Abstract
This study aimed to determine the in vitro cytotoxicity and understand possible cytotoxic mechanisms via an in silico study of eleven chalcones synthesized from two acetophenones. Five were synthesized from a prenylacetophenone isolated from a plant that grows in the Andean region of the Atacama Desert. The cytotoxic activity of all the synthesized chalcones was tested against breast cancer cell lines using an MTT cell proliferation assay. The results suggest that the prenyl group in the A-ring of the methoxy and hydroxyl substituents of the B-ring appear to be crucial for the cytotoxicity of these compounds. The chalcones 12 and 13 showed significant inhibitory effects against growth in MCF-7 cells (IC50 4.19 ± 1.04 µM and IC50 3.30 ± 0.92 µM), ZR-75-1 cells (IC50 9.40 ± 1.74 µM and IC50 8.75 ± 2.01µM), and MDA-MB-231 cells (IC50 6.12 ± 0.84 µM and IC50 18.10 ± 1.65 µM). Moreover, these chalcones showed differential activity between MCF-10F (IC50 95.76 ± 1.52 µM and IC50 95.11 ± 1.97 µM, respectively) and the tumor lines. The in vitro results agree with molecular coupling results, whose affinity energies and binding mode agree with the most active compounds. Thus, compounds 12 and 13 can be considered for further studies and are candidates for developing new antitumor agents. In conclusion, these observations give rise to a new hypothesis for designing chalcones with potential cytotoxicity with high potential for the pharmaceutical industry.
1. Introduction
Despite substantial advances in early detection and treatment, breast cancer is a critical global public health problem, the second leading cause of death in women [1,2]. Furthermore, chemotherapeutic agents traditionally used to treat cancer have high toxicity, multidrug resistance, and lack of selectivity, limiting their effectiveness [3,4]. So, the search and development of new drugs play an essential role in cancer control. Ideally, any anticancer drug should exert a cytotoxic effect on malignant cells with a minimal impact on normal cells. Most drugs developed to combat cancer are of natural origin or inspired by them [5]. The compounds isolated from natural sources have a relevant role in developing new palliative therapies.
In this context, chalcones and their derivatives maintain constant interest among scientists due to their broad spectrum of pharmacological activities, such as anticancer properties [6,7], among others. Chalcones, in their structure, have two aromatic rings linked by a three-carbon α, β-unsaturated carbonyl system and are fundamental, intermediate compounds in the biosynthesis of flavonoids and isoflavonoids in plants. Synthetic and natural chalcones are considered pharmacologically important compounds [8]. Numerous chalcones have been isolated from natural sources and have been shown to affect each level of carcinogenesis and exhibit activity against cancer cells (Figure 1). Amor et al. isolated the compound 2,4-dihydroxy-6-methoxy-3,5-dimethylchalcone from Syzygium samarangense (Myrtaceae) and determined that this compound has antiproliferative activity in MCF-7 and SKBR-3 [9]. Other naturally occurring compounds that show antiproliferative activity in cancer cell lines (SW-480) are estercurensin and cardamomine [10]. Chalcone-type compounds, such as xanthohumol, a compound isolated from Humulus lupulus (Cannabaceae), have growth-inhibitory effects on prostate (PC-3 and DU-145) and cervical (HeLa) cancer cells [11,12]. Other chalcones that exhibit antiproliferative activity in cancer cells are isoliquiritigenin and 2′-O-methyl isoliquiritigenin [13]. In this context, natural products have relatively high safety profiles. However, in most cases, the cytotoxic effect, chemical stability, oral bioavailability, and metabolic stability of natural compounds are far from synthetic compounds.
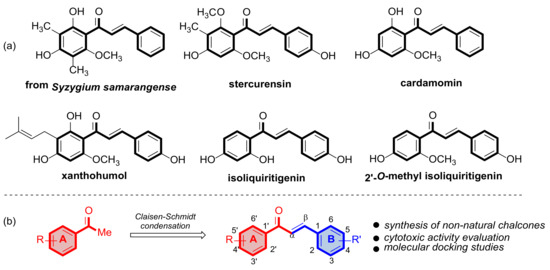
Figure 1.
(a) Chalcones of natural origin with antiproliferative activity in various cancer lines. (b) Strategy to synthesize chalcones using a Claisen–Schmidt reaction.
