Potential Role of Quercetin in Polycystic Ovary Syndrome and Its Complications: A Review
Abstract
1. Introduction
2. Retrieval Methods
3. QUR in the Treatment of PCOS
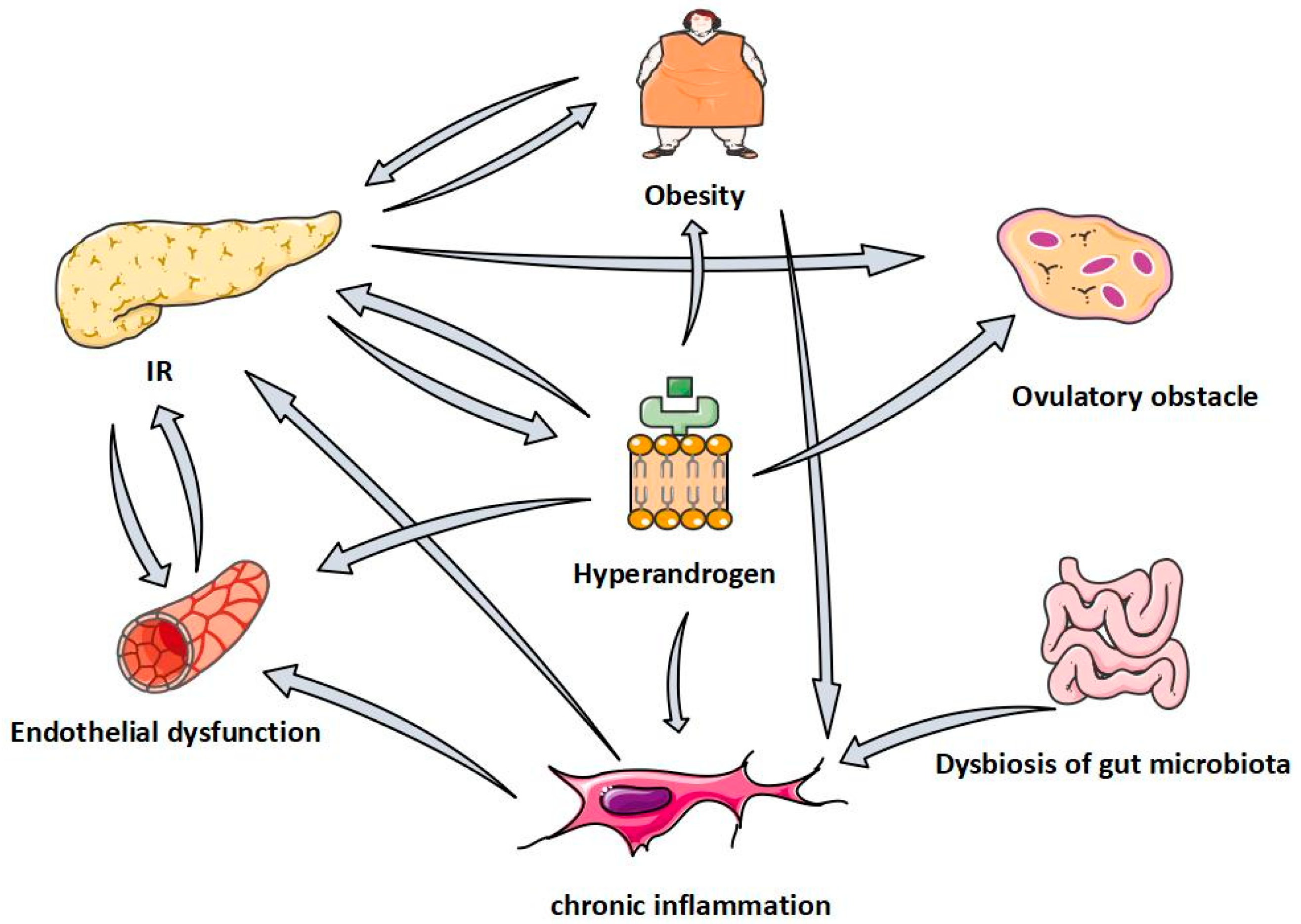
4. Effect and Mechanism of Quercetin in Polycystic Ovary Syndrome
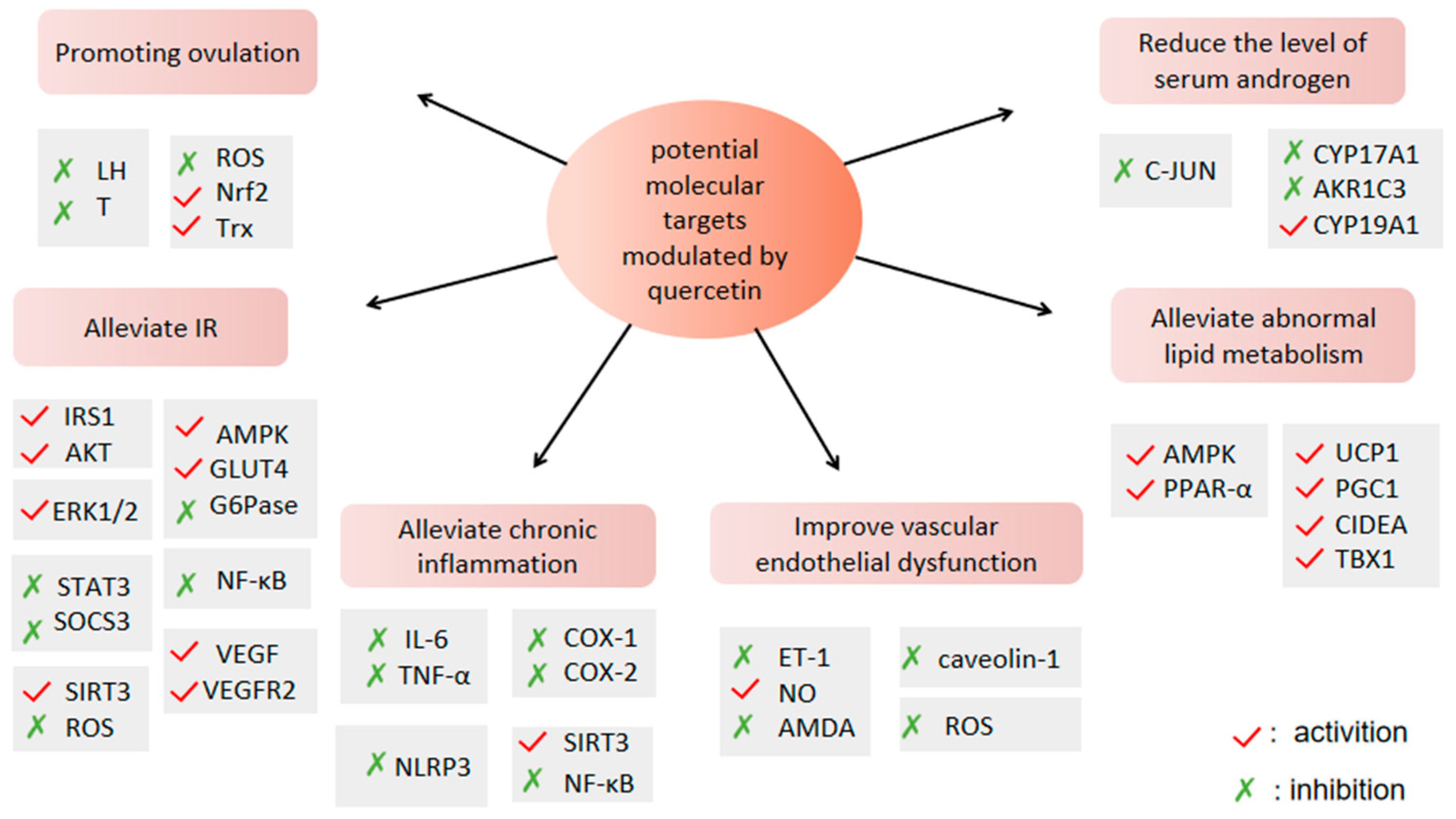
4.1. QUR Can Improve Ovulation Disorders
4.1.1. QUR Can Regulate the Sex Hormone Secretion of HPO Axis
4.1.2. QUR Can Inhibit Apoptosis of Granulosa Cells
4.2. QUR Can Alleviate IR
4.2.1. QUR Activates Insulin Signaling Pathway
4.2.2. QUR Can Maintain Glucose Homeostasis
4.2.3. QUR Can Protect Pancreatic β Cells
4.3. QUR Can Reduce the Level of Serum Androgens
4.3.1. QUR Can Inhibit the Expression of AR
4.3.2. QUR Can Inhibit Androgen Synthesis
4.4. QUR Can Improve Abnormal Lipid Metabolism
4.4.1. QUR Can Promote Fatty Acid Oxidation
4.4.2. QUR Can Regulate Adipokine Production
4.4.3. QUR Can Induce Browning of Adipose Tissue
4.5. QUR Can Regulate Chronic Inflammation
4.6. QUR Can Regulate Intestinal Flora
4.7. QUR Can Improve Vascular Endothelial Dysfunction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dokras, A.; Stener-Victorin, E.; Yildiz, B.O.; Li, R.; Ottey, S.; Shah, D.; Epperson, N.; Teede, H. Androgen Excess- Polycystic Ovary Syndrome Society: Position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 2018, 109, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Q.; Yang, D.; Li, S.; Lu, S.; Wu, X.; Wei, Z.; Song, X.; Wang, X.; Fu, S.; et al. Prevalence of polycystic ovary syndrome in women in China: A large community-based study. Hum. Reprod. 2013, 28, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Kilpatrick, E.S.; Mann, V.; Corless, L.; Abouda, G.; Rigby, A.S.; Atkin, S.L.; Sathyapalan, T. A Randomized, Controlled Trial of Vitamin D Supplementation on Cardiovascular Risk Factors, Hormones, and Liver Markers in Women with Polycystic Ovary Syndrome. Nutrients 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hultgren, K.E. Complementing the US Food and Drug Administration Adverse Event Reporting System with Adverse Drug Reaction Reporting From Social Media: Comparative Analysis. JMIR Public Health Surveill. 2020, 6, e19266. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-T.; Wu, M.-H.; Tsai, L.-C.; Chen, T.-S.; Ou, H.-T. Co-Administration of Clomiphene Citrate and Letrozole in Mild Ovarian Stimulation Versus Conventional Controlled Ovarian Stimulation Among POSEIDON Group 4 Patients. Front. Endocrinol. 2021, 12, 780392. [Google Scholar] [CrossRef]
- Barcroft, J.F.; Galazis, N.; Jones, B.P.; Getreu, N.; Bracewell-Milnes, T.; Grewal, K.J.; Sorbi, F.; Yazbek, J.; Lathouras, K.; Smith, J.R.; et al. Fertility treatment and cancers—The eternal conundrum: A systematic review and meta-analysis. Hum. Reprod. 2021, 36, 1093–1107. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2022, 1–22. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Hussain, A.; Sameen, A.; Sahar, A.; Khan, S.; Siddique, R.; Aadil, R.M.; Xu, B. Novel extraction, rapid assessment and bioavailability improvement of quercetin: A review. Ultrason. Sonochem. 