Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology
Abstract
:1. Introduction
2. Results and Discussion
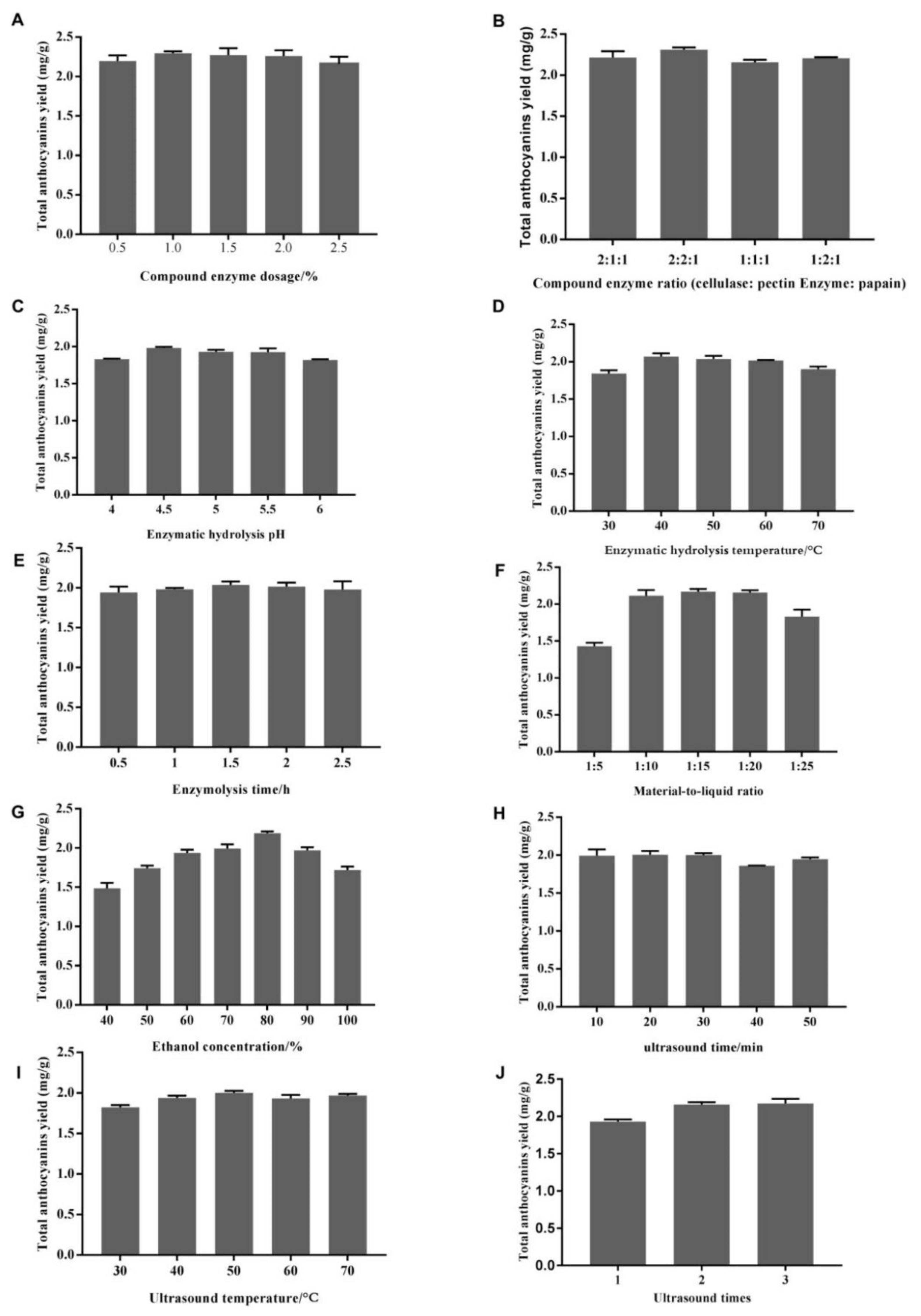
2.1. Single-Factor Tests of Total Anthocyanin Extraction
2.2. Screening of Significant Variables Using the Plackett-Burman Design (PBD)
2.3. Optimization by BBD
0.02 X1 X4 − 0.09 X2 X3 + 0.048 X2 X4 + 0.061 X3 X4 − 0.2 X12 − 0.056 X22
− 0.06 X32 − 0.14 X42
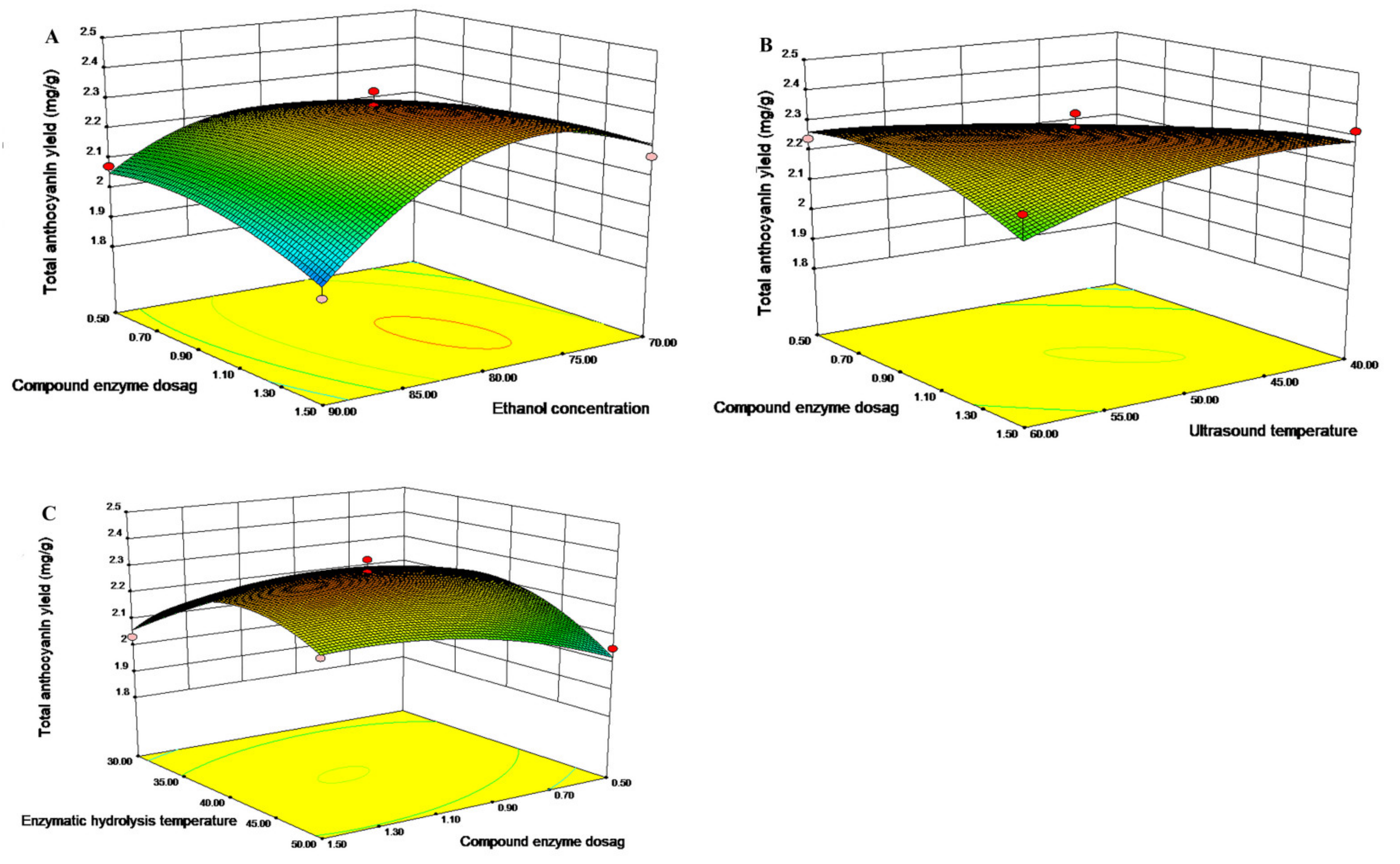
2.4. Analysis Contour and Response Surface
2.5. Model Optimization and Verification
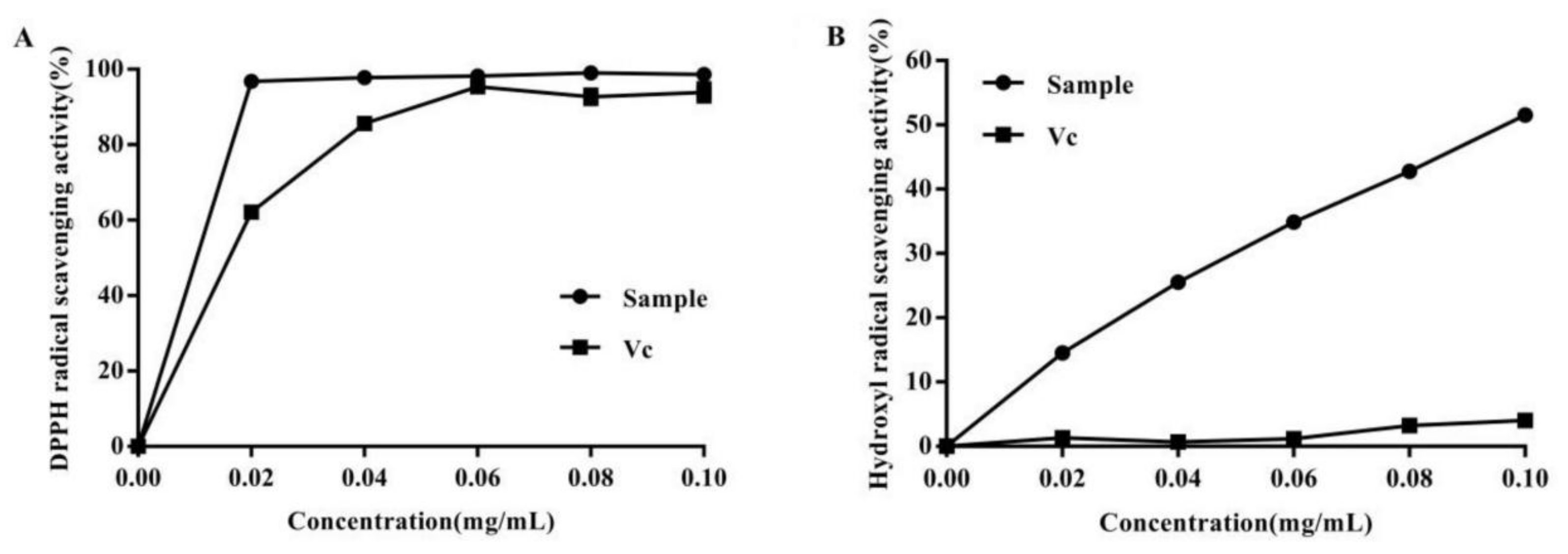
2.6. Antioxidant Activities of the Extracted Anthocyanins
2.7. Identification of Anthocyanins by UPLC-MS
3. Materials and Methods
3.1. Plants
3.2. Ultrasound-Assisted Enzymatic Extraction of Total Anthocyanins
3.3. Total Anthocyanin Level Assessment
3.4. Single-Factor Experiments
3.5. Plackett-Burman Design (PBD)
3.6. Box-Behnken Design (BBD)
3.7. Antioxidant Activity Assessment
3.7.1. DPPH Radical Scavenging Assay
3.7.2. Hydroxyl Radical Scavenging Capacity
3.8. Purifcation of Anthocyanins
3.9. Identification of Anthocyanins by HPLC-MS
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.O.; Hwang, S.J.; Bae, C.S.; Park, S.H.; Heo, B.G.; Gorinstein, S. Extraction and characterization of some natural plant pigments. Ind. Crops Prod. 2012, 40, 129–135. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef]
- Musilova, J.; Lidikova, J.; Vollmannova, A.; Frankova, H.; Urminska, D.; Bojnanska, T.; Toth, T. Influence of heat treatments on the content of bioactive substances and antioxidant properties of sweet potato (Ipomoea batatas L.) tubers. J. Food Quality 2020, 2020, 8856260. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Tao, W.; Han, Q.; Dong, H.; Zhang, J.; Jing, Y.; Wang, Y.; Xiong, Q.; Xu, T. An effective method for extracting anthocyanins from blueberry based on freeze-ultrasonic thawing technology. Ultrason. Sonochem. 2020, 68, 105192. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, H.; Zhao, Y.; Jiao, R.; Lei, L.; Chen, J.; Wang, X.; Zhang, Z.; Huang, Y.; Wang, T.; et al. Cranberry anthocyanin as an herbal medicine lowers plasma cholesterol by increasing excretion of fecal sterols. Phytomedicine 2018, 38, 98–106. [Google Scholar] [CrossRef]
- Jezek, M.; Zorb, C.; Merkt, N.; Geilfus, C.M. Anthocyanin management in fruits by fertilization. J. Agric. Food Chem. 2018, 66, 753–764. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.L.; Zhou, Q. Targets and mechanisms of dietary anthocyanins to combat hyperglycemia and hyperuricemia: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1119–1143. [Google Scholar] [CrossRef]
- Wong, P.Y.; Tan, S.T. Comparison of total phenolic content and antioxidant activities in selected coloured plants. Br. Food J. 2020, 122, 3193–3201. [Google Scholar] [CrossRef]
- Kim, H.J.; Koo, K.A.; Park, W.S.; Kang, D.M.; Kim, H.S.; Lee, B.Y.; Goo, Y.M.; Kim, J.H.; Lee, M.K.; Woo, D.K.; et al. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.; Liu, J.; Zhang, X.; Wu, X.; Tang, S.; Sun, R.; Qian, C.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zeng, M.; Chen, J.; Jiao, Y.; Niu, F.; Tao, G.; Zhang, S.; Qin, F.; He, Z. Identification and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in China by UPLC-PDA and UPLC-QTOF-MS/MS. J. Agric. Food Chem. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Truong, V.D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 404–410. [Google Scholar] [CrossRef]