Given the need for new drugs with high selectivity, low toxicity, and good metabolic stability against breast cancer, prenylated acetophenone 1b, isolated from a plant that grows in the Andean region of the Atacama Desert, was used as starting material to obtain chalcones. This prenylated acetophenone has demonstrated good cytotoxic activity [14], suggesting that synthesized chalcones based on this scaffold could potentially have better activity and selectivity than their precursor. As far as we know, there is no information on the cytotoxic activity of chalcones that contain 1b in their A ring in their structure. Therefore, this study aims to determine the in vitro cytotoxicity and understand the possible cytotoxic mechanisms through in vitro and in silico study from chalcones synthesized from a naturally occurring acetophenone.
2. Results and Discussion
2.1. Cytotoxic Activity of Chalcone Compounds against Breast Cancer
This study continues the search for biologically active compounds from a naturally occurring prenyl acetophenone [15,16]. However, to expand our knowledge about chalcones and how their substituents influence their bioactivity, we will use 11 compounds that we have previously synthesized from readily available starting materials (Scheme 1).
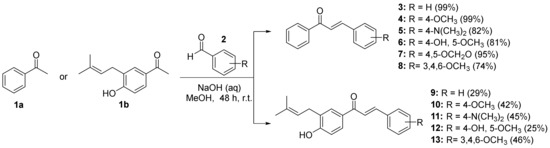
Scheme 1.
Synthesis of chalcone compounds via Claisen–Schmidt condensation.
The cytotoxic activity of all the synthesized chalcones was tested against breast cancer cell lines using an MTT cell proliferation assay. All compounds were tested in vitro at 10 and 100 µM using the cell viability assay in MCF-7 for 24 h in normoxia (Table 1). Firstly, the cytotoxic effect of the chalcone compounds modified in ring B and with an unsubstituted ring A was tested (compounds 3–8). As shown in Table 1, compounds 5, 6, and 7 were the most interesting, with a cell viability percentage between 47–42% in the treatment at 10 µM in MCF-7. Secondly, compounds with modifications in the A and B rings (9–13) were tested. The substitution of the A-ring with a hydroxyl group at C-4′ and a prenyl group at C-5′ shows a potentiating effect on cytotoxic activity compared to unsubstituted chalcones in the A-ring. Furthermore, it was observed that the substitution with a methoxy group at C-5 in the B-ring was beneficial for cytotoxic effects, as indicated by percentage values of 3 (81.3%) vs. 4 (74.5%) and 9 (93.8%) vs. 10 (73.7%).

Table 1.
10 and 100 µM treatments in MCF-7 cells with the synthesized chalcones.
Compound 12 bearing a methoxy group at C-5′ and hydroxyl at C-4 of the B-ring showed a greater cellular growth inhibition effect (10.3%). However, the results also suggested that compounds bearing a nitrigen (such as –N(Me)2; 42.0%) at the C-4 site of the B-ring could be of interest [17]. Moreover, we also found that the introduction of methoxy groups on B-ring in C-3, C-4, and C-6 sites decreased cell viability (13). Because of the results, we considered changing the hydroxy group of the A-ring for a methoxy group to evaluate the activity. However, all the synthesized compounds did not show relevant cytotoxic activity. According to the result in Figure 2, the compounds that exhibited more promising cytotoxicity in MCF-7 cells (cell viability ≤ 50%) are marked with an asterisk.
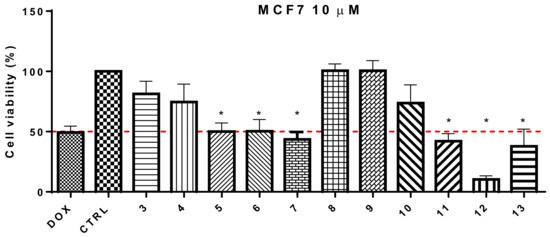
Figure 2.
Cell viability of the synthesized chalcones with 10 µM treatments in MCF-7 cells. *—cell viability ≤ 50%.
Table 2 shows IC50 of the most active chalcones in different breast cancer lines. Compounds 5 and 7 do not show significant activity relative to the other compounds. The compound 6 (21.55 ± 2.71 µM) was more active than the starting material 1b (79.40 ± 12.25 µM), and it is recognized for its cytotoxic activity in cells MCF-7 [14]. Moreover, this chalcone showed slight differential activity between MCF-10F (72.60 ± 10.31 µM) and the tumor lines. However, the prenylated chalcones were most active in all breast cancer lines than chalcones without this substituent. The compounds 12 and 13 possess a similar activity in the MCF-7 cells (4.19 ± 1.04 µM and 3.30 ± 0.92 µM) and ZR-75-1 cells (9.40 ± 1.74 µM and 8.75 ± 2.01 µM), but the MDA-MB-231 cells show slightly different inactivity (6.12 ± 0.84 µM and 18.10 ± 1.65 µM). Compounds 12 and 13 presented IC50 values that make them highly cytotoxic compounds against all cell lines with a differential effect.

