ||||
| IC50 =< 20 µM in NB4, U937, K562s, K562r cells. IC50 = >20 µM in HL60, THP-1, U937, MOLM-13 cells. IC50 = 75.5 µM in HCT116 cells. IC50 = 44.1 µM in SW480 cells. IC50 = 128.3 µM in Tca8113 cells. IC50 = 16.5 µM in HepG2 cells. IC50 = 13.2 µM in Hep3B cells. IC50 =< 40 µM in MCF-7, ZR-75–1, MDA-MB-231 cells. IC50 = 26.2 µM in PC-3 cells. IC50 = >50 µM in LNCaP cells. IC50 = 15.1 µM in PC-3 cells. IC50 = >50 µM in HeLa cells [180]. | Inhibition of NO production in LPS-activated RAW 264.7 cells, IC50 = 17 µM [181]. | Inhibition of NADPH-, ascorbate-, t-BuOOH-, and CCl4-induced lipid peroxidation in microsomes, IC50 = 57.3, 20.8, 61.7, 17.6 μM, respectively [182]. | ||
| 3.12 µg/mL inhibited NO production by 79.57% in LPS-activated RAW 264.7 cells. 15-LOX inhibitor, IC50 = 25.9 µg/mL [183]. | ||||
| Attenuated Sephadex-induced lung injury in rats, inhibition of NF-κB-mediated upregulation of A20 and activation of NRF2/HO-1 signalling pathway [184]. | ||||
| IC50 = 31.6 µM in L-02 IC50 = 31.3 µM in HUVEC [185]. | ||||
| IC50 = >100 µM in cerebellar granule cells [186]. | ||||
| ↓ of the cell viability of HaCaT cells at 25 µg/mL after 24 h [145]. | Inhibitory effect on IL-6-induced STAT3 promoter activity in Hep3B cells, IC50 = 2.5 µM [161]. | |||
| Isosophoranone | CC50 in the range of 25–62 µM in human tumour cells (HSC-2, HSG) and human normal cells (HGF, HPC, HPLF) [187]. | Inhibition of NO production in LPS-activated RAW 264.7 cells, (IC50 = 17 µM) [187]. | ||
| Kazinol B | (2S)-Kazinol B IC50 = >100 µM in Bcap37, MCF-7, U251, A549 cells. IC50 = 58.4 µM in HepG2 cells. IC50 = 38.9 µM in Hep3B cells. (2R)-Kazinol B IC50 = >100 µM in Bcap37, MCF-7, U251, A549 cells. IC50 = 64.2 µM in HepG2 cells. IC50 = 30.3 µM in Hep3B cells [188]. | Inhibition of NO production in LPS-activated RAW 264.7 cells (IC50 = 21.6 µM) via inhibition of iNOS activity [189]. | Protection of mitochondria from injury through direct Fyn inhibition [190]. | |
| Kuraridin | Noncytotoxic when compared with the drug-free control in the range of 0.3–64 µg/mL in PBMC cells [123]. | COX-1 inhibitor IC50 = 0.6–1 µM. 5-LOX inhibitor IC50 = 5.4–6.9 µM [191]. | Additive effect with ciprofloxacin, erythromycin, gentamicin, kanamycin, oxacillin [123]. | |
| IC50 = 37.8 μg/mL in HepG2 cells [114]. | ||||
| Kurarinone | Inhibition of fatty acid β-oxidation through the reduction of l-carnitine and the inhibition of the PPAR-α pathway → lipid accumulation and liver injury (hepatotoxicity) [192]. | COX-1 inhibitor IC50 = 0.6–1 µM. 5-LOX inhibitor IC50 = 22 µM [191]. | Activation of Nrf2 and ↑ expression of antioxidant enzymes, including HO-1 [193]. | |
| Little toxic effects in BEAS-2B. In vivo apparent signs of toxicity [194]. | Inhibition of the expression of interleukin IL-1β, iNOS in LPS-stimulated RAW 264.7 cells [193]. | |||
| IC50 = 2–62 µM in cervical, lung (non-small and small), hepatic, esophageal, breast, gastric, cervical, and prostate cancer cells 20–500 mg/kg in vivo in lungs (non-small and small) cancer. Higher selectivity toward cancer cells in comparison with respective normal cells [195]. | Psoriasis-like skin disease induced by IL-23 and contact dermatitis induced by TNCB. Repression of disease development by inhibiting the expression of proinflammatory mediators and through the suppression of pathogenic CD4+T-cell differentiation and the overall immune response [196]. | |||