- Ding, F.; Yu, J.L.; Yu, H.K.Z.; Chen, N.W.; Zou, X.; Liu, L.F. Breeding and industrialization of high anthocyanin sweet potato variety Mianzishu 9. Hubei Agric. Sci. 2017, 56, 2613–2615. [Google Scholar]
- Zevallos, L.; Caldas, C.; Flores, A.; Obregón, J.; Miano, A.C.; Barraza-Jáuregui, G. Mixing design for optimizing ultrasound-assisted extraction of phenolic components and anthocyanins from blue berries and grape marc. Int. J. Fruit Sci. 2020, 20 (Suppl. S3), S1313–S1327. [Google Scholar] [CrossRef]
- Lao, F.; Cheng, H.; Wang, Q.; Wang, X.; Liao, X.; Xu, Z. Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: Comparison to traditional aqueous and ethanolic extraction. J. CO2 Util. 2020, 40, 101188. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [Green Version]
- Ly Thi Phi, T.; Choi, Y.S.; Bae, H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crops Prod. 2018, 125, 261–268. [Google Scholar]
- Zhou, C.; Mei, X.; Rothenberg, D.O.N.; Yang, Z.; Zhang, W.; Wan, S.; Yang, H.; Zhang, L. Metabolome and transcriptome analysis reveals putative genes involved in anthocyanin accumulation and coloration in white and pink tea (Camellia sinensis) flower. Molecules 2020, 25, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paladines-Quezada, D.F.; Fernandez-Fernandez, J.I.; Moreno-Olivares, J.D.; Bleda-Sanchez, J.A.; Gomez-Martinez, J.C.; Martinez-Jimenez, J.A.; Gil-Munoz, R. Application of elicitors in two ripening periods of Vitis vinifera L. cv Monastrell: Influence on anthocyanin concentration of grapes and wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef] [PubMed]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Shu, G.; Mei, S.; Zhang, Q.; Xin, N.; Chen, H. Application of the Plackett-Burman design to determine the main factors affecting the anti-oxidative activity of goat’s milk casein hydrolyzed by Alcalase and papain. Acta Sci. Polon.-Technol. 2018, 17, 257–266. [Google Scholar]
