A Synergistic Effect of Phthalimide-Substituted Sulfanyl Porphyrazines and Carbon Nanotubes to Improve the Electrocatalytic Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results and Discussion
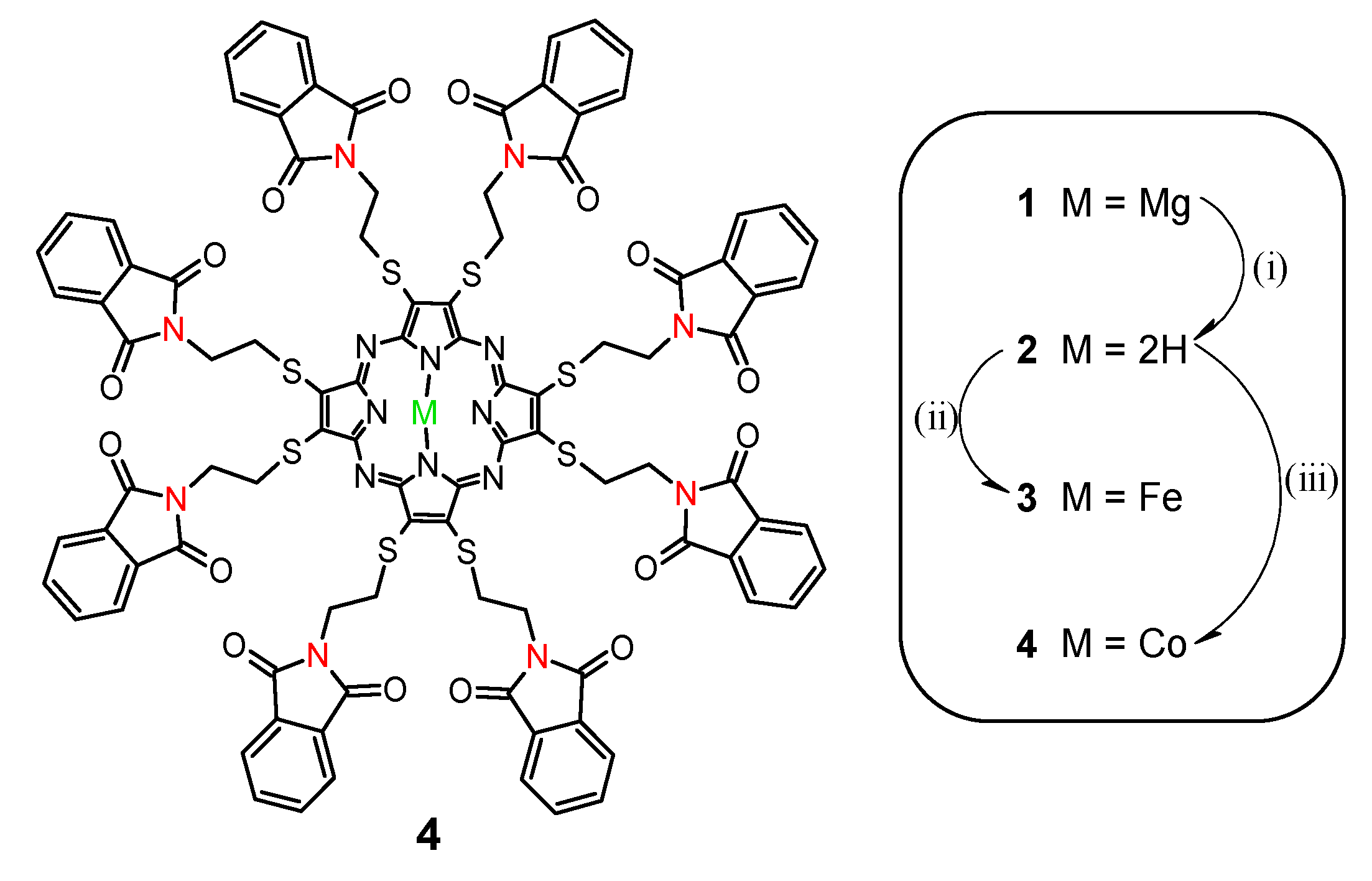
2.1. Synthesis and Characterization
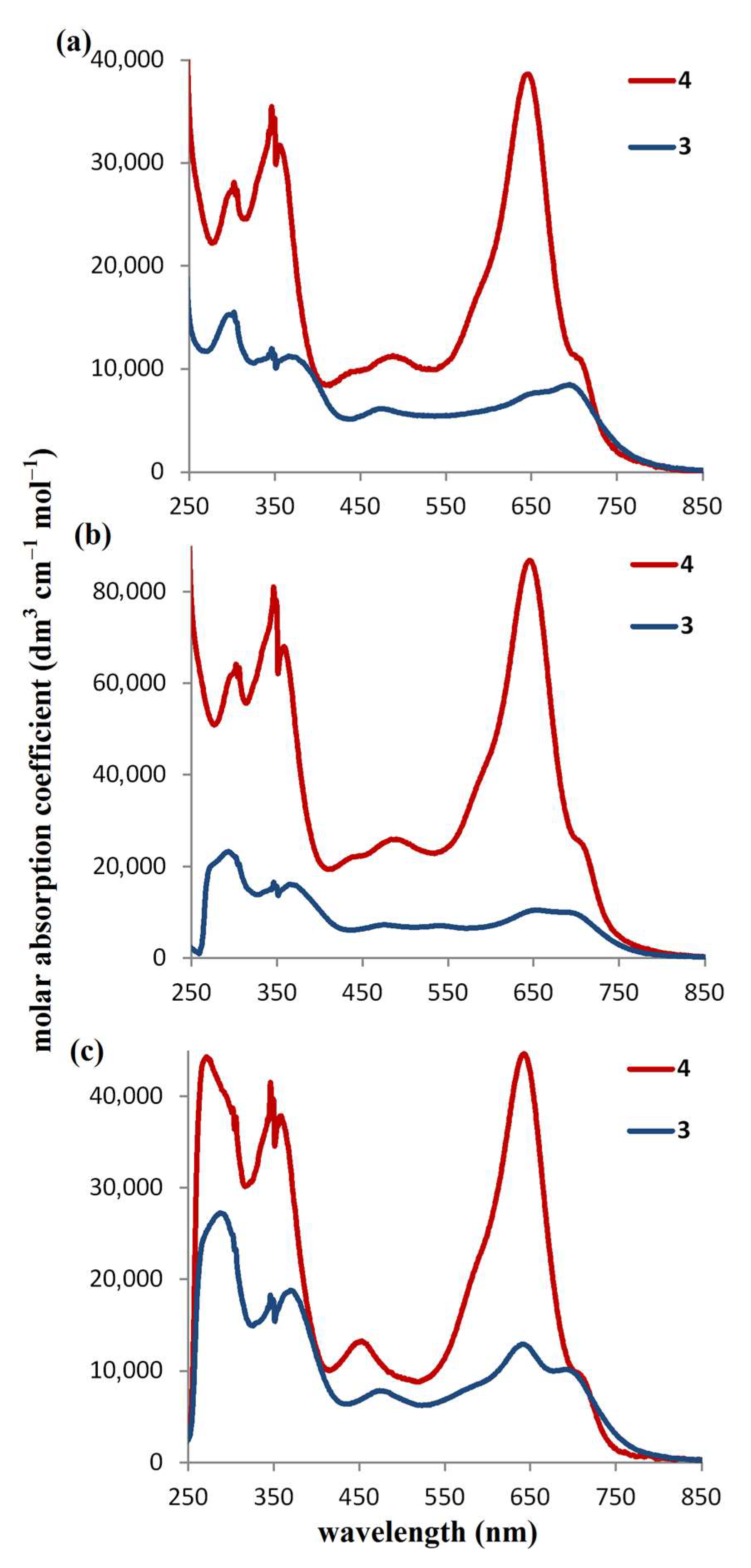
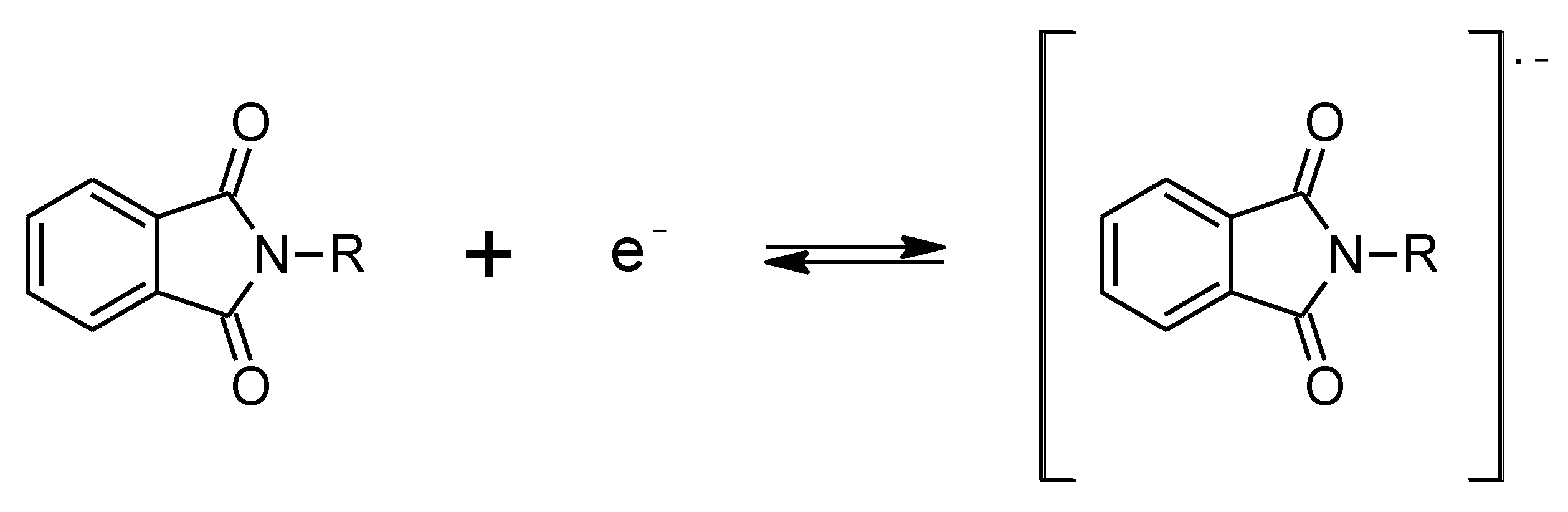
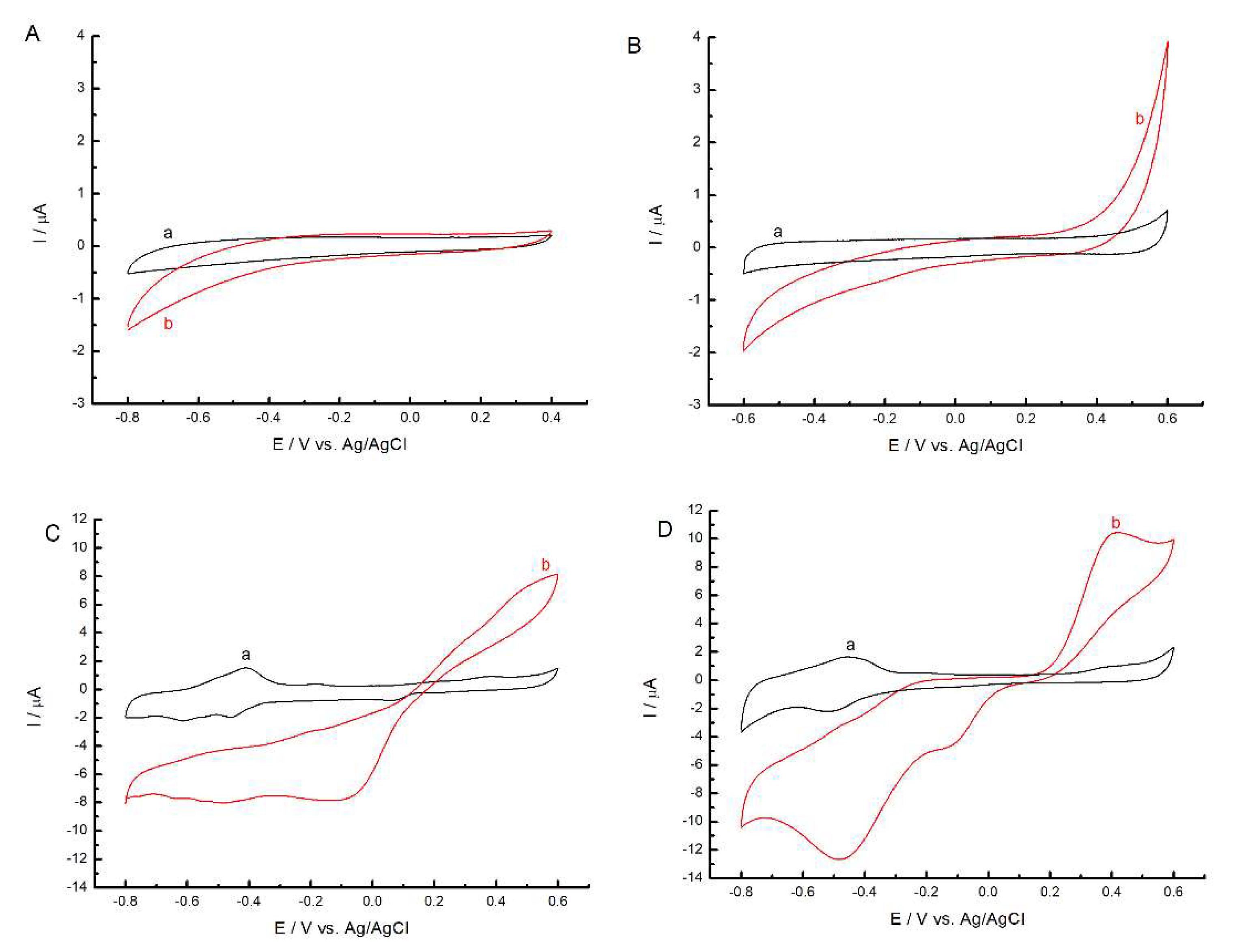
2.2. Electrochemical Characterization of Porphyrazines in Organic Electrolyte
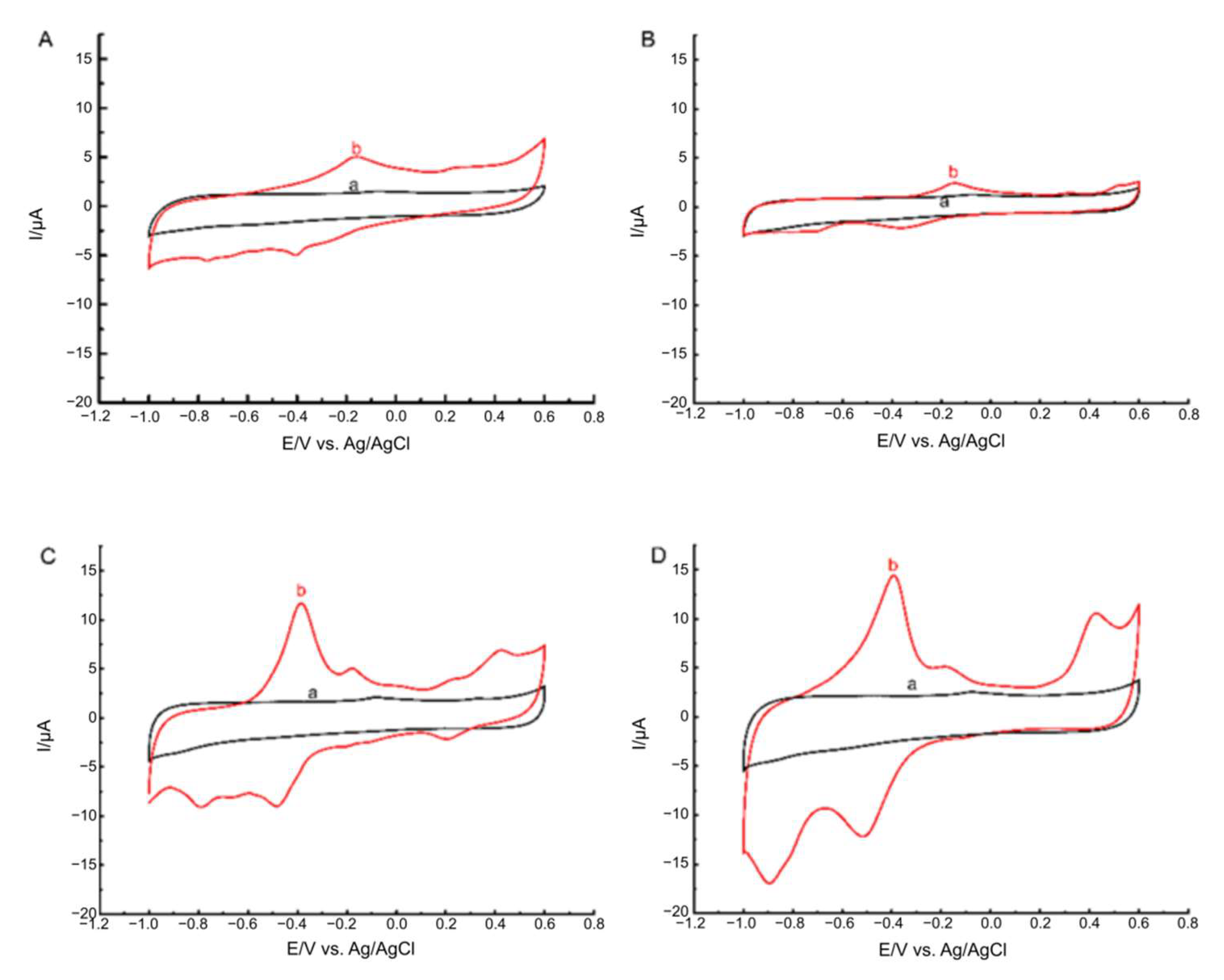
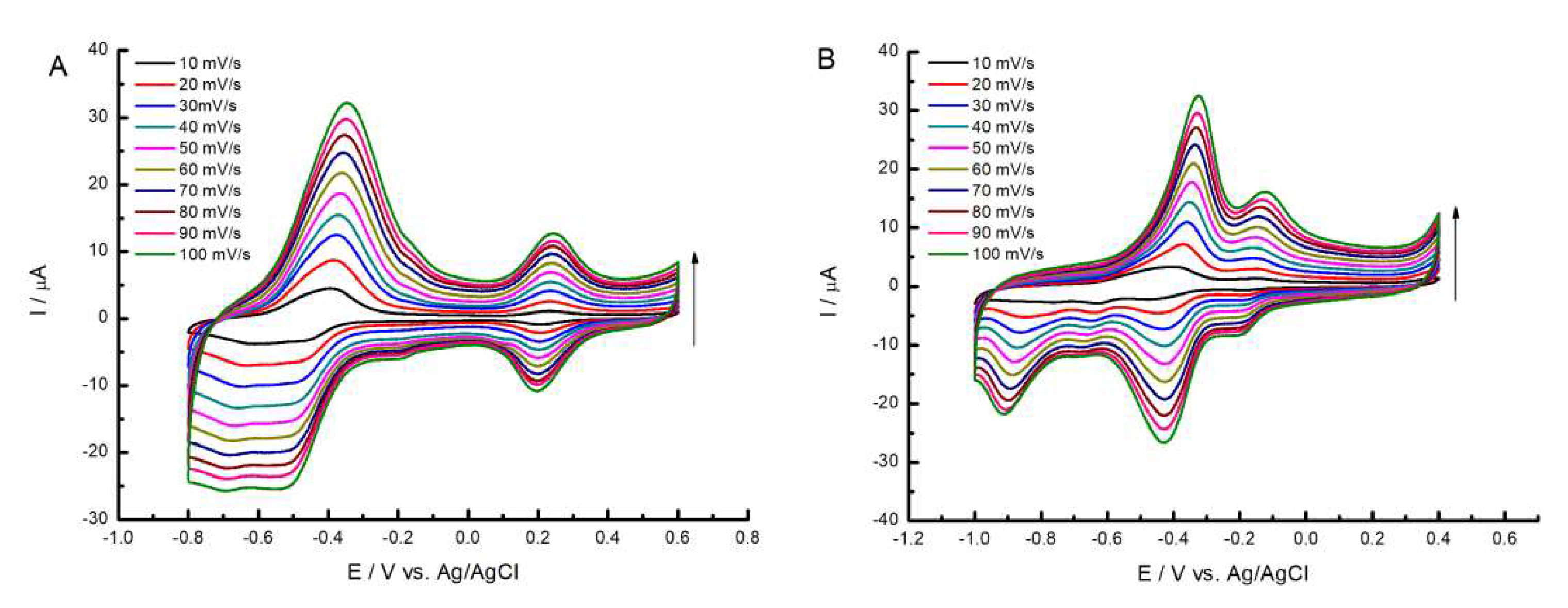
2.3. Electrochemical Study of Porphyrazines Deposited on MWCNT
2.4. The Influence of Hydrogen Peroxide on the Modification of the Surface of the GC Electrode
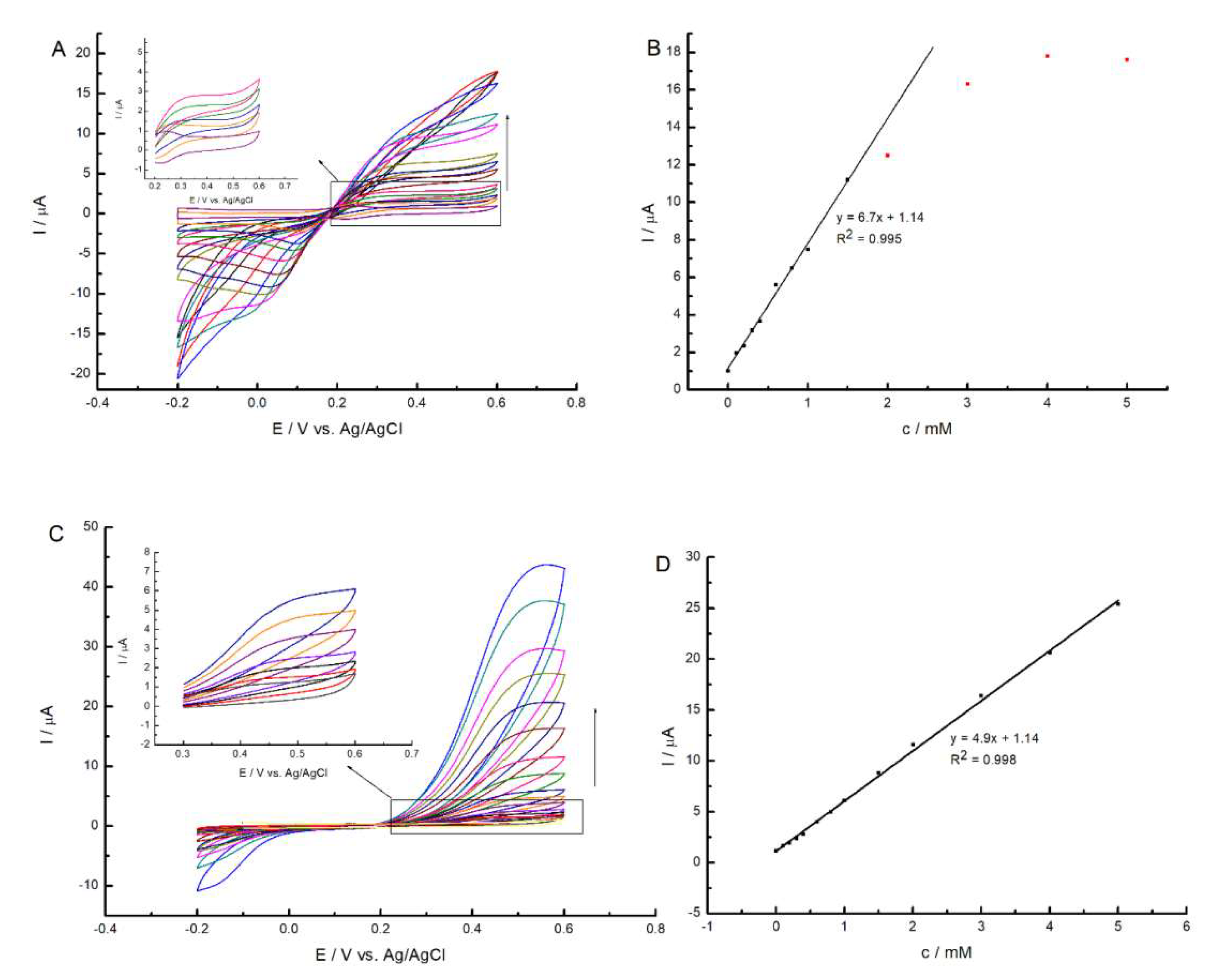
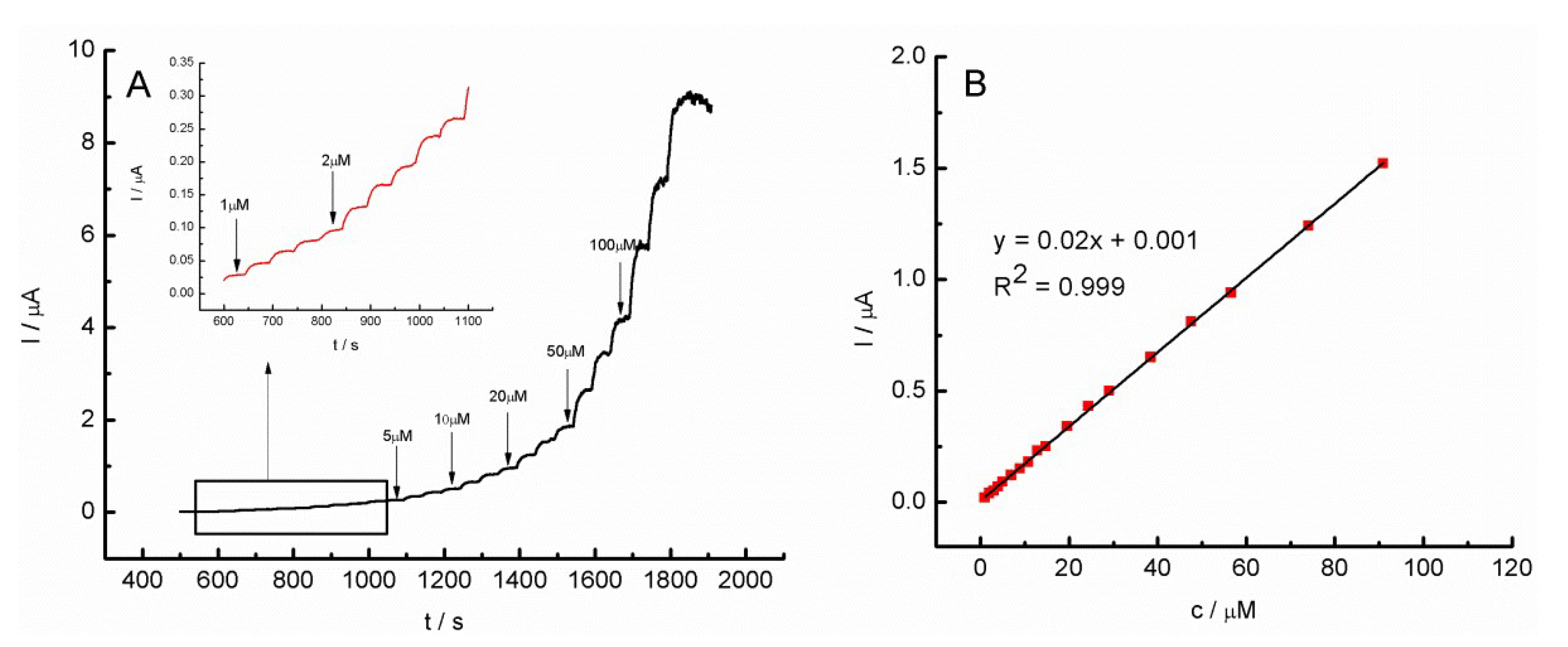
2.5. Chronoamperometric Measurements of GC/MWCNT/Pz3- and GC/MWCNT/Pz4-Modified Electrode in the Presence of H2O2
3. Materials and Methods
3.1. General Procedures
3.2. Synthetic Procedures and Characterization
3.3. Materials and Reagents for Electrochemical Testing
3.4. Electrochemical Methods
3.5. Fabrication of GC/MWCNT, GC/MWCNT/Pz1, GC/MWCNT/Pz2, GC/MWCNT/Pz3, and GC/MWCNT/Pz4 Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and Application of Electrochemical Sensor for Analyzing Hydrogen Peroxide in Food System and Biological Samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef]
- Deng, Z.; Zhao, L.; Zhou, H.; Xu, X.; Zheng, W. Recent Advances in Electrochemical Analysis of Hydrogen Peroxide towards in Vivo Detection. Process Biochem. 2022, 115, 57–69. [Google Scholar] [CrossRef]
- Byon, C.H.; Heath, J.M.; Chen, Y. Redox Signaling in Cardiovascular Pathophysiology: A Focus on Hydrogen Peroxide and Vascular Smooth Muscle Cells. Redox Biol. 2016, 9, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghalkhani, M.; Gharagozlou, M.; Sohouli, E.; Marzi Khosrowshahi, E. Preparation of an Electrochemical Sensor Based on a HKUST-1/CoFe2O4/SiO2-Modified Carbon Paste Electrode for Determination of Azaperone. Microchem. J. 2022, 175, 107199. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Khosrowshahi, E.M.; Sohouli, E.; Eskandari, K.; Aghaei, M.; Rahimi-Nasrabadi, M.; Sobhani-Nasab, A.; Banafshe, H.; Kouchaki, E. Electrochemical Monitoring of Carbamazepine in Biological Fluids by a Glassy Carbon Electrode Modified with CuO/ZnFe2O4/RGO Nanocomposite. Surf. Interfaces 2022, 30, 101943. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, P.; Roy, S.; Bhardwaj, K.; Jaiswal, A. Superior Peroxidase-Like Activity of Gold Nanorattles in Ultrasensitive H2O2 Sensing and Antioxidant Screening. ChemBioChem 2022, 23, e202100691. [Google Scholar] [CrossRef]
- Ullah, R.; Rasheed, M.A.; Abbas, S.; Rehman, K.; Shah, A.; Ullah, K.; Khan, Y.; Bibi, M.; Ahmad, M.; Ali, G. Electrochemical Sensing of H2O2 Using Cobalt Oxide Modified TiO2 Nanotubes. Curr. Appl. Phys. 2022, 38, 40–48. [Google Scholar] [CrossRef]
- Virbickas, P.; Kavaliauskaitė, G.; Valiūnienė, A.; Plaušinaitienė, V.; Rekertaitė, A.I.; Ramanavičius, A. Cobalt Hexacyanoferrate Based Optical Sensor for Continuous Optical Sensing of Hydrogen Peroxide. Electrochim. Acta 2020, 362, 137202. [Google Scholar] [CrossRef]
- Mounesh; Reddy, K.R.V. Detection of Nanomolar Concentrations H2O2 Using Cobalt (II) Phthalocyanine Modified GCE with MWCNTs. Anal. Chem. Lett. 2020, 10, 33–48. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Plesu, N.; Lascu, A.; Anghel, D.; Cazacu, M.; Ianasi, C.; Fagadar-Cosma, G.; Fratilescu, I.; Epuran, C. Novel Platinum-Porphyrin as Sensing Compound for Efficient Fluorescent and Electrochemical Detection of H2O2. Chemosensors 2020, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, M.; Rebis, T.; Kryjewski, M.; Popenda, L.; Lijewski, S.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. An Enhanced Electrochemical Nanohybrid Sensing Platform Consisting of Reduced Graphene Oxide and Sulfanyl Metalloporphyrazines for Sensitive Determination of Hydrogen Peroxide and L-Cysteine. Dye. Pigment. 2017, 138, 190–203. [Google Scholar] [CrossRef]
- Płócienniczak, P.; Rębiś, T.; Nowicki, M.; Milczarek, G. A Green Approach for Hybrid Material Preparation Based on Carbon Nanotubes/Lignosulfonate Decorated with Silver Nanostructures for Electrocatalytic Sensing of H2O2. J. Electroanal. Chem. 2021, 880, 114896. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, P.K.; Manna, S.; Satpati, A.K. A Novel Low Cost Nonenzymatic Hydrogen Peroxide Sensor Based on CoFe2O4/CNTs Nanocomposite Modified Electrode. J. Electroanal. Chem. 2020, 876, 114504. [Google Scholar] [CrossRef]