| Inhibition of LPS-induced macrophage activation and expression of proinflammatory genes, while ↑ anti-inflammatory gene expression including IL-10 in an AhR-dependent manner. An immunomodulatory activity in the treatment of IBS [197]. | ||||
| Kuwanon A | Inhibition of NO production stimulated by LPS and IFN-γ in RAW 264.7 cells, IC50 = 10.5 μM [198]. | |||
| COX-2 inhibitor IC50 = 14 μM [199]. | ||||
| Kuwanon C | IC50 = 14.2 µM in B16 melanoma cells [200]. | Inhibition of NO production stimulated by LPS and IFN-γ in RAW 264.7 cells, IC50 = 12.6 μM [198]. | ||
| IC50 = 1.7 µM in THP-1 cells [201]. | 5-LOX inhibitor IC50 = 12 µM. 12-LOX inhibitor IC50 = 19 µM [191]. | |||
| IC50 = 3.9 μM in MCF-7 cells IC50 = 9.54 μM in HepG2 cells [202]. | Anti-inflammatory effects of kuwanon C are regulated by HO-1 expression [203]. | |||
| Kuwanon E | Noncytotoxic EC50 > 10 µM in MCF-7, CEM, RPMI8226, U266, HeLa, BJ, THP-1 cells [172]. | Inhibition of NO production stimulated by LPS and IFN-γ in RAW 264.7 cells, IC50 = 14.9 μM [198]. | Synergy with amikacin and etimicin [139]. | |
| Inhibition of IL-6 production, IC50 = 47.5 μM without a cytotoxic effect in A549 cells [204]. | ||||
| COX-2 inhibitor IC50 = 34 µM [199]. | ||||
| Kuwanon G | Toxic effect in RAW 264.7 cells ≥ 50 μM. The viability of cells was not affected at concentrations of 2, 5, 10, and 20 μM [205]. | Inhibition of NO production at 100 μM (79.9%) [204]. | ||
| ↓ of the release of RANTES/CCL5, TARC/CCL17, and MDC/CCL22 via downregulation of STAT1 and NF-κB p65 signalling in TNF-α- and IFN-γ-stimulated HaCaT keratinocytes. Inhibition of histamine production and 5-LOX activation in PMA- and A23187-stimulated MC/9 mast cells [206]. | ||||
| 20 µM ↓ the ox-LDL induced inflammatory response by suppressing the NF-κB activation in RAW 264.7 cells [205]. | ||||
| Licochalcone A | Cytotoxic activity for: HepG2 cells (10 μM) = 14%. SW480 cells (10 μM) = 7%. MCF7 cells (10 μM) = 10% [207]. | Inhibition of NF-κB transcription, IC50 = 13.9 μM [207]. | Inhibition of peroxyl radical-induced DCFH oxidation without a PBS wash (EC50 = 58.8 μg/mL) and with a PBS wash (EC50 = 46.3 μg/mL) [208]. | Subinhibitory concentrations ↓ the secretion of SEA and SEB by both MSSA and MRSA [209]. |
| IC50 for 24 and 48 h = 6.0 and 13.7 μg/mL, respectively, for the HepG2 cells [208]. | Inhibitor for P. acnes-induced NLRP3 inflammasome activation. Block of C. acnes-induced production of caspase-1 (p10) and IL-1β in macrophages and SZ95. Suppression of C. acnes-induced ASC speck formation and mitochondrial reactive oxygen species [210]. | |||
| IC50 = 4.8 μM in A549 cells. IC50 = 4.6 μM in SK-OV-3 cells. IC50 = 2.7 μM in SK-MEL-2 cells. IC50 = 3.4 μM in HCT-15 cells [211]. | Suppression of ORAI1, Kv1.3, and KCa3.1 channels, IC50 = 3, 0.8, and 11.2 µM, respectively. Suppressive effects on the IL-2 secretion and proliferation in CD3 and CD28 antibody-induced T-cells [212]. | |||