- Golmakani, M.-T.; Moayyedi, M. Comparison of microwave-assisted hydrodistillation and solvent-less microwave extraction of essential oil from dry and fresh Citruslimon (Eureka variety) peel. J. Essent. Oil Res. 2016, 28, 272–282. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.H.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Han, D.; Kim, B.; Baek, N.; Baik, B.-K. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Int. J. Food Sci. Technol. 2013, 48, 984–991. [Google Scholar] [CrossRef]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Li, Z.-H.; Wang, Z.-J.; Liu, L.-L.; Sun, T.-T.; Ma, J.-Z.; Zhang, Y. Ultrasound-assisted extraction of total anthocyanins from Rubia sylvatica Nakai fruit and radical scavenging activity of the extract. Ind. Crop. Prod. 2020, 150, 112420. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wang, H.; Fan, X.H.; Yue, W.; Wu, Q.N. Waste Euryale ferox Salisb. Leaves as a potential source of anthocyanins: Extraction optimization, identification and antioxidant activities evaluation. Waste Biomass Valori 2020, 11, 4327–4340. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef]
- Wang, H.B.; Race, E.J.; Shrikhande, A.J. Characterization of anthocyanins in grape juices by ion trap liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2003, 51, 1839–1844. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research advances of purple sweet potato anthocyanins: Extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef] [Green Version]