- Maringa, A.; Antunes, E.; Nyokong, T. Electrochemical Behaviour of Gold Nanoparticles and Co Tetraaminophthalocyanine on Glassy Carbon Electrode. Electrochim. Acta 2014, 121, 93–101. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Akhond, M.; Ghorbankhani, G.A.; Absalan, G. Electrochemical Sensing of Hydrogen Peroxide Using a Glassy Carbon Electrode Modified with Multiwalled Carbon Nanotubes and Zein Nanoparticle Composites: Application to HepG2 Cancer Cell Detection. Microchim. Acta 2020, 187, 105. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Q.; Pan, J.; Tsounis, C.; Esmailpour, A.A.; Xi, S.; Yang, H.Y.; Han, Z.; Yun, J.; Amal, R.; et al. Atomic Co Decorated Free-Standing Graphene Electrode Assembly for Efficient Hydrogen Peroxide Production in Acid. Energy Environ. Sci. 2022, 15, 1172–1182. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-Based Molecular Materials as Catalysts for Electrochemical Reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Fuchter, M.J.; Zhong, C.; Zong, H.; Hoffman, B.M.; Barrett, A.G.M. Porphyrazines: Designer Macrocycles by Peripheral Substituent Change. Aust. J. Chem. 2008, 61, 235–255. [Google Scholar] [CrossRef]
- Rodríguez-Morgade, M.S.; Stuzhin, P.A. The Chemistry of Porphyrazines: An Overview. J. Porphyr. Phthalocyanines 2004, 08, 1129–1165. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Falkowski, M.; Popenda, L.; Kryjewski, M.; Szczolko, W.; Jurga, S.; Mielcarek, J.; Goslinski, T. Tribenzoporphyrazines with Dendrimeric Peripheral Substituents and Their Promising Photocytotoxic Activity against Staphylococcus aureus. J. Photochem. Photobiol. B Biol. 2020, 204, 111803. [Google Scholar] [CrossRef]
- Piskorz, J.; Lijewski, S.; Gierszewski, M.; Gorniak, K.; Sobotta, L.; Wicher, B.; Tykarska, E.; Düzgüneş, N.; Konopka, K.; Sikorski, M.; et al. Sulfanyl Porphyrazines: Molecular Barrel-like Self-Assembly in Crystals, Optical Properties and in Vitro Photodynamic Activity towards Cancer Cells. Dye. Pigment. 2017, 136, 898–908. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Highly Selective Oxidation of Glucose to Gluconic Acid and Glucaric Acid in Water Catalyzed by an Efficient Synergistic Photocatalytic System. Catal. Sci. Technol. 