2021, 78, 105686. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Tajbakhsh, A.; Seidel, V.; Zirak, M.R.; Tabeshpour, J.; Shakeri, A. Quercetin and its role in modulating endoplasmic reticulum stress: A review. Phytother. Res. 2022, 36, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; Elfiky, A.M.; Abo-Zeid, F.S. The anti-androgenic effect of quercetin on hyperandrogenism and ovarian dysfunction induced in a dehydroepiandrosterone rat model of polycystic ovary syndrome. Steroids 2022, 177, 108936. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, Y.; Ma, M.; Li, M. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J. Formos. Med Assoc. 2022, 121, 1081–1092. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef]
- Olaniyan, O.T.; Bamidele, O.; Adetunji, C.O.; Priscilla, B.; Femi, A.; Ayobami, D.; Okotie, G.; Oluwaseun, I.; Olugbenga, E.; Mali, P.C. Quercetin modulates granulosa cell mRNA androgen receptor gene expression in dehydroepiandrosterone-induced polycystic ovary in Wistar rats via metabolic and hormonal pathways. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef]
- Khorchani, M.J.; Zal, F.; Neisy, A. The phytoestrogen, quercetin, in serum, uterus and ovary as a potential treatment for dehydroepiandrosterone-induced polycystic ovary syndrome in the rat. Reprod. Fertil. Dev. 2020, 32, 313–321. [Google Scholar] [CrossRef]
- Neisy, A.; Zal, F.; Seghatoleslam, A.; Alaee, S. Amelioration by quercetin of insulin resistance and uterine GLUT4 and ERα gene expression in rats with polycystic ovary syndrome (PCOS). Reprod. Fertil. Dev. 2019, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Abid, A.; Khalid, S.; Afsar, T.; Ain, Q.U.; Shaheen, G.; Almajwal, A.; Razak, S. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: A histological and a biochemical study. J. Ovarian Res. 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N.; Patel, S.S. Phosphatidylinositide 3-kinase inhibition: A new potential target for the treatment of polycystic ovarian syndrome. Pharm. Biol. 2016, 54, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, M.; Moini, A.; Alipoor, E.; Rezvan, N.; Gorgani-Firuzjaee, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother. Res. 2018, 32, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, N.; Moini, A.; Gorgani-Firuzjaee, S.; Hosseinzadeh-Attar, M.J. Oral Quercetin Supplementation Enhances Adiponectin Receptor Transcript Expression in Polycystic Ovary Syndrome Patients: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Cell J. 2018, 19, 627–633. [Google Scholar]
- Rezvan, N.; Moini, A.; Janani, L.; Mohammad, K.; Saedisomeolia, A.; Nourbakhsh, M.; Gorgani-Firuzjaee, S.; Mazaherioun, M.; Hosseinzadeh-Attar, M.J. Effects of Quercetin on Adiponectin-Mediated Insulin Sensitivity in Polycystic Ovary Syndrome: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Horm. Metab. Res. 2017, 49, 115–121. [Google Scholar] [CrossRef]
