Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
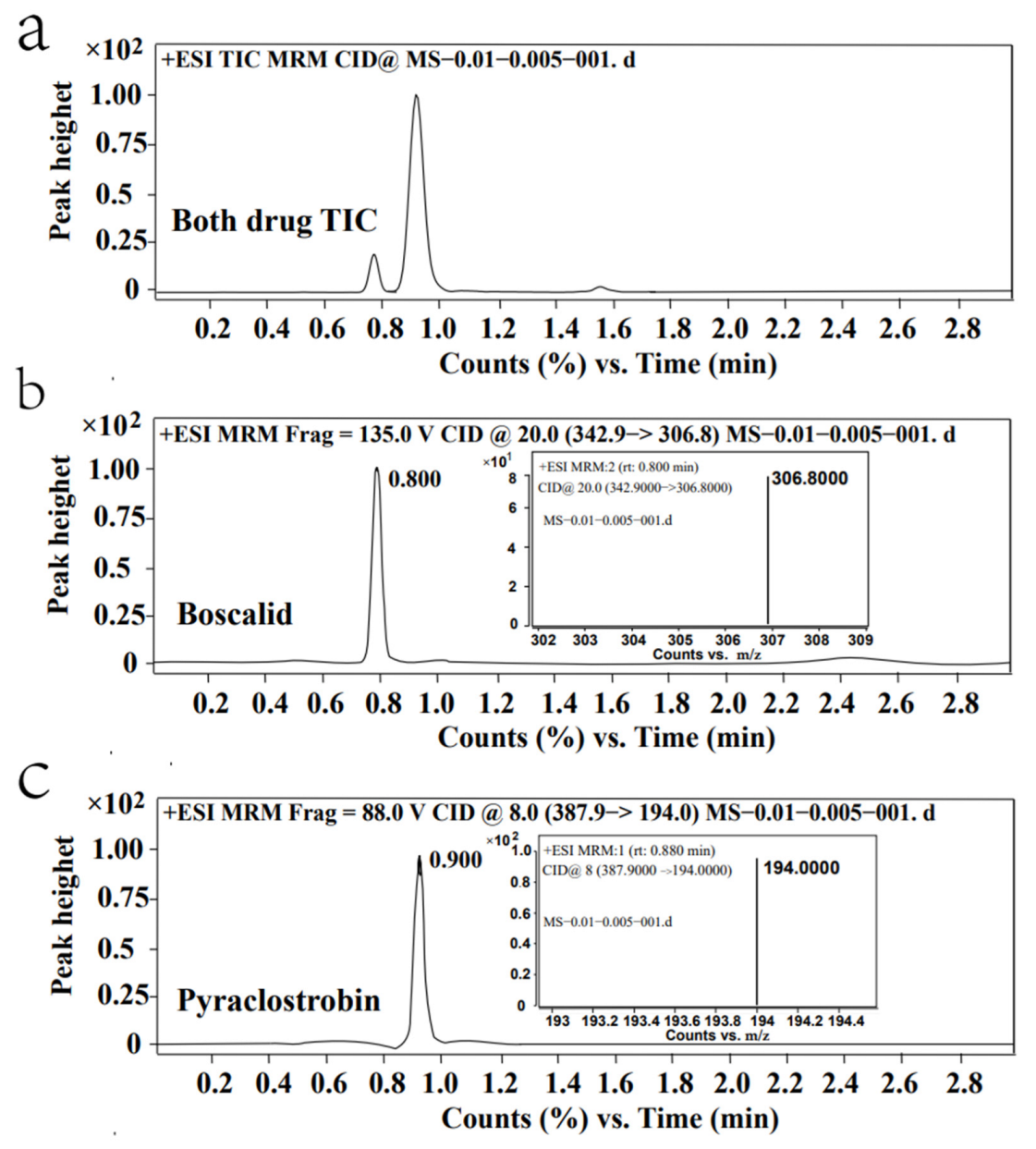
2.1. Optimization of Instrument Conditions, Extraction, and Purification
2.2. Method Validation
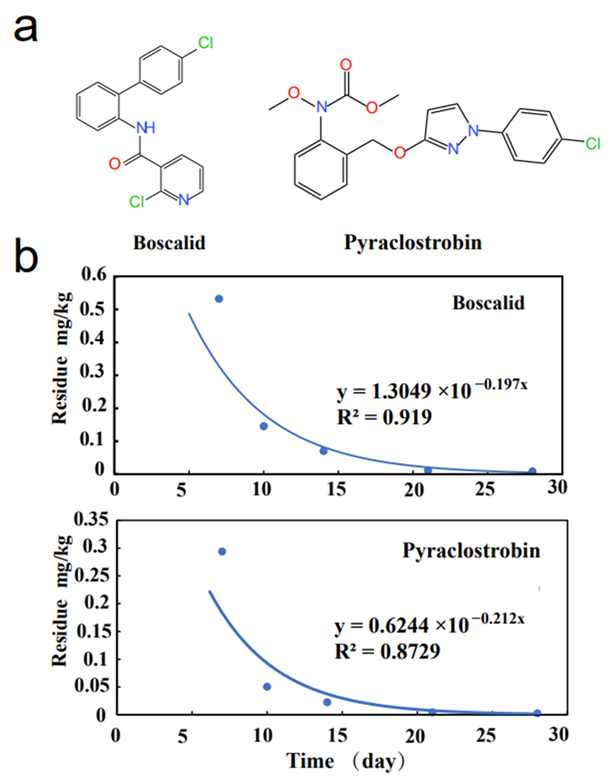
2.3. Dissipation Behaviors of Boscalid and Pyraclostrobin in Watermelon
2.4. Residue Distributions of Boscalid and Pyraclostrobin in Watermelon
2.5. Dietary Exposure Risk Assessment
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Sample Pretreatment
3.3. Instrumental Parameters
3.4. Recovery Experiments
3.5. Field Trials
3.6. Statistical Analysis
3.7. Dietary Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Odelade, K.A.; Oladeji, O.S. Isolation of phytopathogenic fungi associated with the post-harvest deterioration of watermelon fruits. Sci. Afr. 2020, 8, e00366. [Google Scholar] [CrossRef]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.S.S.L.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Bu, S.; Munir, S.; He, P.; Li, Y.; Wu, Y.; Li, X.; Kong, B.; He, P.; He, Y. Bacillus subtilis L1-21 as a biocontrol agent for postharvest gray mold of tomato caused by Botrytis cinerea. Biol. Control 2021, 157, 104568. [Google Scholar] [CrossRef]
- Avenot, H.; Morgan, D.P.; Michailides, T.J. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine®(pyraclostrobin+ boscalid) fungicide in Alternaria alternata causing alternaria late blight of pistachios in California. Plant Pathol. 2008, 57, 135–140. [Google Scholar] [CrossRef]
- Luz, A.L.; Kassotis, C.D.; Stapleton, H.M.; Meyer, J.N. The high-production volume fungicide pyraclostrobin induces triglyceride accumulation associated with mitochondrial dysfunction, and promotes adipocyte differentiation independent of PPARγ activation, in 3T3-L1 cells. Toxicology 2018, 393, 150–159. [Google Scholar] [CrossRef]
- Stammler, G. Mode of action, biological performance and latest monitoring results of boscalid sensitivity. In Abstracts of the 18th Symposium of the Research Committee on Fungicide Resistance; The Phytopathological Society of Japan: Shimane, Japan, 2008; pp. 30–43. [Google Scholar]
- Karlsson, A.S.; Weihermüller, L.; Tappe, W.; Mukherjee, S.; Spielvogel, S. Field scale boscalid residues and dissipation half-life estimation in a sandy soil. Chemosphere 2016, 145, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Cui, F.; Yang, Y.; Liu, Y.; Qi, S.; Wang, C. Mechanisms of developmental toxicity in zebrafish embryos (Danio rerio) induced by boscalid. Sci. Total Environ. 2018, 634, 478–487. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Li, B.; Wang, A.; Li, H.; Li, X.; Luo, J.; Liu, F.; Mu, W. Comparative toxicity of multiple exposure routes of pyraclostrobin in adult zebrafish (Danio rerio). Sci. Total Environ. 2021, 777, 145957. [Google Scholar] [CrossRef]
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Hautier, L. Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci. Rep. 2018, 8, 7241. [Google Scholar] [CrossRef]