- Rapisarda, F.; Fanella, F.; Maccarone, E. Reliability of analytical methods for determining anthocyanins in blood orange. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, H.; Shi, J.; Neethirajan, S.; Lin, Y.; Chen, Y.; Wang, H.; Lin, Y. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Hao, L.J.; Wu, G.; Wang, S.; Duan, J.a.; Xie, G.Y.; Qin, M.J. Effects of drying methods on the phytochemicals contents and antioxidant properties of chrysanthemum flower heads harvested at two developmental stages. J. Funct. Foods 2015, 19, 786–795. [Google Scholar] [CrossRef]
- Mau, J.L.; Chang, C.N.; Huang, S.J.; Chen, C.C. Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004, 87, 111–118. [Google Scholar] [CrossRef]
| Scheme | Sum of Squares | df | Mean Squares | F-Value | Regression Coefficient | p-Value |
|---|---|---|---|---|---|---|
| Model b | 0.16 | 6 | 0.027 | 28.21 | 1.96 | 0.0011 |
| X1 | 0.035 | 1 | 0.035 | 37.13 | −0.054 | 0.0017 ** |
| X2 | 0.0002 | 1 | 0.0002 | 0.21 | 0.00408 | 0.6664 |
| X3 | 0.0095 | 1 | 0.0095 | 9.94 | 0.028 | 0.0253 * |
| X4 | 0.00017 | 1 | 0.00017 | 0.18 | 0.00379 | 0.6885 |
| X5 | 0.11 | 1 | 0.11 | 111.94 | −0.19 | 0.0001 ** |
| X6 | 0.0094 | 1 | 0.0094 | 9.85 | 0.028 | 0.0257 * |
| Residual | 0.00477 | 5 | 0.000955 | |||
| Pure Error | 0.17 | 11 |
| Run | Extraction Conditions | Total Anthocyanins/mg/g | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||
| 1 | 0 | −1 | 0 | −1 | 2.17 ± 0.03 |
| 2 | 1 | 1 | 0 | 0 | 1.97 ± 0.02 |
| 3 | 0 | 1 | −1 | 0 | 2.24 ± 0.02 |
| 4 | −1 | 0 | 1 | 0 | 2.16 ± 0.02 |
| 5 | −1 | −1 | 0 | 0 | 2.06 ± 0.04 |
| 6 | 0 | 1 | 0 | −1 | 2.05 ± 0.03 |
| 7 | 0 | 0 | −1 | −1 | 2.15 ± 0.03 |
| 8 | 0 | 0 | −1 | 1 | 2.05 ± 0.08 |
| 9 | 0 | 0 | 0 | 0 | 2.35 ± 0.03 |
| 10 | 0 | 0 | −1 | 1 | 2.03 ± 0.04 |
| 11 | 1 | 0 | 1 | 0 | 1.89 ± 0.10 |
| 12 | 0 | 1 | 1 | 0 | 2.20 ± 0.03 |
| 13 | 0 | 0 | 0 | 0 | 2.30 ± 0.02 |
| 14 | 0 | 0 | 0 | 0 | 2.25 ± 0.02 |
| 15 | 1 | 0 | −1 | 0 | 2.07 ± 0.05 |
| 16 | 1 | 0 | 0 | −1 | 1.90 ± 0.03 |