| IC50 = 36.6 µg/mL in HepG2 cells. IC50 = 26.9 µg/mL in Vero cells [144]. | Inhibition of LPS-induced phosphorylation at serine 276 and transcriptional activation of NF-κB. Inhibition of LPS-induced activation of PKA [213]. | ↑ protein expression of SOD1, CAT, and GPx1 in a concentration-dependent manner (2–8 μg/mL for 24 h) [208]. | ||
| Attenuation of LPS-induced kidney histopathologic changes, serum BUN, and creatinine levels. Suppression of LPS-induced TNF-α, IL-6, and IL-1β production in both serum and kidney tissues. Inhibition of LPS-induced NF-κB activation [214]. | ||||
| Inhibition of PGE2 and NO production and iNOS and COX-2 expression, induced by IL-1β. Inhibition of MMP-1, MMP-3, and MMP-13 production in IL-1β-stimulated chondrocytes. Inhibition of phosphorylation of NF-κB p65 and IκBα. Upregulation of the expression of Nrf2 and HO-1 [215]. | ||||
| Inhibition of sUV-induced COX-2 expression and PGE2 generation through the inhibition of AP-1 transcriptional activity. Suppression of sUV-induced phosphorylation of Akt/mTOR and ERK1/2/p90 ribosomal protein S6 kinase in HaCaT cells. Suppression of the activity of PI3K, (MEK)1, and B-Raf, but not Raf-1 in cell-free assays [216]. | ||||
| Licochalcone B | Inhibition of LPS-induced phosphorylation at serine 276 and transcriptional activation of NF-kappaB. Inhibition of LPS-induced activation of PKA. Reduction of the LPS-induced production of NO, TNFα, and MCP-1 [217]. | Suppression of the oxidative stress and inflammation, manifesting as the enhancement of SOD, GSH, and IL-4, but the decline of MDA, iNOS, and TNF-α [218]. | ||
| A specific inhibitor of the activation of the NLRP3 inflammasome in macrophages, no effect on the activation of AIM2 or NLRC4 inflammasome. It binds to NEK7 and inhibits the interaction between NLRP3 and NEK7 → suppressing NLRP3 inflammasome activation. Protective effects in mouse models of NLRP3 inflammasome-mediated diseases → LPS-induced septic shock, MSU-induced peritonitis, and nonalcoholic steatohepatitis [219]. | ||||
| Licochalcone C | ↓ NF-κB translocation and several downstream molecules, -iNOS, ICAM-1, and VCAM-1. Upregulation of the PI3K/Akt/eNOS signalling pathway [220]. | 50 µM attenuates inflammatory response by influencing extracellular O2− production and by modulating the antioxidant network activity of SOD, CAT, and GPx activity [221]. | ||
| Attenuation of the LPS-IFN-γ-induced inflammatory response by ↓ the expression and activity of iNOS via NF-κB [221]. | ||||
| Licochalcone E | IC50 = 5.9 μM in A549 cells. IC50 = 5.2 μM in SK-OV-3 cells. IC50 = 2.9 μM in SK-MEL-2 cells. IC50 = 3.4 μM in HCT-15 cells [211]. | Dose-dependent inhibition of IL-12p40 production from LPS-stimulated RAW 264.7 cells. ↓ binding to the NF-κB site in RAW 264.7. Inhibition of the ↑ IL-12p40 expression and ear thickness induced by oxazolone in chronic allergic contact dermatitis model [222]. | Subinhibitory concentrations → a dose-dependent decrease in α-toxin expression in S. aureus [146]. | |