- Salazar, G.; Cooper, E.M.; Stapleton, H.M.; Zylka, M.J. Choice of vehicle affects pyraclostrobin toxicity in mice. Chemosphere 2019, 218, 501–506. [Google Scholar] [CrossRef]
- Chen, X.; He, S.; Gao, Y.; Ma, Y.; Hua, J.; Liu, X. Dissipation behavior, residue distribution and dietary risk assessment of field-incurred boscalid and pyraclostrobin in grape and grape field soil via MWCNTs-based QuEChERS using an RRLC-QqQ-MS/MS technique. Food Chem. 2019, 274, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Chen, Y.; Zhang, Q.; Lu, P.; Hu, D. Dissipation, residues and risk assessment of oxine-copper and pyraclostrobin in citrus. Food Addit. Contam. Part A 2019, 36, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.; Chys, M.; De Rop, J.; Goeteyn, L.; Spanoghe, P.; Sampers, I. Pesticide residues in (treated) wastewater and products of Belgian vegetable-and potato processing companies. Chemosphere 2021, 280, 130619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, A.W.; Huang, M.; Yu, W.; Li, Z.; Wu, S.; Zheng, K.; Zhang, K.; Hu, D. Simultaneous determination of boscalid and fludioxonil in grape and soil under field conditions by gas chromatography/tandem triple quadrupole mass spectrometry. Biomed. Chromatogr. 2018, 32, e4091. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Hu, J. Dissipation behaviour and dietary risk assessment of boscalid, triflumizole and its metabolite (FM-6-1) in open-field cucumber based on QuEChERS using HPLC–MS/MS technique. J. Sci. Food Agric. 2018, 98, 4501–4508. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Yu, Y.; Chen, Y.; Zeng, S.; Lu, P.; Hu, D. Residues, dissipation kinetics, and dietary intake risk assessment of two fungicides in grape and soil. Regul. Toxicol. Pharmacol. 2018, 100, 72–79. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, R.; Su, Y.; Hu, J.; Liu, X. Fate, residues and dietary risk assessment of the fungicides epoxiconazole and pyraclostrobin in wheat in twelve different regions, China. Ecotoxicol. Environ. Saf. 2021, 207, 111236. [Google Scholar] [CrossRef]
- Fu, J.; Li, Z.; Huang, R.; Wang, S.; Huang, C.; Cheng, D.; Zhang, Z. Dissipation, residue, and distribution of pyraclostrobin in banana and soil under field conditions in South China. Int. J. Environ. Anal. Chem. 2016, 96, 1367–1377. [Google Scholar] [CrossRef]
- Kwon, H.; Lehotay, S.J.; Geis-Asteggiante, L. Variability of matrix effects in liquid and gas chromatography–mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops. J. Chromatogr. A 2012, 1270, 235–245. [Google Scholar] [CrossRef]