2020, 10, 2231–2241. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Efficient Visible-Light-Driven Selective Conversion of Glucose to High-Value Chemicals over Bi2WO6/Co-Thioporphyrazine Composite in Aqueous Media. Appl. Catal. A Gen. 2021, 623, 118265. [Google Scholar] [CrossRef]
- Belviso, S.; Cammarota, F.; Rossano, R.; Lelj, F. Effect of Polyfluorination on Self-Assembling and Electronic Properties of Thioalkyl-Porphyrazines. J. Porphyr. Phthalocyanines 2016, 20, 223–233. [Google Scholar] [CrossRef]
- Koczorowski, T.; Szczolko, W.; Teubert, A.; Goslinski, T. Sulfanyl Porphyrazines with Morpholinylethyl Periphery—Synthesis, Electrochemistry, and Photocatalytic Studies after Deposition on Titanium(IV) Oxide P25 Nanoparticles. Molecules 2021, 26, 2280. [Google Scholar] [CrossRef]
- Porolnik, W.; Kasprzycka, M.; Teubert, A.; Piskorz, J. Serendipitous Synthesis of Unsymmetrical Porphyrazine: Incomplete Transesterification during Macrocyclization. Inorg. Chem. Commun. 2021, 133, 108953. [Google Scholar] [CrossRef]
- Rębiś, T.; Falkowski, M.; Milczarek, G.; Goslinski, T. Electrocatalytic NADH Sensing Using Electrodes Modified with 2-[2-(4-Nitrophenoxy)Ethoxy]Ethylthio-Substituted Porphyrazine/Single-Walled Carbon Nanotube Hybrids. ChemElectroChem 2020, 7, 2838–2850. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Piskorz, J.; Popenda, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Multiwalled Carbon Nanotube/Sulfanyl Porphyrazine Hybrids Deposited on Glassy Carbon Electrode—Effect of Nitro Peripheral Groups on Electrochemical Properties. J. Porphyr. Phthalocyanines 2017, 21, 295–301. [Google Scholar] [CrossRef]
- Rębiś, T.; Falkowski, M.; Kryjewski, M.; Popenda, L.; Sobotta, L.; Jurga, S.; Marszall, M.P.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Single-Walled Carbon Nanotube/Sulfanyl Porphyrazine Hybrids Deposited on Glassy Carbon Electrode for Sensitive Determination of Nitrites. Dye. Pigment. 2019, 171, 107660. [Google Scholar] [CrossRef]