| Topical application of 0.5–2 mg inhibited TPA-induced ear oedema formation; phosphorylation of SAPK/JNK, c-Jun, and extracellular signal regulated kinase ½, and expression of iNOS and COX-2 in mouse skin. 2.5–7.5 μmol/L → ↓ in LPS-induced release of NO and PGE2; ↓ mRNA expression and secretion of IL-6, IL-1β, and TNF-α; ↓ promoter activity of iNOS and COX-2 and expression of their corresponding mRNAs and proteins; ↓ activation of AKT, MAPK, SAPK/JNK, and c-Jun; ↓ phosphorylation of IκB kinase-αβ and IκBα, degradation of IκBα, translocation of p65 to the nucleus and transcriptional activity of NF-κB; and transcriptional activity of AP-1 in RAW 264.7 cells [223]. | ||||
| Licoflavanone | Inhibition of NO production in LPS-stimulated RAW 264.7 cells, IC50 = 37.7 µM [224]. | IC50 = 59.6 µM in ABTS assay [224]. | ||
| ↓ of NF-kB translocation into the nucleus→ ↓ proinflammatory cytokines and COX-2/iNOS expression levels. ↓ of p38, JNK, and ERK1/2 phosphorylation and activation. Disruption of the NF-kB/MAPKs signal transduction pathway → ↓ in mRNA levels of TNFα, IL 1β, and IL 6 [224]. | ||||
| Licoflavone C = 8-prenylapigenin | IC50 = 9 µg/mL in Hep-2 cells [122]. | Inhibition of the LPS-induced gene expression for TNF-α, iNOS, COX-2, and release of TNF-α, NO, and PGE2, through the inhibition of NF-κB activation and reactive oxygen species accumulation in RAW 264.7 cells [225]. | ↓ of increase in the cellular ROS levels at 3 μM in RAW 264.7 cells [225]. | |
| IC50 = 121.4 μM in RAW 264.7 cells [225]. | ||||
| IC50 = 41.6 μM in RAW 264.7 cells [153]. | ||||
| IC50 = 42 μmol/L in H4IIE cells. IC50 = 37 μmol/L in C6 glioma cells [226]. | ||||
| Inhibition of NO production in LPS-activated RAW 264.7 cells, IC50 = 20.4 µM [153]. | ||||
| Lonchocarpol A | COX-1 inhibitor IC50 = 16.9 μM. COX-2 inhibitor IC50 = 9.5 μM [227]. | |||
| ↓ of NO production, IC50 = 2.5 μM and ↓ NOX activity, IC50 = 24.4 μM in murine microglial cells [228]. | ||||
| Lupalbigenin | IC50 = 11.630–37.712 µM in MCF-7, MDA-MB-231, MDA-MB-468, SW-620, and the mouse fibroblast cell line L-929, [229]. | 1.25 and 2.5 mM effectively inhibited the LPS-induced TNF-α, COX-2, iNOS, and NF-κB [230]. | ||
| Mimulone | Cytotoxicity < 50% of DMSO in WB-F344 [164]. | COX-1 inhibitor IC50 = 3.6 μM. COX-2 inhibitor IC50 = 6.0 μM [168]. | TEACABTS = 1.7 [171]. | Synergy with oxacillin, additive effect with tetracycline and ciprofloxacin [231]. |
| IC50 = 6.6 µM in THP-1 cells [232]. | 25 mg/kg prior and after induction of colitis ameliorated its symptoms and delayed the onset. ↓ of the levels of COX-2 and ↑ the ratio of pro-MMP2/MMP2 activity, ↓ of SOD2, and CAT [170]. | DPPH quenching activity TEAC 0.4 at 10 µM [164]. | ||
| Morusin | IC50 = 0.6 µM in HeLa. IC50 = 7.9 µM in MCF-7. IC50 = 9.2 µM in Hep3B [233]. | Inhibition of NO production stimulated by LPS and IFN-γ in RAW 264.7 cells, IC50 = 10.6 μM [198]. | ||
| Inhibition of RANTES/CCL5 and TARC/CCL17 secretion via the suppression of STAT1 and NF-κB p65 phosphorylation in TNF-α- and IFN-γ-stimulated HaCaT keratinocytes, and the release of histamine and LTC4 by suppressing 5-LOX activation in PMA- and A23187-stimulated MC/9 mast cells [206]. | ||||
| Morusinol | IC50 = 4.3 µM in THP-1 cells [201]. | The attenuation of LPS-induced secretion of TNF-α → an effect nearly twice that of prednisone [201]. | Synergy with amikacin, ciprofloxacin, vancomycin, streptomycin [139]. | |