- Cho, J.; Lee, J.; Lim, C.U.; Ahn, J. Quantification of pesticides in food crops using QuEChERS approaches and GC-MS/MS. Food Addit. Contam. Part A 2016, 33, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, W.; Hu, J.; Liu, X. Dissipation behavior, residues distribution and dietary risk assessment of tembotrione and its metabolite in maize via QuEChERS using HPLC-MS/MS technique. Ecotoxicol. Environ. Saf. 2020, 191, 110187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Pang, K.; Fan, X.; Ma, Y.; Hu, J. Dissipation behavior and residue distribution of fluazaindolizine and its seven metabolites in tomato ecosystem based on SAX SPE procedure using HPLC-QqQ-MS/MS technique. J. Hazard. Mater. 2018, 342, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. NY/T 788-2018 Guideline for the Testing of Pesticide Residues in Crops. 2018. Available online: https://www.sdtdata.com/fx/fmoa/tsLibCard/169332.html (accessed on 1 March 2022).
- Jankowska, M.; Kaczyński, P.; Łozowicka, B. Dissipation kinetics and processing behavior of boscalid and pyraclostrobin in greenhouse dill plant (Anethum graveolens L.) and soil. Pest Manag. Sci. 2021, 77, 3349–3357. [Google Scholar] [CrossRef] [PubMed]
- Jabot, C.; Daniele, G.; Giroud, B.; Tchamitchian, S.; Belzunces, L.P.; Casabianca, H.; Vulliet, E. Detection and quantification of boscalid and its metabolites in honeybees. Chemosphere 2016, 156, 245–251. [Google Scholar] [CrossRef]
- Liberato, P.A.; Okumura, L.L.; de Souza Silva, A.F.; Aleixo, H.; Silva, J.G.; Diniz, J.A.; Oliveira, A.F. Direct determination of boscalid in grape samples by differential pulse voltammetry using a carbon paste electrode. Anal. Methods 2021, 13, 5195–5203. [Google Scholar] [CrossRef]
- Sandín-España, P.; Mateo-Miranda, M.; López-Goti, C.; Seris-Barrallo, E.; Alonso-Prados, J.L. Analysis of Pesticide Residues by QuEChERS Method and LC-MS/MS for a New Extrapolation of Maximum Residue Levels in Persimmon Minor Crop. Molecules 2022, 27, 1517. [Google Scholar] [CrossRef]
- Han, L.; Wu, Q.; Wu, X. Dissipation and Residues of Pyraclostrobin in Rosa roxburghii and Soil under Filed Conditions. Foods 2022, 11, 669. [Google Scholar] [CrossRef]
- Yu, J.; Hou, J.; Yu, R.; Hu, X.; Xu, Z.; Zhao, X.; Chen, L. Dissipation and dietary exposure risk assessment of pyraclostrobin, fluxapyroxad, difenoconazole, and azoxystrobin in the Fritillaria field ecosystem. Environ. Sci. Pollut. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Han, S.; Bi, Y.; Han, L.; Song, S.; Ye, Z.; Qin, F.; Lv, X. Residue Behavior and Risk Assessment of Pyraclostrobin and Thifluzamide in Cowpea. Bull. Environ. Contam. Toxicol. 2022, 108, 786–790. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Hyne, R.V. Comparison of environmental risks of pesticides between tropical and nontropical regions. Integr. Environ. Assess. Manag. 2011, 7, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, N.K.; Kaur, P. Dissipation of bispyribac sodium in aridisols: Impact of soil type, moisture and temperature. Ecotoxicol. Environ. Saf. 2019, 170, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Muskus, A.M.; Miltner, A.; Hamer, U.; Nowak, K.M. Microbial community composition and glyphosate degraders of two soils under the influence of temperature, total organic carbon and pH. Environ. Pollut. 2022, 297, 118790. [Google Scholar] [CrossRef] [PubMed]