| 17 | 0 | −1 | 0 | 1 | 2.05 ± 0.03 |
| 18 | 1 | −1 | 0 | 0 | 2.00 ± 0.05 |
| 19 | −1 | 1 | 0 | 0 | 2.16 ± 0.02 |
| 20 | 0 | −1 | 1 | 0 | 2.32 ± 0.02 |
| 21 | 0 | 0 | 1 | 1 | 2.18 ± 0.05 |
| 22 | 1 | 0 | 0 | 1 | 1.95 ± 0.08 |
| 23 | −1 | 0 | −1 | 0 | 2.01 ± 0.06 |
| 24 | −1 | 0 | 0 | −1 | 2.03 ± 0.06 |
| 25 | −1 | 0 | 0 | 1 | 2.00 ± 0.03 |
| 26 | 0 | 0 | 0 | 0 | 2.30 ± 0.03 |
| 27 | 0 | −1 | −1 | 0 | 1.99 ± 0.07 |
| 28 | 0 | 0 | 0 | 0 | 2.30 ± 0.03 |
| 29 | 0 | 1 | 0 | 1 | 2.13 ± 0.04 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.45 | 14 | 0.032 | 16.93 | <0.0001 |
| X1 | 0.033 | 1 | 0.033 | 17.50 | 0.0009 ** |
| X2 | 0.002061 | 1 | 0.002061 | 1.08 | 0.3167 |
| X3 | 0.005889 | 1 | 0.005889 | 3.08 | 0.1011 |
| X4 | 0.00006225 | 1 | 0.00006225 | 0.033 | 0.88594 |
| X1 X2 | 0.004239 | 1 | 0.0004239 | 2.22 | 0.1587 |
| X1 X3 | 0.028 | 1 | 0.028 | 14.82 | 0.0018 ** |
| X1 X4 | 0.00167 | 1 | 0.00167 | 0.87 | 0.3659 |
| X2 X3 | 0.032 | 1 | 0.032 | 16.97 | 0.0010 ** |
| X2 X4 | 0.009034 | 1 | 0.009034 | 4.72 | 0.0474 |
| X3 X4 | 0.015 | 1 | 0.017 | 7.83 | 0.0143 * |
| X12 | 0.25 | 1 | 0.25 | 132.09 | <0.0001 ** |
| X22 | 0.020 | 1 | 0.020 | 10.54 | 0.0055 ** |
| X32 | 0.024 | 1 | 0.024 | 12.39 | 0.0034 ** |
| X42 | 0.12 | 1 | 0.12 | 64.47 | <0.0001 ** |
| Residual | 0.027 | 14 | 0.001912 | ||
| Lack of Fit | 0.022 | 10 | 0.002194 | 1.82 | 0.2964 |
| Pure Error | 0.004827 | 4 | 0.001207 | ||
| Sum | 0.48 | 28 |
| Input Variables | Levels | |
|---|---|---|
| −1 | 1 | |
| compound enzyme dosage (%) (X1) | 0.5 | 1.5 |
| enzymatic hydrolysis pH (X2) | 4 | 5 |
| enzymatic hydrolysis temperature (°C) (X3) | 30 | 50 |
| material-to-liquid ratio (g/mL) (X4) | 1:15 | 1:25 |
| ethanol concentration (%) (X5) | 80 | 90 |
| ultrasound temperature (°C) (X6) | 40 | 60 |