- Homburg, R. Management of infertility and prevention of ovarian hyperstimulation in women with polycystic ovary syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 773–788. [Google Scholar] [CrossRef]
- Cadagan, D.; Khan, R.; Amer, S. Thecal cell sensitivity to luteinizing hormone and insulin in polycystic ovarian syndrome. Reprod. Biol. 2016, 16, 53–60. [Google Scholar] [CrossRef]
- Franks, S.; Hardy, K. Aberrant follicle development and anovulation in polycystic ovary syndrome. Ann. Endocrinol. 2010, 71, 228–230. [Google Scholar] [CrossRef]
- Shu, X.; Hu, X.-J.; Zhou, S.-Y.; Xu, C.-L.; Qiu, Q.-Q.; Nie, S.-P.; Xie, M.-Y. Effect of quercetin exposure during the prepubertal period on ovarian development and reproductive endocrinology of mice. Yao Xue Xue Bao 2011, 46, 1051–1057. [Google Scholar]
- Naseer, Z.; Ahmad, E.; Epikmen, E.T.; Uçan, U.; Boyacioğlu, M.; İpek, E.; Akosy, M. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 2017, 96, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, Z.; Aleyasin, A.; Eslami, M.; Nekoonam, S.; Zendedel, A.; Bahramrezaie, M.; Amidi, F. Quercetin protects human granulosa cells against oxidative stress via thioredoxin system. Reprod. Biol. 2019, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Catteau-Jonard, S.; Dewailly, D. Pathophysiology of Polycystic Ovary Syndrome: The Role of Hyperandrogenism. Front. Horm. Res. 2013, 40, 22–27. [Google Scholar]
- Willis, D.S.; Watson, H.; Mason, H.D.; Galea, R.; Brincat, M.; Franks, S. Premature Response to Luteinizing Hormone of Granulosa Cells from Anovulatory Women with Polycystic Ovary Syndrome: Relevance to Mechanism of Anovulation. J. Clin. Endocrinol. Metab. 1998, 83, 3984–3991. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Shi, B.; Feng, D.; Sagnelli, M.; Jiao, J.; Sun, X.; Wang, X.; Li, D. Fructose levels are elevated in women with polycystic ovary syndrome with obesity and hyperinsulinemia. Hum. Reprod. 2020, 35, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Eseberri, I.; Laurens, C.; Miranda, J.; Louche, K.; Lasa, A.; Moro, C.; Portillo, M. Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes. Int. J. Mol. Sci. 2021, 22, 1384. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Pan, Y.; Wang, R.; Kang, L.-L.; Xue, Q.-C.; Wang, X.-N.; Kong, L.-D. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J. Nutr. Biochem. 2014, 25, 420–428. [Google Scholar] [CrossRef]
- Khodarahmi, A.; Eshaghian, A.; Safari, F.; Moradi, A. Quercetin Mitigates Hepatic Insulin Resistance in Rats with Bile Duct Ligation Through Modulation of the STAT3/SOCS3/IRS1 Signaling Pathway. J. Food Sci. 2019, 84, 3045–3053. [Google Scholar] [CrossRef] [PubMed]
- Fryer, L.G.D.; Parbu-Patel, A.; Carling, D. The Anti-diabetic Drugs Rosiglitazone and Metformin Stimulate AMP-activated Protein Kinase through Distinct Signaling Pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Nistor, L.; Afshar, A.; Arnason, J.T.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.S.; Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tam, C.; Rolston, M.; Alves, P.; Chen, L.; Meng, S.; Hong, H.; Chang, S.; Yokoyama, W. Quercetin Ameliorates Insulin Resistance and Restores Gut Microbiome in Mice on High-Fat Diets. Antioxidants 2021, 10, 1251. [Google Scholar] [CrossRef]