- Kaka, H.; Opute, P.A.; Maboeta, M.S. Potential Impacts of Climate Change on the Toxicity of Pesticides towards Earthworms. J. Toxicol. 2021, 2021, 8527991. [Google Scholar] [CrossRef]
- Schabacker, J.; Hahne, J.; Ludwigs, J.D.; Vallon, M.; Foudoulakis, M.; Murfitt, R.; Ristau, K. Residue levels of pesticides on fruits for use in wildlife risk assessments. Integr. Environ. Assess. Manag. 2021, 17, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Szpyrka, E.; Piechowicz, B.; Sadło, S.; Sudoł, M. Assessment of boscalid and pyraclostrobin disappearance and behavior following application of effective microorganisms on apples. J. Environ. Sci. Health Part B 2018, 53, 652–660. [Google Scholar] [CrossRef]
- EFSA. 2021. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/index.cfm?event=details&pest_res_ids=244&product_ids=&v=1&e=search.pr&p=&v=1 (accessed on 6 March 2022).
- EFSA. 2020. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=details&pest_res_ids=195&product_ids=&v=1&e=search.pr (accessed on 6 March 2022).
- National Health Commission of the People’s Republic of China. GB 2763-2019 National Food Safety Standard- Maximum Residue Limits for Pesticides in Food. 2019. Available online: https://www.sdtdata.com/fx/fmoa/tsLibCard/173701.html (accessed on 2 March 2022).
- Kapoor, U.; Srivastava, M.K.; Srivastava, A.K.; Patel, D.K.; Garg, V.; Srivastava, L.P. Analysis of imidacloprid residues in fruits, vegetables, cereals, fruit juices, and baby foods, and daily intake estimation in and around Lucknow, India. Environ. Toxicol. Chem. 2013, 32, 723–727. [Google Scholar] [CrossRef]
| Compounds | Spiked Level (mg/kg) | Watermelon | |
|---|---|---|---|
| Average Recoveries (%) n = 5 | RSD (%) | ||
| Boscalid | 0.05 | 108 | 5.4 |
| 3 | 101 | 7.7 | |
| 10 | 97 | 7.8 | |
| Pyraclostrobin | 0.05 | 103 | 2.5 |
| 0.5 | 97 | 7.3 | |
| 5 | 93 | 9.1 | |
| Compounds | Matrix | Method | LOQ (mg/kg) | Retention Times (mins) | Reference |
|---|---|---|---|---|---|
| Boscalid and Pyraclostrobin | Watermelon | LC–MS/MS | 0.01,0.005 | 0.8–0.9 | This study |
| Grape | RRLC–MS/MS | 0.001 | 0.80, 0.97 | [13] | |
| Greenhouse dill | LC–MS/MS | 0.001 | 13.19, 14.60 | [26] | |
| Boscalid | Honeybees | UHPLC–QqQ | 0.0001 | 6.50 | [27] |
| Grape pulp | ACSV * | 2.73 | No data | [28] | |
| Persimmon | LC–MS/MS | 0.001 | 4.59 | [29] | |
| Pyraclostrobin | Rosa roxburghii | LC–MS/MS | 0.00024 | 5.00 | [30] |
| Fritillaria | LC–MS/MS | 0.01 | 1.98 | [31] | |
| Cowpea | LC–MS/MS | 0.01 | No data | [32] |
| Location | Dose (g a.i./ha) | Spray Times | Intervals (d) | Average Residue (mg/kg) n = 3 * | |
|---|---|---|---|---|---|