Table 2.
IC50 of synthesized chalcones in different lines of breast cancer.
Among the tested chalcones, the hydroxyl group in ring A is essential for cytotoxicity, as has been studied in various biological assays, predominantly for its anticancer properties [18,19]. The presence of electron-withdrawing and donor groups affects the α,β-unsaturated system [18,20], which in turn affects cytotoxicity. Additionally, the prenyl group increases lipophilicity, improves membrane binding, and increases transmembrane transport, and these characteristics favor increased biological activity [12,21]. Besides, this study observed the speed with which the compounds act on cells, causing their death (see Figure 3).
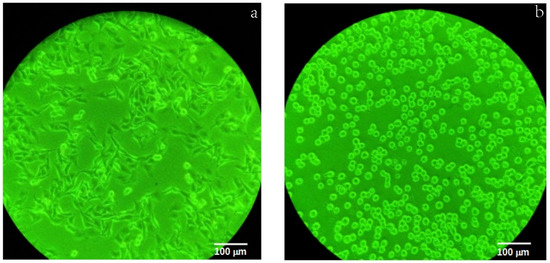
Figure 3.
Change in the morphology of MDA-MB-231 cells at (a) 0 h and (b) after 6 h of incubation after addition of compound 6.
On the other hand, compounds 6 and 12 share the B-ring’s structure, similar to that found in ferulic acid. This substitution could increase biological activity since this acid decreases cell viability, induces apoptosis, and suppresses potential metastasis in MDA-MB-231 cells [22]. Moreover, the trimethoxyphenyl substitution presented by chalcone 13 improves the cytotoxic activity on all cells except on MDA-MB-231 compared to compound 12, so that the portion derived from ferulic acid would have a better effect on the activity. Chalcones 12 and 13 presented IC50 values higher than those reported by the chalcone compounds synthesized by Abosalim et al. as possible anticancer agents [23]. Furthermore, these chalcones were more active in the MCF-7 and MDA-MB231 cell lines than the compounds synthesized by Patel et al. [24]. Moreover, the chalcones selected in this work showed significant cytotoxicity against these cancer cell lines with an IC50 between 4 to 3 μM on the growth of the MCF-7 cells, which resulted in better results than Paratocarpin E for the same cell lines (19.6 μM) [25].
2.2. Understanding the Possible Cytotoxic Mechanism, an In Silico Study
Ng et al. correlated the cytotoxic activity of a series of chalcones with dihydrofolate reductase (DHFR) [26,27]. Based on that evidence, molecular docking was performed at the catalytic site of this enzyme with compounds 6, 12, and 13. The results in Table 3 show an affinity trend for DHFR similar to the inhibition trend shown in Table 2. We can see these effects in the binding energy (∆G Bind), where compound 13 is the most active with −59.16 kcal/mol, compound 12 with −54.66 kcal/mol, and compound 6 with −51.29 kcal/mol. All three compounds have indispensable chalcone A-ring interactions such as hydrogen bonding with Ala9 and carbonyl group, hydrophobic interactions with Leu22, and π-stacking between phenone group and Phe34 residue.

Table 3.
Binding energies of the synthesized compounds.
Compound 6 has better ∆G Coulomb (−21.50 kcal/mol) due to the interaction with the hydroxyl (phenyl B) with residues Gly20 and Ser188; in addition, there is a second hydrogen bond with Thr56 and the methoxyl (Figure 4A). Compound 12 has higher hydrogen bonding energy (∆G HBond −2.04 kcal/mol) and more significant lipophilic (∆G Lipo −23.80 kcal/mol) and van der Waals (∆G vdW −48.46 kcal/mol) characteristics; this can be explained by the 3-methylbutenyl (prenyl group) radical interacting favorably with residues Leu67 (Figure 4B). These same characteristics can be seen in compound 13, where the difference in binding energy is due to the methoxyl substituents of the chalcone B-ring, which causes ligand displacement, enhancing hydrophobic interactions with the Leu67 and Thr56 residues (Figure 4C); we can see this in the ∆G Lipo energy of −27.34 kcal/mol and the ∆G vdW energy of −53.45 kcal/mol, which are the highest compared to the others.