- Stolarska, M.; Glowacka-Sobotta, A.; Ziental, D.; Dlugaszewska, J.; Falkowski, M.; Mielcarek, J.; Goslinski, T.; Sobotta, L. Photochemical Properties and Photocytotoxicities against Wound Bacteria of Sulfanyl Porphyrazines with Bulky Peripheral Substituents. J. Organomet. Chem. 2021, 934, 121669. [Google Scholar] [CrossRef]
- Cai, C.; Wang, L.; Hu, M.; Li, L.; Fu, J.; Zhao, X.; Zhang, Y.; Hu, Y.; Yuan, Z. Solution-Processable Silicon Naphthalocyanine Tetraimides as near Infrared Electron Acceptors in Organic Solar Cells. Dye. Pigment. 2022, 197, 109846. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Diz, V.E.; Awruch, J.; Dicelio, L.E. Photophysics of Zinc (II) Phthalocyanine Polymer and Gel Formulation. Photochem. Photobiol. 2010, 86, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2012, 2013, e676815. [Google Scholar] [CrossRef]
- Basova, T.V.; Polyakov, M.S. Hybrid Materials Based on Carbon Nanotubes and Polyaromatic Molecules: Methods of Functionalization and Sensor Properties. MHC 2020, 13, 91–112. [Google Scholar] [CrossRef]
- Hosu, I.S.; Wang, Q.; Vasilescu, A.; Peteu, S.F.; Raditoiu, V.; Railian, S.; Zaitsev, V.; Turcheniuk, K.; Wang, Q.; Li, M.; et al. Cobalt Phthalocyanine Tetracarboxylic Acid Modified Reduced Graphene Oxide: A Sensitive Matrix for the Electrocatalytic Detection of Peroxynitrite and Hydrogen Peroxide. RSC Adv. 2014, 5, 1474–1484. [Google Scholar] [CrossRef]
- Wang, H.; Bu, Y.; Dai, W.; Li, K.; Wang, H.; Zuo, X. Well-Dispersed Cobalt Phthalocyanine Nanorods on Graphene for the Electrochemical Detection of Hydrogen Peroxide and Glucose Sensing. Sens. Actuators B Chem. 2015, 216, 298–306. [Google Scholar] [CrossRef]
- Mashazi, P.; Mugadza, T.; Sosibo, N.; Mdluli, P.; Vilakazi, S.; Nyokong, T. The Effects of Carbon Nanotubes on the Electrocatalysis of Hydrogen Peroxide by Metallo-Phthalocyanines. Talanta 2011, 85, 2202–2211. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Piskorz, J.; Popenda, L.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. Improved Electrocatalytic Response toward Hydrogen Peroxide Reduction of Sulfanyl Porphyrazine/Multiwalled Carbon Nanotube Hybrids Deposited on Glassy Carbon Electrodes. Dye. Pigment. 2016, 134, 569–579. [Google Scholar] [CrossRef]