| Boscalid | Pyraclostrobin | ||||
| Hunan | 270 | 2 | 7 | 0.5323 | 0.2938 |
| 10 | 0.1455 | 0.0505 | |||
| 14 | 0.0704 | 0.0225 | |||
| 21 | 0.0118 | 0.0044 | |||
| 28 | 0.0083 | 0.0027 | |||
| Compounds | Matrix | Half-Lives (DT50, Day) | Reference |
|---|---|---|---|
| Boscalid | Loamy sand soil | 104–182 | [8] |
| Greenhouse dill | 1.90–2.01 | [26] | |
| Topsoil from dill cultivation | 2.64–4.85 | [26] | |
| Grape | 18.1–18.8 | [13] | |
| Grape field soil | 9.7–17.6 | [13] | |
| Watermelon | 3.52 | This study | |
| Pyraclostrobin | Greenhouse dill | 1.62–1.73 | [26] |
| Topsoil from dill cultivation | 2.08–2.11 | [26] | |
| Grape | 17.8–25.9 | [13] | |
| Grape field soil | 8.9–13.7 | [13] | |
| Rosa roxburghii | 6.20–7.79 | [30] | |
| Rosa roxburghii soil | 3.86–5.95 | [30] | |
| Fritillaria | 6.3 | [31] | |
| Cowpea | 1.5–2.3 | [32] | |
| Watermelon | 3.27 | This study |
| Food Classification | Fi (kg) | Boscalid | Pyraclostrobin | ||
|---|---|---|---|---|---|
| Reference Residue Limits or STMR (mg/kg) | NEDI (mg) | Reference Residue Limits or STMR (mg/kg) | NEDI (mg) | ||
| Rice and its products | 0.2399 | 0.02 (EU) | 0.004798 | ||
| Flour and its products | 0.1385 | 0.2 (CN) | 0.0277 | ||
| Other grains | 0.0233 | 0.02 (CAC) | 0.000466 | ||
| Tubers | 0.0495 | 1 (CN) | 0.0495 | 0.2 (CN) | 0.0099 |
| Dried beans and their products | 0.016 | ||||
| Dark vegetables | 0.0915 | 2 (CN) | 0.183 | 1 (CN) | 0.0915 |
| Light vegetable | 0.1837 | 5 (CN) | 0.9185 | 5 (CN) | 0.9185 |
| Pickles | 0.0103 | ||||
| Fruits | 0.0457 | 0.05 (STMR) | 0.002285 | 0.05 (STMR) | 0.002285 |
| Nuts | 0.0039 | ||||
| Livestock and poultry | 0.0795 | ||||
| Milk and its products | 0.0263 | ||||
| Egg and its products | 0.0236 | ||||
| Fish and shrimp | 0.0301 | ||||
| Vegetable oil | 0.0327 | 2 (CN) | 0.0654 | 0.2 (CN) | 0.00654 |
| Animal oil | 0.0087 | ||||
| Sugar, starch | 0.0044 | ||||
| Salt | 0.012 | 10 (CN) | 0.12 | ||
| Soy sauce | 0.009 | 0.15 (CAC) | 0.00135 | ||
| Total | 1.0286 | 1.2187 | 1.1830 | ||
| ADI × 63 (mg) | 2.52 | 1.89 | |||
| Risk quotient (%) | 48.4 | 62.6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, L.; Su, Y.; Dong, B.; Lu, W.; Hu, J.; Liu, X. Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS. Molecules 2022, 27, 4410. https://doi.org/10.3390/molecules27144410
Lv L, Su Y, Dong B, Lu W, Hu J, Liu X. Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS. Molecules. 2022; 27(14):4410. https://doi.org/10.3390/molecules27144410
Chicago/Turabian StyleLv, Le, Yue Su, Bizhang Dong, Wang Lu, Jiye Hu, and Xiaolu Liu. 2022. "Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS" Molecules 27, no. 14: 4410. https://doi.org/10.3390/molecules27144410
APA StyleLv, L., Su, Y., Dong, B., Lu, W., Hu, J., & Liu, X. (2022). Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS. Molecules, 27(14), 4410. https://doi.org/10.3390/molecules27144410