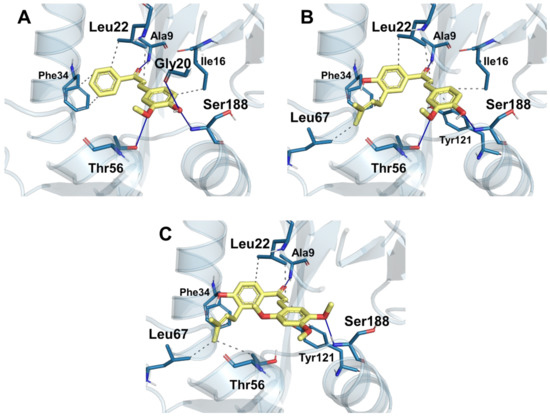
Figure 4.
Interactions of the synthesized chalcones with the catalytic site of DHFR. (A) Binding mode of compound 6 and most residues represented. (B) Binding mode of compound 12 and most residues represented. (C) Binding mode of compound 13 and most residues represented.
3. Materials and Methods
3.1. Chemistry
All reactions were carried out under anhydrous conditions in an argon atmosphere with dry, freshly distilled solvents. Analytical thin-layer chromatography was performed on SiO2 (Merck silica gel 60 F254), and the spots were located with 1% aqueous KMnO4. Chromatography, referring to flash chromatography, was carried out on SiO2 (SDS silica gel 60 ACC, 35–75 mm, 230–240 mesh ASTM). Drying of organic extracts during workup of reactions was performed over anhydrous MgSO4 except where stated otherwise. Evaporation of solvent was accomplished with a rotatory evaporator. NMR spectra were recorded in CDCl3 or MeOD on a Varian VNMRS 400 (Varian, Palo Alto, CA, USA). Chemical shifts of 1H and 13C NMR spectra are reported in ppm downfield (δ) from Me4Si. Electron-spray ionization mass spectra in positive mode (ESI-MS) data were recorded on a Bruker Esquire 3000 + spectrometer (Bruker Daltonics Inc., Billerica, MA, USA).
3.2. Synthesis of Chalcones
To the mixture of a commercial benzaldehyde (1.2 mmol), acetophenone 1a or 1b (1.22 and 2.08 mmol) in MeOH (5 mL), a NaOH saturated solution, was added (in 10 mL of methanol), and the mixture was stirred for 48 h at room temperature. After the disappearance of the reactant (TLC), 5% HCl solution was added until pH ~7 to end the reaction, the mixture was extracted with EtOAc (3 × 50 mL), and the combined organic layers were dried (anhydrous Na2SO4). Removal of the solvent and purification of the residue by column chromatography or recrystallization gave the target products.
3.3. Characterizacion Data
(E)-1-(4-hydroxy-3-(3-methylbut-2-en-1-yl)phenyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one (12). Yellow solid (25% yield). 1H NMR (400 MHz, MeOD) δ: 7.85 (1H, m, H6), 7.84 (1H, s, H2), 7.67 (1H, d, J = 15.5 Hz, Hβ), 7.56 (1H, d, J = 15.5 Hz, Hα), 7.18 (1H, dd, J = 8.1, 1.8 Hz, H6′), 6.85 (1H, d, J = 8.1 Hz, H5), 6.83 (1H, d, J = 8.1 Hz, H5′), 5.34 (1H, ddt, J = 9.1, 7.4, 1.3 Hz, H2″), 3.92 (3H, s, OCH3), 3.33 (2H, d, J = 7.4 Hz, CH2), 1.74 (3H, s, CH3), 1.73 (3H, s, CH3). 13C NMR (100 MHz, MeOD) δ: 191.0 (C=O), 161.6 (C4), 150.8 (C4′), 149.4 (C3′), 145.9 (Hβ), 133.7 (C3″), 131.7 (C2), 131.1 (C1), 129.9 (C6), 129.8 (C3) 128.5 (C1′), 124.7 (C6′), 123.4 (C2″), 120.0 (Cα), 116.5 (C5′), 115.5 (C5), 112.1 (C2′), 56.5 (OCH3), 29.2 (C1″), 26.0 (C4″), 17.9 (C5″). EI-MS: m/z 339.1556 [M+] (100).