- Falkowski, M.; Kucinska, M.; Piskorz, J.; Wieczorek-Szweda, E.; Popenda, L.; Jurga, S.; Sikora, A.; Mlynarczyk, D.T.; Murias, M.; Marszall, M.P.; et al. Synthesis of Sulfanyl Porphyrazines with Bulky Peripheral Substituents—Evaluation of Their Photochemical Properties and Biological Activity. J. Photochem. Photobiol. A Chem. 2021, 405, 112964. [Google Scholar] [CrossRef]
- Nar, I.; Atsay, A.; Karaoğlu, H.P.; Altındal, A.; Hamuryudan, E. π-Extended Hexadeca-Substituted Cobalt Phthalocyanine as an Active Layer for Organic Field-Effect Transistors. Dalton Trans. 2018, 47, 15017–15023. [Google Scholar] [CrossRef]
- Sevim, A.M.; Arıkan, S.; Koca, A.; Gül, A. Synthesis and Spectroelectrochemistry of New Phthalocyanines with Ester Functionalities. Dye. Pigment. 2012, 92, 1114–1121. [Google Scholar] [CrossRef]
- Tuncer, S.; Koca, A.; Gül, A.; Avcıata, U. Synthesis, Characterization, Electrochemistry and Spectroelectrochemistry of Novel Soluble Porphyrazines Bearing Unsaturated Functional Groups. Dye. Pigment. 2012, 92, 610–618. [Google Scholar] [CrossRef]
- Nas, A.; Kantekin, H.; Koca, A. Novel 4-(2-(Benzo[d]Thiazol-2-Yl)Phenoxy) Substituted Phthalocyanine Derivatives: Synthesis, Electrochemical and in Situ Spectroelectrochemical Characterization. J. Organomet. Chem. 2014, 757, 62–71. [Google Scholar] [CrossRef]
- Koca, A.; Gonca, E.; Gül, A. Voltammetric and Spectroelectrochemical Characterization of Porphyrazines: Electrochemical Metal Sensor. J. Electroanal. Chem. 2008, 612, 231–240. [Google Scholar] [CrossRef]
- Adebayo, A.I.; Nyokong, T. Synthesis, Spectroscopic and Electrochemical Properties of Manganese, Nickel and Iron Octakis-(2-Diethylaminoethanethiol)-Phthalocyanine. Polyhedron 2009, 28, 2831–2838. [Google Scholar] [CrossRef]
- Tuncer, S.; Koca, A.; Gül, A.; Avcıata, U. 1,4-Dithiaheterocycle-Fused Porphyrazines: Synthesis, Characterization, Voltammetric and Spectroelectrochemical Properties. Dye. Pigment. 2009, 81, 144–151. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Kobak, R.Z.U.; Barut, B.; Bayrak, R.; Koca, A.; Kantekin, H. Synthesis and Electrochemical Characterization of Tetra-(5-Chloro-2-(2,4-Dichlorophenoxy)Phenol) Substituted Ni(II), Fe(II) and Cu(II) Metallophthalocyanines. Synth. Met. 2016, 215, 7–13. [Google Scholar] [CrossRef]