- Pereira, D.F.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.R.B.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R.M.B. Effects of flavonoids on α-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Nie, Y.-X.; Dong, B.-Z.; Cai, Z.-C.; Zeng, X.-K.; Du, L.; Zhu, X.; Yin, X.-X. Quercetin protects islet β-cells from oxidation-induced apoptosis via Sirt3 in T2DM. Iran. J. Basic Med Sci. 2021, 24, 629–635. [Google Scholar] [CrossRef]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Li, Y. Quercetin and quercitrin protect against cytokine induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2013, 31, 265–271. [Google Scholar] [CrossRef]
- Kim, J.W.; Kang, K.M.; Yoon, T.K.; Shim, S.H.; Lee, W.S. Study of circulating hepcidin in association with iron excess, metabolic syndrome, and BMP-6 expression in granulosa cells in women with polycystic ovary syndrome. Fertil. Steril. 2014, 102, 548–554.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef] [PubMed]
- Suganya, N.; Dornadula, S.; Chatterjee, S.; Mohanram, R.K. Quercetin improves endothelial function in diabetic rats through inhibition of endoplasmic reticulum stress-mediated oxidative stress. Eur. J. Pharmacol. 2018, 819, 80–88. [Google Scholar] [CrossRef]
- Sirmans, S.M.; Pate, K.A. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 2013, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, F.-F.; Li, Y.-M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef]
- Wang, F.; Pan, J.; Liu, Y.; Meng, Q.; Lv, P.; Qu, F.; Ding, G.-L.; Klausen, C.; Leung, P.C.K.; Chan, H.C.; et al. Alternative splicing of the androgen receptor in polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 4743–4748. [Google Scholar] [CrossRef]
- Yuan, H.; Pan, Y.; Young, C.Y. Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2004, 213, 155–163. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Ujah, G.A.; Nna, V.U.; Agah, M.I.; Omue, L.O.; Leku, C.B.; Osim, E.E. Effect of quercetin on cadmium chloride-induced impairments in sexual behaviour and steroidogenesis in male Wistar rats. Andrologia 2018, 50, e12866. [Google Scholar] [CrossRef]
- Škarydová, L.; Živná, L.; Xiong, G.; Maser, E.; Wsól, V. AKR1C3 as a potential target for the inhibitory effect of dietary flavonoids. Chem. Biol. Interact. 2009, 178, 138–144. [Google Scholar] [CrossRef]