3.4. Cell Culture
The cell culture methodology was used with modifications [14]. Three human breast cancer cell lines were used for this study: MCF-7 (ATCC® HTB-22™), ZR-75-1 (ATCC® CRL-1500™), MDA-MB-231 (ATCC® HTB-26™), and the non-tumorigenic MCF-10F (ATCC® CRL-10318™) cell lines. Cells were cultured in specific media according to ATCC recommendations. The incubation condition was established at 37 °C, a complete humid atmosphere, 5% CO2 and 95% O2.
3.5. Cytotoxic Assays
The cytotoxic effect of the chalcone compounds was assessed in MCF-10F cells in a dose- and time-dependent manner [14]. Cells (1 × 104 and 4 × 104) cultured under normoxic conditions were seeded in 24-well plates in quadruplicate and incubated for 4 days until 70% confluence. After this incubation period, cells were exposed to concentrations of the synthesized compounds dissolved in ethanol (50%) at concentrations ranging from 0 to 96.8 µg/mL (dissolved in 0.5% DMSO). Doxorubicin was used as the control drug, and for all data a p < 0.05 value was considered statistically significant. Cell viability was assessed using a neutral red uptake assay after 24 h of treatment.
3.6. Molecular Docking
Molecular docking studies were performed to understand the binding modes of the synthesized compounds to the dihydrofolate reductase protein using the Gilde software [28]. This program allows for determining the most suitable positions of the ligands in the binding site of interest of the protein, considering the total flexibility of the ligand [29].
The preparation for the protein (PDB ID: 4M6J; resolution 1.2 Å [30]) was minimized to a gradient of 0.01 kcal/mol Å. The default parameters in the Glide docking program were: 5000 poses per ligand for the initial docking phase; standard precision (SP) method; RMS deviation less than 5.0 Å; and maximal atomic displacement less than 1.3 Å. The binding site was defined as a cubic region (Grid) encompassing all protein atoms from the co-crystallized ligand (NADPH) within 15.0 Å. The three compounds’ structures were built using the 2D sketcher module of the Schrodinger Suite, and ligand preparation was performed using the LigPrep module [31]. For the five best poses achieved with the described procedure, Induced Fit Docking was performed in order to improve the interactions between the synthesized ligands and the receptor [32,33]; the same Grid parameters were maintained with a refinement of the residues at 6.0 Å distance from the ligand poses and a rescoring of the best poses achieved with the eXtra Precision (XP) method [34]. Finally, the ligand-binding energies and ligand strain energies for the chalcones were estimated using Prime MM-GBSA (Molecular Mechanics/Generalized Born Model and Solvent Accessibility), which includes the OPLS4 force field [35], VSGB solvent model [36,37], and rotamer search algorithms. The MM/GBSA method is used to calculate the relative binding affinity of ligands to the receptor (in kcal/mol). Because the MM/GBSA [38] binding energies are estimates of binding free energies, a lower number indicates greater binding. The energies obtained for the complexes were estimated automatically using the energy terms and equation systems presented in the following.
where ΔGbind represents the total binding free energy upon ligand–receptor binding; ΔEMM is the total gas phase energy in the molecular mechanics (MM) and force field (OPLS4); ΔGCoulomb, ΔGPacking, ΔGLipo, and ΔGvdW correspond, respectively, to the electrostatic, π-π packing correction, lipophilic and van der Waals energies; and ΔGSolvGB is the polar electrostatic solvation energy calculated via the generalized Born (GB) method.
3.7. Statistical Analysis
All data were analyzed using OriginPro 9.1 software packages (Originlab Corporation, Northampton, MA, USA). Significant difference comparisons were made by the Tukey test; the statistical significance was defined as p ≤ 0.05.
4. Conclusions
The results presented in this study suggest that the synthesized chalcones 12 and 13 showed significant inhibitory effects against the growth of human cancer cell lines in vitro. Both compounds showed differential activity between non-tumor and the tumor lines. Furthermore, the prenyl group appears to be crucial for the cytotoxicity of these compounds. This evidence coincides with the molecular docking results, whose affinity energies and binding mode agree with the most active compounds (compounds 12 and 13) due to enhanced binding of the methoxyl groups of the A-ring via hydrogen bond formation and enhanced hydrophobic interactions of the prenyl group with the amino acids Leu67 and Thr56. These observations give rise to a new hypothesis for designing new chalcones with potential antineoplastic activity. Therefore, these molecules based on the natural compound 1b scaffold represent good lead compounds in the search for new pharmaceuticals to fight breast cancer.