| Papyriflavonol A | IC50 = 20.9 µg/mL in HepG2 cells [114]. | 5-LOX inhibitor IC50 = 7 µM [191]. | ||
| Inhibition of sPLA2s-IIA (IC50 = 3.9 µM) and -V (IC50 = 4.5 µM). Inhibition of LTC4 (IC50 = 0.6 µM) production in mouse bone marrow mast cells. 12.5–50 mg/kg i.p. reduced IgE-dependent PCA [234]. | ||||
| Propolin D = nymphaeol B | 12 µM inhibited cell proliferation in RAW 264.7 cells [174]. | Inhibition of albumin denaturation, IC50 = 0.5 µM. Inhibition of nitrite production stimulated by LPS in RAW 264.7 cells, IC50 = 5.4 µM. COX-2 inhibitor = 17.9 µM [174]. | DPPH radical scavenging activity, IC50 = 7.1 µM. The inhibition of linoleic acid oxidation by β-carotene bleaching systems, IC50 = 5.8 µM [175]. | Dose-dependent inhibition of S. aureus and C. albicans biofilm formation [107]. |
| At concentrations up to 200 µg/mL, nontoxic in C. elegans model, slightly reduced nematode survival at 500 µg/mL [107]. | ||||
| Propolin F = isonymphaeol B | 12 µM inhibited cell proliferation in RAW 264.7 cells [174]. | Inhibition of albumin denaturation, IC50 = 0.4 µM. Inhibition of nitrite production stimulated by LPS in RAW 264.7 cells, IC50 = 6.2 µM. COX-2 inhibitor = 23.8 µM [174]. | DPPH radical scavenging activity, IC50 = 8.5 µM. The inhibition of linoleic acid oxidation by β-carotene bleaching systems, IC50 = 5.9 µM [175]. | Dose-dependent inhibition of S. aureus biofilm formation. Inhibition of C. albicans biofilm formation [107]. |
| Propolin G = nymphaeol C | At tested concentrations did not inhibit cell proliferation; it induced cell proliferation to some extent in RAW 264.7 cells [174]. | Inhibition of albumin denaturation, IC50 = 0.37 µM. Inhibition of nitrite production stimulated by LPS in RAW 264.7 cells, IC50 = 2.4 µM. COX-2 inhibitor = 15.5 µM [174]. | DPPH radical scavenging activity IC50 = 9.8 µM. The inhibition of linoleic acid oxidation by β-carotene bleaching systems, IC50 = 10.3 µM [175]. | Inhibition of C. albicans biofilm formation [107]. |
| Scandenone = warangalone | At 10 µM (86.5 % cell viability) in RAW 264.7 cells [119]. | Inhibition of LPS-stimulated NO production in RAW 264.7 cells, IC50 = 8.5 µM [119]. | ||
| 20 µM inhibited proliferation in MDA-MB-231 cells and 15 µM in MCF-7 cells. Promotion of proliferation in MCF-10A [235]. | Anti-inflammatory and antinociceptive activity in carrageenan-induced hind paw oedema model and TPA-induced mouse ear oedema model at 100 mg/kg dose [236]. | |||
| Sophoraflavanone G | Toxic from 4 to 64 µg/mL in human PBMC with >50% cellular activity inhibition. IC50 = 3.2 µg/mL [123]. | COX-1 inhibitor IC50 = 0.1–0.6 µM. 5-LOX inhibitor IC50 = 0.1–0.3 µM. 12-LOX inhibitor IC50 = 20 µM [191]. | Additive effect with ciprofloxacin, erythromycin, gentamicin, fusidic acid, oxacillin [123]. | |
| Interruption of the NF-κB and MAPK signalling pathways [237]. | ||||