- Farnia, G.; Romanin, A.; Capobianco, G.; Torzo, F. Electrochemical Reduction Mechanism of Phthalimide and Some of Its N-Derivatives in DMF. J. Electroanal. Chem. Interfacial Electrochem. 1971, 33, 31–44. [Google Scholar] [CrossRef]
- Morozan, A.; Campidelli, S.; Filoramo, A.; Jousselme, B.; Palacin, S. Catalytic Activity of Cobalt and Iron Phthalocyanines or Porphyrins Supported on Different Carbon Nanotubes towards Oxygen Reduction Reaction. Carbon 2011, 49, 4839–4847. [Google Scholar] [CrossRef]
- Coates, M.; Nyokong, T. Characterization of Glassy Carbon Electrodes Modified with Carbon Nanotubes and Iron Phthalocyanine through Grafting and Click Chemistry. Electrochim. Acta 2013, 91, 158–165. [Google Scholar] [CrossRef]
- Wu, C.; Sun, H.; Li, Y.; Liu, X.; Du, X.; Wang, X.; Xu, P. Biosensor Based on Glucose Oxidase-Nanoporous Gold Co-Catalysis for Glucose Detection. Biosens. Bioelectron. 2015, 66, 350–355. [Google Scholar] [CrossRef]
- Welch, C.M.; Banks, C.E.; Simm, A.O.; Compton, R.G. Silver Nanoparticle Assemblies Supported on Glassy-Carbon Electrodes for the Electro-Analytical Detection of Hydrogen Peroxide. Anal. Bioanal. Chem. 2005, 382, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Achraf Ben Njima, M.; Legrand, L. Ag Nanoparticles-Oxidized Green Rust Nanohybrids for Novel and Efficient Non-Enzymatic H2O2 Electrochemical Sensor. J. Electroanal. Chem. 2022, 906, 116015. [Google Scholar] [CrossRef]
- Paulraj, P.; Umar, A.; Rajendran, K.; Manikandan, A.; Kumar, R.; Manikandan, E.; Pandian, K.; Mahnashi, M.H.; Alsaiari, M.A.; Ibrahim, A.A.; et al. Solid-State Synthesis of Ag-Doped PANI Nanocomposites for Their End-Use as an Electrochemical Sensor for Hydrogen Peroxide and Dopamine. Electrochim. Acta 2020, 363, 137158. [Google Scholar] [CrossRef]
- Ning, R.; Lu, W.; Zhang, Y.; Qin, X.; Luo, Y.; Hu, J.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. A Novel Strategy to Synthesize Au Nanoplates and Their Application for Enzymeless H2O2 Detection. Electrochim. Acta 2012, 60, 13–16. [Google Scholar] [CrossRef]
- Bo, X.; Bai, J.; Ju, J.; Guo, L. A Sensitive Amperometric Sensor for Hydrazine and Hydrogen Peroxide Based on Palladium Nanoparticles/Onion-like Mesoporous Carbon Vesicle. Anal. Chim. Acta 2010, 675, 29–35. [Google Scholar] [CrossRef]
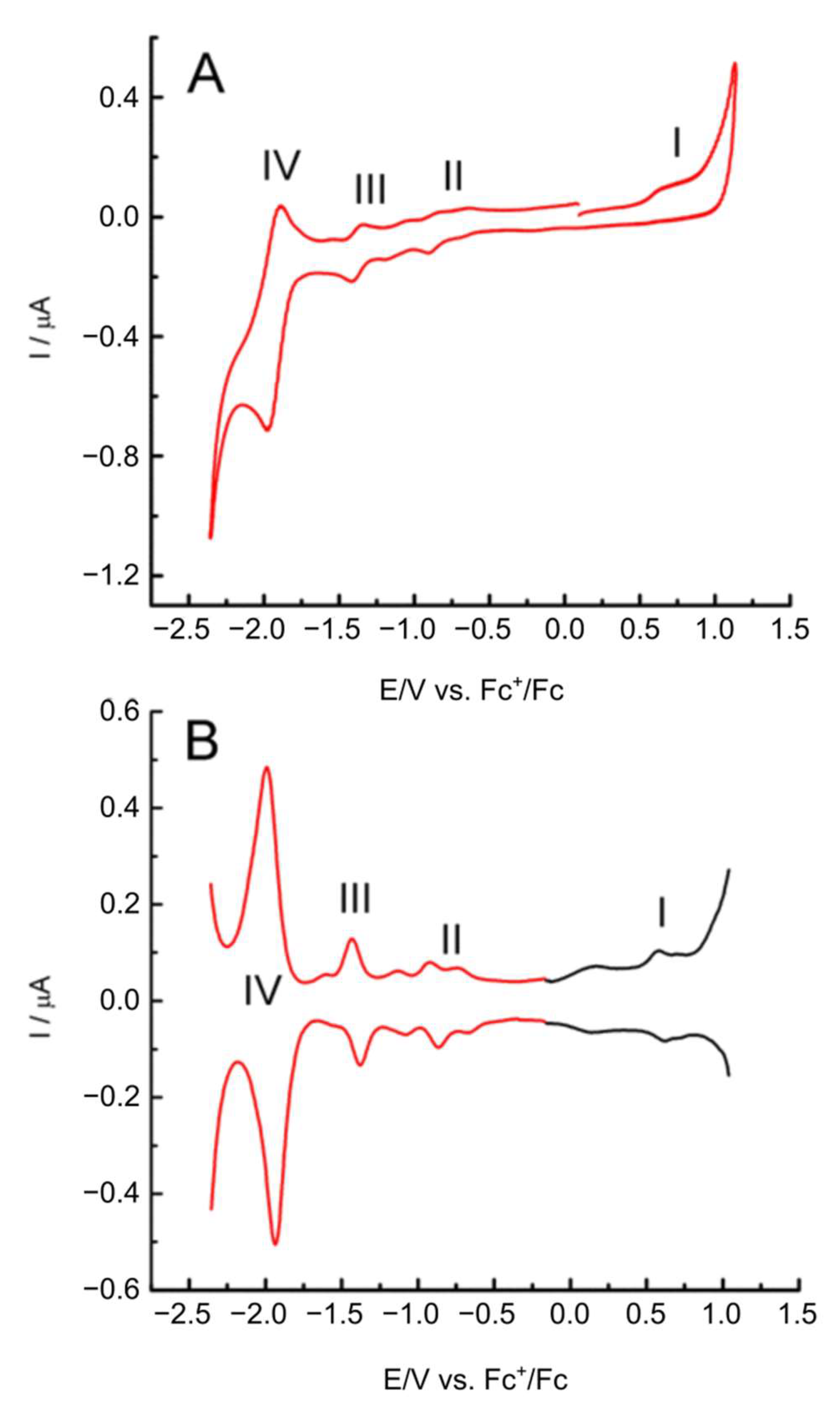