- Solak, K.A.; Wijnolts, F.M.; Nijmeijer, S.M.; Blaauboer, B.J.; Berg, M.V.D.; van Duursen, M.B. Excessive levels of diverse phytoestrogens can modulate steroidogenesis and cell migration of KGN human granulosa-derived tumor cells. Toxicol. Rep. 2014, 1, 360–372. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and polycystic ovary syndrome. Clin. Endocrinol. 2021, 95, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, W.; Qiu, S.; Shu, J. Research progress on the relationship of brown adipose tissue with polycystic ovary syndrome. Zhejiang Da Xue Xue Bao Yi Xue Ban 2017, 46, 315–320. [Google Scholar] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Lee, J.-S.; Cha, Y.-J.; Lee, K.-H.; Yim, J.-E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract. 2016, 10, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Otieno, D.; Gu, I.; Lee, S.-O.; Parks, J.S.; Schimmel, K.; Kang, H.W. Effect of quercetin on nonshivering thermogenesis of brown adipose tissue in high-fat diet-induced obese mice. J. Nutr. Biochem. 2021, 88, 108532. [Google Scholar] [CrossRef]
- Chen, X.-F.; Wang, L.; Wu, Y.-Z.; Song, S.-Y.; Min, H.-Y.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 2018, 8, 1. [Google Scholar] [CrossRef]
- Jiang, H.; Horiuchi, Y.; Hironao, K.-Y.; Kitakaze, T.; Yamashita, Y.; Ashida, H. Prevention effect of quercetin and its glycosides on obesity and hyperglycemia through activating AMPKα in high-fat diet-fed ICR mice. J. Clin. Biochem. Nutr. 2020, 67, 74–83. [Google Scholar] [CrossRef]
- Sun, X.; Yamasaki, M.; Katsube, T.; Shiwaku, K. Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice. Nutr. Res. Pract. 2015, 9, 137–143. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Güneş, M.; Bukan, N. Examination of angiopoietin-like protein 4, neuropeptide Y, omentin-1 levels of obese and non-obese patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Gursoy Calan, O.; Calan, M.; Yesil Senses, P.; Unal Kocabas, G.; Ozden, E.; Sari, K.R.; Kocar, M.; Imamoglu, C.; Senses, Y.M.; Bozkaya, G.; et al. Increased adipsin is associated with carotid intima media thickness and metabolic disturbances in polycystic ovary syndrome. Clin. Endocrinol. 2016, 85, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Fujiwara, Y.; Kondo, T.; Nishikawa, T.; Ogawa, R.; Matsumura, T.; Ishii, N.; Nagai, R.; Miyata, K.; Tabata, M.; et al. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem. Biophys. Res. Commun. 2009, 379, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Stoemmer, K.; Muendlein, A.; Saely, C.H.; Kinz, E.; Brandtner, E.M.; Fraunberger, P.; Drexel, H. Quercetin Impacts Expression of Metabolism- and Obesity-Associated Genes in SGBS Adipocytes. Nutrients 2016, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scotti, A.; Fang, J.; Yin, L.; Xiong, T.; He, W.; Qin, Y.; Liew, C.; Khayyat, N.; Zhu, W.; et al. Characterization of brown adipose tissue (BAT) in polycystic ovary syndrome (PCOS) patients by Z-Spectral Imaging (ZSI). Eur. J. Radiol. 2020, 123, 108777. [Google Scholar] [CrossRef]
- Choi, H.; Kim, C.-S.; Yu, R. Quercetin Upregulates Uncoupling Protein 1 in White/Brown Adipose Tissues through Sympathetic Stimulation. J. Obes. Metab. Syndr. 2018, 27, 102–109. [Google Scholar] [CrossRef]
- Gil Lee, S.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef]