| Inhibition of cell proliferation in: A549, NCI-H460, SK-OV-3, SK-MEL-2, XF498, HCT-15, HL60, SPC-A-1 cells with IC50 = 2–36 μg/mL [238]. | Inhibition of PGE2 production in LPS-induced RAW cells by COX-2 downregulation at 1–50 μM. 10–250 µg/ear in mouse croton oil-induced ear oedema and 2–250 mg/kg in rat carrageenan paw oedema → effect far less than prednisolone, but higher when applied topically [69]. | Synergy with ampicillin and oxacillin [125]. | ||
| IC50 = 12.5 μM in HL-60 cells [239]. | Inhibition of NO, PGE2, IL-1β, IL-6, TNFα production in Ag I/II-N-stimulated RAW 264.7 cells via the downregulation of iNOS and COX-2 expression. Inhibition of the phosphorylation of IκB-α, nuclear translocation of p65, and subsequent activation of NF- κB. Inhibition of MAPK-mediated pathways [240]. | |||
| IC50 = 12.5 μM in HL-60 cells. IC50 = 13.3 μM in HepG2 cells [241]. | ||||
| CC50 =< 19 μM in HSC-2 cells. CC50 = 19 μM in HSG cells. CC50 = 19 μM in HGF cells [242]. | ||||
| Tomentodiplacone B | IC50 = >20 μM in THP-1 cells, viability at 30 µM [232]. | Reduction of TNF-α secretion as much as or more than the prednisone. IC50 >20 μM [232]. | TEACABTS = 1.0, TEACInhibition of. peroxynitrite-induced tyrosine nitration = 0.8 [171]. | |
| Xanthoangelol | IC50 = 23.6 μM in THP-1. IC50 = 21.7 μM in MRC-5. IC50 = 21.5 μM in HEK293. IC50 = 13.7 μM in HepG2. IC50 = 58.8 μM in CLS-54. IC50 = 1.0 μM in MRSA [109]. | Inhibition of LPS-stimulated NO production, IC50 = 5 μM. Suppression of AP-1. Reduction of the phosphorylation (at serine 536) level of the p65 subunit of NF-κB [243]. | ||
| IC50 = 25 μM in MRC-5. IC50 = 25 μM in THP-1 [108]. | ||||
| Xanthohumol | IC50 = 5.2 μg/mL in NHLF cells [148]. | NF-κB activity was reduced in vitro as well as in hepatic tissue after ischemia/reperfusion [244]. | TEACABTS = 0.3 μmol/l TEACFRAP = 0.3 μmol/l [245]. | Synergy with oxacillin against S. aureus [147]. |
| IC50 = 11.0 μM in MCF-7. IC50 = 10.7 μM in PC-3. IC50 = 91.3 μM in HT-29 [246]. | 10 μg/mL inhibits 91.7% of the NO production by suppressing iNOS induced by a combination of LPS and IFN-γ [247]. | DPPH radical scavenging activity, IC50 = 2.0 µM [246]. | At MIC reduced biofilm viability by 86.5% [147]. | |
| IC50 = 40.8 μM in HCT116. IC50 = 50.2 μM in HT-29. IC50 = 25.4 μM in HepG2. IC50 = 37.2 μM in Huh7 [248]. | ↓ the expression of the LPS receptor components - TLR4 and MD2 → suppression of NF-κB activation in LPS-activated RAW 264.7 cells. Inhibition of the binding activity of STAT-1alpha and IRF-1 In IFN-γ-stimulated RAW 264.7 cells [249]. | TEACABTS = 0.2, IC50 = 0.7 mg/mL, TEACDPPH = 0.04, IC50 = >1.2 mg/mL [250]. | 15–30 μg/mL → ↓ release of planktonic bacteria from the 24 h old biofilm by more than 90%. BBC = 60–125 μg/mL [148]. | |
| IC50 = 3.6 μM in HCT-15 [251]. | ↓ the release of MCP-1 and TNF-α in LPS-stimulated RAW 264.7 and U937 human monocytes [252]. | ↓ reactive oxygen species in vitro. Levels of enzymatic and nonenzymatic antioxidants were restored after pretreatment in postischemic hepatic tissue, and lipid peroxidation was attenuated [244]. | ||
| 0.5–10 µM inhibited melanogenesis induced by isobutyl-methylxanthine in B16 melanoma cells [253]. | Inhibition of IL-12 production in stimulated macrophages through the downregulation of NF-κB. In an oxazolone-induced chronic dermatitis model in mouse ear → attenuated dermatitis [254]. | |||