| Compound | E1/2 I/V | E1/2 II/V | E1/2 III/V | E1/2 IV/V |
|---|---|---|---|---|
| Pz1 (metal-free) | 0.90 | −0.89 | −1.13 | −1.98 |
| Pz2 (Mg) | 0.63 | −1.14 | −1.40 | −1.99 |
| Pz3 (Fe) | 0.61 | −0.82/−0.62 * | −1.37 | −1.96 |
| Pz4 (Co) | 0.63 | −0.62 | −1.50 | −1.96 |
| Electrode | Sensitivity/μA mM−1 cm−2 | LOD/μM | Linear Range/mM | Ref. |
|---|---|---|---|---|
| MWCNT/LS/NAg | 252 | 1.17 | 6–486 | [12] |
| AgNPs/GC | 169 | 2.0 | 5–50 | [52] |
| Ag-exGRc-CI/SS | 115 | 5.0 | 100–8000 | [53] |
| PANI-Ag/GCE | - | 0.23 | 10–90 | [54] |
| AuP/GCE | - | 4 | 100–50,000 | [55] |
| Pd/MCV/Nafion/GC | - | 0.079 | 0.1–6100 | [56] |
| GC/MWCNT/Pz3 | 636 | 0.20 | 1–90 | This work |
| GC/MWCNT/Pz4 | 640 | 0.18 | 1–90 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falkowski, M.; Leda, A.; Rebis, T.; Piskorz, J.; Popenda, L.; Hassani, M.; Mlynarczyk, D.T.; Marszall, M.P.; Milczarek, G. A Synergistic Effect of Phthalimide-Substituted Sulfanyl Porphyrazines and Carbon Nanotubes to Improve the Electrocatalytic Detection of Hydrogen Peroxide. Molecules 2022, 27, 4409. https://doi.org/10.3390/molecules27144409
Falkowski M, Leda A, Rebis T, Piskorz J, Popenda L, Hassani M, Mlynarczyk DT, Marszall MP, Milczarek G. A Synergistic Effect of Phthalimide-Substituted Sulfanyl Porphyrazines and Carbon Nanotubes to Improve the Electrocatalytic Detection of Hydrogen Peroxide. Molecules. 2022; 27(14):4409. https://doi.org/10.3390/molecules27144409
Chicago/Turabian StyleFalkowski, Michal, Amanda Leda, Tomasz Rebis, Jaroslaw Piskorz, Lukasz Popenda, Mina Hassani, Dariusz T. Mlynarczyk, Michal P. Marszall, and Grzegorz Milczarek. 2022. "A Synergistic Effect of Phthalimide-Substituted Sulfanyl Porphyrazines and Carbon Nanotubes to Improve the Electrocatalytic Detection of Hydrogen Peroxide" Molecules 27, no. 14: 4409. https://doi.org/10.3390/molecules27144409
APA StyleFalkowski, M., Leda, A., Rebis, T., Piskorz, J., Popenda, L., Hassani, M., Mlynarczyk, D. T., Marszall, M. P., & Milczarek, G. (2022). A Synergistic Effect of Phthalimide-Substituted Sulfanyl Porphyrazines and Carbon Nanotubes to Improve the Electrocatalytic Detection of Hydrogen Peroxide. Molecules, 27(14), 4409. https://doi.org/10.3390/molecules27144409