- Regidor, P.-A.; Mueller, A.; Sailer, M.; Gonzalez Santos, F.; Rizo, J.M.; Egea, F.M. Chronic Inflammation in PCOS: The Potential Benefits of Specialized Pro-Resolving Lipid Mediators (SPMs) in the Improvement of the Resolutive Response. Int. J. Mol. Sci. 2020, 22, 384. [Google Scholar] [CrossRef]
- Alissa, E.M.; Algarni, S.A.; Khaffji, A.J.; Al Mansouri, N.M. Role of inflammatory markers in polycystic ovaries syndrome: In relation to insulin resistance. J. Obstet. Gynaecol. Res. 2021, 47, 1409–1415. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Keane, K.N.; Scheinpflug, A.L.; Cordeiro, R.; Soares, M.J.; Newsholme, P. Alanyl-glutamine improves pancreatic β-cell function following ex vivo inflammatory challenge. J. Endocrinol. 2015, 224, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhai, D.; Zhang, D.; Bai, L.; Yao, R.; Yu, J.; Cheng, W.; Yu, C. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reprod. Sci. 2017, 24, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Tang, Y.; Lu, H.; Qi, Y.; Li, G.; He, H.; Lu, F.; Yang, Y.; Sun, H. Quercetin Alleviates Osteoarthritis Progression in Rats by Suppressing Inflammation and Apoptosis via Inhibition of IRAK1/NLRP3 Signaling. J. Inflamm. Res. 2021, 14, 3393–3403. [Google Scholar] [CrossRef]
- Luo, X.; Bao, X.; Weng, X.; Bai, X.; Feng, Y.; Huang, J.; Liu, S.; Jia, H.; Yu, B. The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sci. 2022, 291, 120064. [Google Scholar] [CrossRef]
- Li, J.; Sun, Z.; Luo, G.; Wang, S.; Cui, H.; Yao, Z.; Xiong, H.; He, Y.; Qian, Y.; Fan, C. Quercetin Attenuates Trauma-Induced Heterotopic Ossification by Tuning Immune Cell Infiltration and Related Inflammatory Insult. Front. Immunol. 2021, 12, 649285. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, S.; Chen, J.; Zheng, J.; Ren, J.; Qi, P.; Zhu, Z.; Li, Z. Quercetin Nanoparticle Ameliorates Lipopolysaccharide-Triggered Renal Inflammatory Impairment by Regulation of Sirt1/NF-KB Pathway. J. Biomed. Nanotechnol. 2021, 17, 230–241. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Zhuang, S.; Fan, K.; Liu, Z. Quercetin Attenuates Adhesion Molecule Expression in Intestinal Microvascular Endothelial Cells by Modulating Multiple Pathways. Dig. Dis. Sci. 2018, 63, 3297–3304. [Google Scholar] [CrossRef]
- Warren, C.A.; Paulhill, K.J.; Davidson, L.A.; Lupton, J.R.; Taddeo, S.S.; Hong, M.Y.; Carroll, R.J.; Chapkin, R.S.; Turner, N.D. Quercetin May Suppress Rat Aberrant Crypt Foci Formation by Suppressing Inflammatory Mediators That Influence Proliferation and Apoptosis. J. Nutr. 2009, 139, 101–105. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Li, X.; Huang, G.; Xu, A. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J. Nutr. Biochem. 2020, 84, 108454. [Google Scholar] [CrossRef]