| Stout beer supplemented with 10 mg/L of xanthohumol for 4 weeks decreased serum VEGF levels (18.4%), N-acetylglucosaminidase activity (27.8%), IL1β concentration (9.1%), and NO released (77.1%), accompanied by a reduced redox state as observed by an increased GSH/GSSG ratio (to 198.8%) [255]. | ||||
| 3′-O-methyldiplacol | IC50 = 7.2 μM in THP-1 cells [232]. | Inhibition of LPS-induced NO production in RAW 264.7 cells, IC50 = 5.9 µM [173]. | TEACABTS = 1.6, TEACDPPH = 0.1, TEACFRAP = 0.1, TEACInhibition of. peroxynitrite-induced tyrosine nitration = 0.7 [171]. | Synergy with oxacillin. Additive effect with ciprofloxacin, tetracycline against MRSA [231]. |
| 3′-O-methyldiplacone | IC50 = 30.2 µM in WB 344 [164]. | ↓ the secretion of TNF-α ≥ than the prednisone [164]. | DPPH quenching activity TEAC 0.8 at 10 µM [164]. | |
| IC50 =< 10 μM in THP-1 cells [232]. | TEACABTS = 1.4, TEACDPPH = 0.1, TEACFRAP = 0.1, TEACInhibition of. peroxynitrite induced tyrosine nitration = 0.8 [171]. | |||
| EC50 =< 10 µM in MCF-7 cells, EC50 =< 10 µM in CEM cells, EC50 = 7.3 µM in RPMI8226 cells, EC50 = 5.5 µM in U266 cells, EC50 = 7.4 µM in HeLa cells, EC50 = 4.7 µM in BJ cells, EC50<10 µM in THP-1 cells [172]. | ||||
| 3′-O-methyl-5′-hydroxydiplacone | Antiproliferative (IC50 = 12.6 μM) and cytotoxic (LC50>30 µM) effect [169]. | Inhibition of LPS-induced NO production in RAW 264.7 cells, IC50 = 1.5 µM [173]. | TEACABTS = 1.7, TEACDPPH = 1.0, TEACFRAP = 0.7, TEACInhibition of. peroxynitrite induced tyrosine nitration = 0.8, Superoxide scavenging activity-Enzymatic = 71.2%, Non-Enzymatic = 29.5% at 50 μM [171]. | |
| COX-1 inhibitor IC50 = 3.3 μM COX-2 inhibitor IC50 = 10.6 μM 5-LOX inhibitor IC50 = 0.1 μM [168]. | ||||
| 3′-O-methyl-5′-O-methyldiplacone | IC50 = 7.9 μM in THP-1 cells [232]. | 5-LOX inhibitor IC50 = 0.4 μM [168]. | TEACABTS 1.6, TEAC DPPH = 0.3, TEAC FRAP = 1.2, TEAC Inhibition of. peroxynitrite-induced tyrosine nitration = 0.8 [171]. | |
| 4-hydroxylonchocarpin | IC50 > 100 μM in RAW 264.7 cells [256]. | Inhibition (66.5%) of NO release from LPS-stimulated RAW 264.7 cells. 10 μM inhibited iNOS activity. In the carrageenan-induced paw oedema model, 10 mg/kg showed comparable activity to indomethacin, and 50 mg/kg showed higher activity than indomethacin [256]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sychrová, A.; Škovranová, G.; Čulenová, M.; Bittner Fialová, S. Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules 2022, 27, 4491. https://doi.org/10.3390/molecules27144491
Sychrová A, Škovranová G, Čulenová M, Bittner Fialová S. Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules. 2022; 27(14):4491. https://doi.org/10.3390/molecules27144491
Chicago/Turabian StyleSychrová, Alice, Gabriela Škovranová, Marie Čulenová, and Silvia Bittner Fialová. 2022. "Prenylated Flavonoids in Topical Infections and Wound Healing" Molecules 27, no. 14: 4491. https://doi.org/10.3390/molecules27144491
APA StyleSychrová, A., Škovranová, G., Čulenová, M., & Bittner Fialová, S. (2022). Prenylated Flavonoids in Topical Infections and Wound Healing. Molecules, 27(14), 4491. https://doi.org/10.3390/molecules27144491