Author Contributions
Conceptualization, M.C., M.J.S. and M.M.; methodology, L.B.; A.C.-A. and C.P.; validation, C.E.-C., L.B. and M.C.; formal analysis, C.P. and M.M.; investigation, C.E.-C. and M.C.; resources, C.E.-C.; writing—original draft preparation, C.P., A.C.-A., B.B. and L.B.; writing—review and editing, B.B., M.C. and M.J.S.; supervision, M.C. and C.E.-C.; project administration, C.P.; funding acquisition, C.E.-C. and M.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID, grant number R15F10011. C.P. acknowledges Fondecyt 11190698. M.J.S. acknowledges Fondecyt 1220075. M.C. acknowledges Programa Formación de Capital Humano Avanzado 21130456. M.M. acknowledges Postdoctoral Fondecyt 3180408.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to express their gratitude to the Rectoría of Universidad de Tarapacá for their financial and administrative support.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Arya, G.C.; Kaur, K.; Jaitak, V. Isoxazole derivatives as anticancer agent: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2021, 221, 113511. [Google Scholar] [CrossRef] [PubMed]
- Moku, B.; Ravindar, L.; Rakesh, K.P.; Qin, H.L. The significance of N-methylpicolinamides in the development of anticancer therapeutics: Synthesis and structure-activity relationship (SAR) studies. Bioorg. Chem. 2019, 86, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.D.; Bernades, A.; Demetrius, G.; Noda-Perez, C.; Vieira, P.C.; Dos Santos, C.Y.; da Silva, J.A.; de Moraes, M.O.; Mousinho, K.C. Synthetic chalcone derivatives as inhibitors of cathepsins K and B, and their cytotoxic evaluation. Chem. Biodivers 2013, 10, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- von Schwarzenberg, K.; Vollmar, A.M. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013, 332, 295–303. [Google Scholar] [CrossRef]
- Kozlowska, J.; Potaniec, B.; Baczynska, D.; Zarowska, B.; Aniol, M. Synthesis and Biological Evaluation of Novel Aminochalcones as Potential Anticancer and Antimicrobial Agents. Molecules 2019, 24, 4129. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, R.; Kodwani, R.; Kapoor, S.; Khare, A.; Bansal, R.; Khurana, S.; Singh, S.; Thomas, J.; Roy, B.; et al. A Review on Mechanisms of Anti-Tumor Activity of Chalcones. Anticancer Agents Med. Chem. 2015, 16, 200–211. [Google Scholar] [CrossRef]
- Amor, E.C.; Villaseñor, I.M.; Antemano, R.; Perveen, Z.; Concepcion, G.P.; Choudhary, M. Cytotoxic C-Methylated Chalcones from Syzygium samarangense. Pharm. Biol. 2007, 45, 777–783. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Basile, M.J.; Gil, R.R.; Weinstein, I.B.; Kennelly, E.J. Cytotoxic chalcones and antioxidants from the fruits of a Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef]
- Ackerstaff, E.; Artemov, D.; Gillies, R.J.; Bhujwalla, Z.M. Hypoxia and the presence of human vascular endothelial cells affect prostate cancer cell invasion and metabolism. Neoplasia 2007, 9, 1138–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Echiburu-Chau, C.; Alfaro-Lira, S.; Brown, N.; Salas, C.O.; Cuellar, M.; Santander, J.; Ogalde, J.P.; Rothhammer, F. The selective cytotoxicity elicited by phytochemical extract from Senecio graveolens (Asteraceae) on breast cancer cells is enhanced by hypoxia. Int. J. Oncol. 2014, 44, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Espinoza, L.; Madrid, A.; Mella, J.; Chavez-Weisser, E.; Diaz, K.; Cuellar, M. Design, synthesis, antifungal activity, and structure-activity relationship studies of chalcones and hybrid dihydrochromane-chalcones. Mol. Divers 2020, 24, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Salas, C.O.; Uriarte, E.; Viña, D.; Jara-Gutiérrez, C.; Matos, M.J.; Cuellar, M. Design, synthesis and docking calculations of prenylated chalcones as selective monoamine oxidase B inhibitors with antioxidant activity. Chem. Select 2019, 4, 7698–7703. [Google Scholar] [CrossRef]