- Sun, L.; Hu, W.; Liu, Q.; Hao, Q.; Sun, B.; Zhang, Q.; Mao, S.; Qiao, J.; Yan, X. Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome Res. 2012, 11, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Insenser, M.; Murri, M.; Del Campo, R.; Martínez-García, M.; Fernández-Durán, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, J.; Lee, A.-R.; Jo, S.V.; Park, C.H.; Han, D.S.; Eun, C.S. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J. Nutr. Biochem. 2022, 101, 108926. [Google Scholar] [CrossRef]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Bayram, F.; Kocer, D.; Ozsan, M.; Muhtaroglu, S. Evaluation of endothelial dysfunction, lipid metabolism in women with polycystic ovary syndrome: Relationship of paraoxonase 1 activity, malondialdehyde levels, low-density lipoprotein subfractions, and endothelial dysfunction. Gynecol. Endocrinol. 2012, 28, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Usselman, C.W.; Yarovinsky, T.O.; Steele, F.E.; Leone, C.A.; Taylor, H.S.; Bender, J.R.; Stachenfeld, N.S. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: Role of the endothelin B receptor. J. Physiol. 2019, 597, 2853–2865. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.; Protogerou, A.; Papaioannou, T.; Piperi, C.; Mastorakos, G.; Lekakis, J.; Panidis, D.; Diamanti-Kandarakis, E. Early microvascular and macrovascular dysfunction is not accompanied by structural arterial injury in polycystic ovary syndrome. Hormones 2006, 5, 126–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vrtacnik-Bokal, E.; Meden-Vrtovec, H. Utero-ovarian arterial blood flow and hormonal profile in patients with polycystic ovary syndrome. Hum. Reprod. 1998, 13, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Chekir, C.; Nakatsuka, M.; Kamada, Y.; Noguchi, S.; Sasaki, A.; Hiramatsu, Y. Impaired uterine perfusion associated with metabolic disorders in women with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2005, 84, 189–195. [Google Scholar] [CrossRef]
- Stone, T.; Stachenfeld, N.S. Pathophysiological effects of androgens on the female vascular system. Biol. Sex Differ. 2020, 11, 45. [Google Scholar] [CrossRef]
- Owusu, J.; Barrett, E. Early Microvascular Dysfunction: Is the Vasa Vasorum a “Missing Link” in Insulin Resistance and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 7574. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Cai, X.; Bao, L.; Ding, Y.; Dai, X.; Zhang, Z.; Li, Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017, 15, 825–832. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Li, Y.; Wang, Y. Protection of vascular endothelial cells from high glucose injury induced by quercetin. Zhong Yao Cai 2002, 25, 268–270. [Google Scholar]
- Taguchi, K.; Tano, I.; Kaneko, N.; Matsumoto, T.; Kobayashi, T. Plant polyphenols Morin and Quercetin rescue nitric oxide production in diabetic mouse aorta through distinct pathways. Biomed. Pharmacother. 2020, 129, 110463. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, J.; Shen, J.; Bai, Q. Quercetin improves endothelial insulin sensitivity in obese mice by inhibiting Drp1 phosphorylation at serine 616 and mitochondrial fragmentation. Acta Biochim. Biophys. Sin. 2019, 51, 1250–1257. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Wang, Z.; Zhou, Q.; Chen, S.; Yang, B.; Yin, D.; He, H.; He, M. Quercetin protects the vascular endothelium against iron overload damages via ROS/ADMA/DDAHII/eNOS/NO pathway. Eur. J. Pharmacol. 2020, 868, 172885. [Google Scholar] [CrossRef] [PubMed]