| Runs | X1 | X2 | X3 | X4 | X5 | X6 | Total Anthocyanins/mg/g |
|---|---|---|---|---|---|---|---|
| 1 | −1 | 1 | −1 | 1 | −1 | 1 | 2.25 ± 0.04 |
| 2 | 1 | −1 | 1 | −1 | 1 | 1 | 1.97 ± 0.04 |
| 3 | 1 | −1 | −1 | 1 | −1 | 1 | 2.10 ± 0.04 |
| 4 | −1 | −1 | 1 | 1 | 1 | 1 | 2.07 ± 0.03 |
| 5 | −1 | −1 | −1 | −1 | −1 | −1 | 2.13 ± 0.06 |
| 6 | 1 | 1 | −1 | −1 | 1 | 1 | 1.90 ± 0.08 |
| 7 | −1 | −1 | −1 | −1 | 1 | −1 | 1.97 ± 0.02 |
| 8 | 1 | 1 | −1 | 1 | 1 | −1 | 1.84 ± 0.03 |
| 9 | −1 | 1 | 1 | 1 | 1 | −1 | 2.03 ± 0.04 |
| 10 | 1 | −1 | 1 | 1 | −1 | −1 | 2.09 ± 0.04 |
| 11 | −1 | 1 | 1 | −1 | −1 | 1 | 2.23 ± 0.05 |
| 12 | 1 | 1 | 1 | −1 | −1 | −1 | 2.13 ± 0.04 |
| Factor Levels | Independent Variable | |||
|---|---|---|---|---|
| X1 (%) | X2 (°C) | X3 (%) | X4 (°C) | |
| −1 | 70 | 40 | 0.5 | 30 |
| 0 | 80 | 50 | 1.0 | 40 |
| +1 | 90 | 60 | 1.5 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, S.; Deng, G.; Xu, K.; Xu, H.; Liu, J. Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology. Molecules 2022, 27, 4344. https://doi.org/10.3390/molecules27144344
Wang F, Zhang S, Deng G, Xu K, Xu H, Liu J. Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology. Molecules. 2022; 27(14):4344. https://doi.org/10.3390/molecules27144344
Chicago/Turabian StyleWang, Fang, Shuo Zhang, Guowei Deng, Kun Xu, Haiyan Xu, and Jialei Liu. 2022. "Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology" Molecules 27, no. 14: 4344. https://doi.org/10.3390/molecules27144344
APA StyleWang, F., Zhang, S., Deng, G., Xu, K., Xu, H., & Liu, J. (2022). Extracting Total Anthocyanin from Purple Sweet Potato Using an Effective Ultrasound-Assisted Compound Enzymatic Extraction Technology. Molecules, 27(14), 4344. https://doi.org/10.3390/molecules27144344