- Dos Santos, M.B.; Bertholin Anselmo, D.; de Oliveira, J.G.; Jardim-Perassi, B.V.; Alves Monteiro, D.; Silva, G.; Gomes, E.; Lucia Fachin, A.; Marins, M.; de Campos Zuccari, D.A.P.; et al. Antiproliferative activity and p53 upregulation effects of chalcones on human breast cancer cells. J. Enzyme Inhib. Med. Chem. 2019, 34, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.W.; Yaeghoobi, M.; Abd-Rahman, N.; Kang, Y.B.; Pichika, M.R. Chalcones with electron-withdrawing and electron-donating substituents: Anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur. J. Med. Chem. 2014, 77, 378–387. [Google Scholar] [CrossRef]
- Pouget, C.; Lauthier, F.; Simon, A.; Fagnere, C.; Basly, J.P.; Delage, C.; Chulia, A.J. Flavonoids: Structural requirements for antiproliferative activity on breast cancer cells. Bioorg. Med. Chem. Lett. 2001, 11, 3095–3097. [Google Scholar] [CrossRef]
- Jin, F.; Jin, X.Y.; Jin, Y.L.; Sohn, D.W.; Kim, S.A.; Sohn, D.H.; Kim, Y.C.; Kim, H.S. Structural requirements of 2′,4′,6′-tris(methoxymethoxy) chalcone derivatives for anti-inflammatory activity: The importance of a 2′-hydroxy moiety. Arch. Pharm. Res. 2007, 30, 1359–1367. [Google Scholar] [CrossRef]
- Shen, G.; Huhman, D.; Lei, Z.; Snyder, J.; Sumner, L.W.; Dixon, R.A. Characterization of an isoflavonoid-specific prenyltransferase from Lupinus albus. Plant Physiol. 2012, 159, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Abosalim, H.M.; Nael, M.A.; El-Moselhy, T.F. Design, synthesis and molecular docking of chalcone derivatives as potential anticancer agents. Chem. Select 2021, 6, 888–895. [Google Scholar] [CrossRef]
- Patel, K.; Karthikeyan, C.; Raja Solomon, V.; Shari Narayana Moorthy, N.; Lee, H.; Sahu, K.; Singh Deora, G.; Trivedi, P. Synthesis of some coumarinyl chalcones and their antiproliferative activity against breast cancer cell lines. Lett. Drug Des. Discov. 2011, 8, 308–311. [Google Scholar] [CrossRef]
- Gao, S.; Sun, D.; Wang, G.; Zhang, J.; Jiang, Y.; Li, G.; Zhang, K.; Wang, L.; Huang, J.; Chen, L. Growth inhibitory effect of paratocarpin E, a prenylated chalcone isolated from Euphorbia humifusa Wild., by induction of autophagy and apoptosis in human breast cancer cells. Bioorg. Chem. 2016, 69, 121–128. [Google Scholar] [CrossRef]
- Ng, H.L.; Chen, S.; Chew, E.H.; Chui, W.K. Applying the designed multiple ligands approach to inhibit dihydrofolate reductase and thioredoxin reductase for anti-proliferative activity. Eur. J. Med. Chem. 2016, 115, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.L.; Ma, X.; Chew, E.H.; Chui, W.K. Design, Synthesis, and Biological Evaluation of Coupled Bioactive Scaffolds as Potential Anticancer Agents for Dual Targeting of Dihydrofolate Reductase and Thioredoxin Reductase. J. Med. Chem. 2017, 60, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef]
- Bhabha, G.; Ekiert, D.C.; Jennewein, M.; Zmasek, C.M.; Tuttle, L.M.; Kroon, G.; Dyson, H.J.; Godzik, A.; Wilson, I.A.; Wright, P.E. Divergent evolution of protein conformational dynamics in dihydrofolate reductase. Nat. Struct. Mol. Biol. 2013, 20, 1243–1249. [Google Scholar] [CrossRef]
- Chen, I.J.; Foloppe, N. Drug-like bioactive structures and conformational coverage with the LigPrep/ConfGen suite: Comparison to programs MOE and catalyst. J. Chem. Inf. Model. 2010, 50, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Lee, M.C.; Yang, R.; Duan, Y. Comparison between Generalized-Born and Poisson-Boltzmann methods in physics-based scoring functions for protein structure prediction. J. Mol. Model. 2005, 12, 101–110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).