- Kondo-Kawai, A.; Sakai, T.; Terao, J.; Mukai, R. Suppressive effects of quercetin on hydrogen peroxide-induced caveolin-1 phosphorylation in endothelial cells. J. Clin. Biochem. Nutr. 2021, 69, 28–36. [Google Scholar] [CrossRef] [PubMed]

| Sample Size | Models | Effect of Experimental Group Compared with Control Group | Daily Dosage and Administration of QUR | Time | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | T | SHBG | LH | LH/FSH | E2 | P | HOMA-IR | INS | CHO | TG | PCO | |||||
| 54 | ① | ↓ | ↓ | / | ↓ | ↓ | ↑ | ↑ | / | / | / | / | improve | 25 mg/kg, dissolved in saline, gavage | 28 days | [16] |
| 270 | ① | / | ↓ | / | ↓ | ↓ | ↓ | / | ↓ | / | / | / | improve | 100 mg/kg, dissolved in 1% CMC, gavage | 28 days | [17] |
| 18 | ② | - | ↓ | / | / | / | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | improve | 100 mg/kg, dissolved in 0.5% CMC, gavage | 30 days | [18] |
| 28 | ① | / | / | / | / | / | ↓ | ↑ | / | / | / | / | / | 100 mg/kg, po | 15 days | [19] |
| / | ① | / | / | / | / | / | ↓ | / | / | / | / | / | / | 15 mg/kg, dissolved in 10% ethanol, gavage | 30 days | [20] |
| 35 | ① | - | / | / | / | / | / | / | ↓ | ↓ | / | / | improve | 15 mg/kg, dissolved in 10% ethanol, gavage | 30 days | [21] |
| 24 | ② | - | ↓ | / | / | / | ↑ | ↑ | / | / | ↓ | ↓ | improve | 30 mg/kg, po | 21 days | [22] |
| 12 | ③ | - | ↓ | / | ↓ | / | / | / | / | ↓ | ↓ | ↓ | improve | 150 mg/kg, po | 6 weeks | [23] |
| Sample Size | Effect of Experimental Group Compared with Control Group | Daily Dosage and Administration of QUR | Time | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | T | SHBG | LH | LH/FSH | E2 | P | HOMA-IR | INS | CHO | TG | PCO | ||||
| 80 | ↓ | ↓ | ↑ | ↓ | / | / | / | ↓ | ↓ | / | / | / | 1000 mg, capsules, po | 12 weeks | [24] |
| 84 | - | ↓ | - | ↓ | / | / | / | ↓ | ↓ | / | / | / | 1000 mg, capsules, po | 12 weeks | [25,26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Jia, F.; Yu, Y.; Zhang, W.; Wang, C.; Zhu, S.; Zhang, N.; Liu, X. Potential Role of Quercetin in Polycystic Ovary Syndrome and Its Complications: A Review. Molecules 2022, 27, 4476. https://doi.org/10.3390/molecules27144476
Chen T, Jia F, Yu Y, Zhang W, Wang C, Zhu S, Zhang N, Liu X. Potential Role of Quercetin in Polycystic Ovary Syndrome and Its Complications: A Review. Molecules. 2022; 27(14):4476. https://doi.org/10.3390/molecules27144476
Chicago/Turabian StyleChen, Tong, Fan Jia, Yue Yu, Wufan Zhang, Chaoying Wang, Shiqin Zhu, Nana Zhang, and Xinmin Liu. 2022. "Potential Role of Quercetin in Polycystic Ovary Syndrome and Its Complications: A Review" Molecules 27, no. 14: 4476. https://doi.org/10.3390/molecules27144476
APA StyleChen, T., Jia, F., Yu, Y., Zhang, W., Wang, C., Zhu, S., Zhang, N., & Liu, X. (2022). Potential Role of Quercetin in Polycystic Ovary Syndrome and Its Complications: A Review. Molecules, 27(14), 4476. https://doi.org/10.3390/molecules27144476






