Development of Green and Efficient Extraction of Bioactive Ginsenosides from Panax ginseng with Deep Eutectic Solvents
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals, Reagents, and Equipment
2.2. Preparation of Mixed Standard Solutions
2.3. Comparison of Different Conventional Solvents
2.4. Preparation of DESs
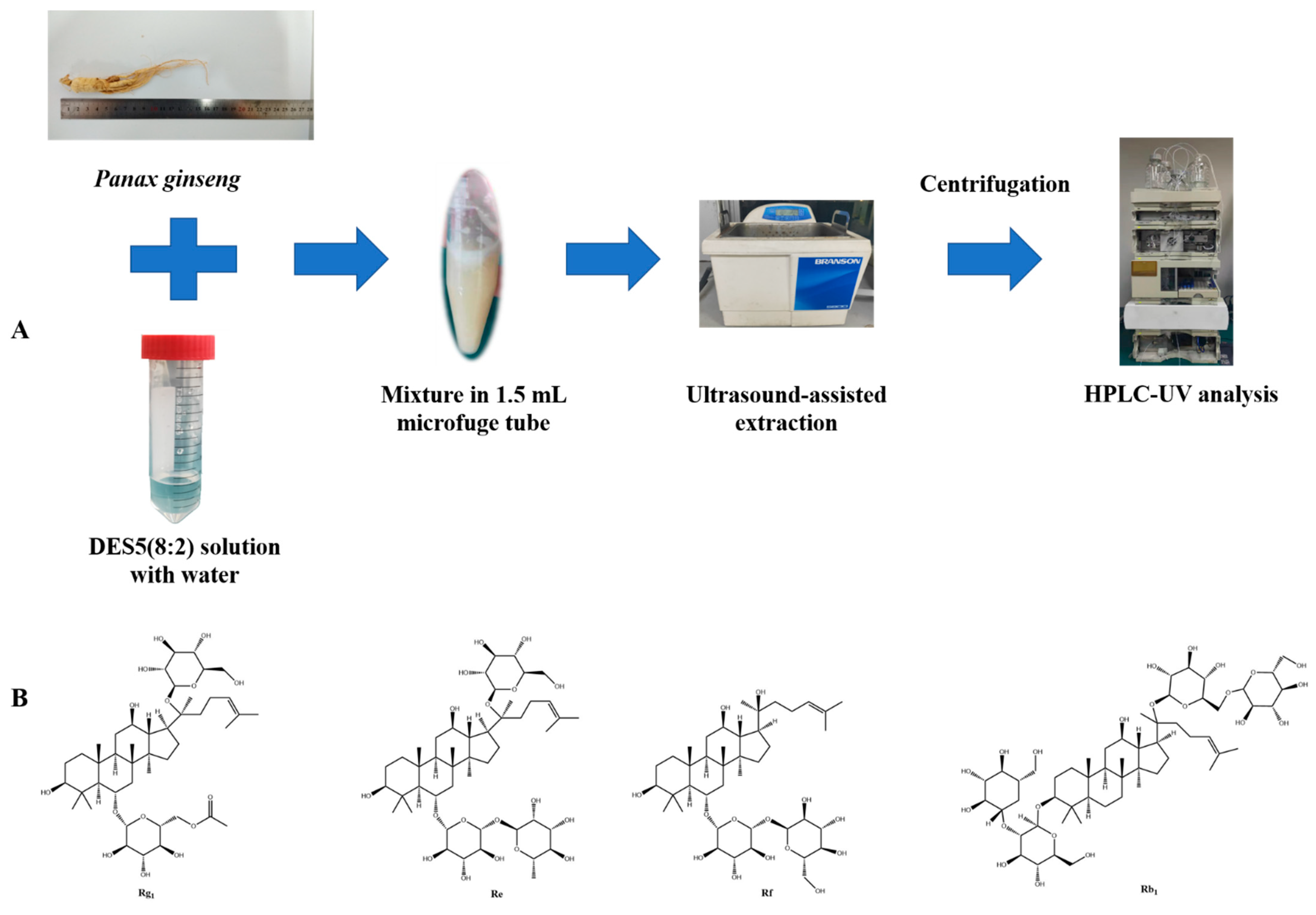
2.5. Green Extraction of Ginsenosides from Panax Ginseng Based on DESs
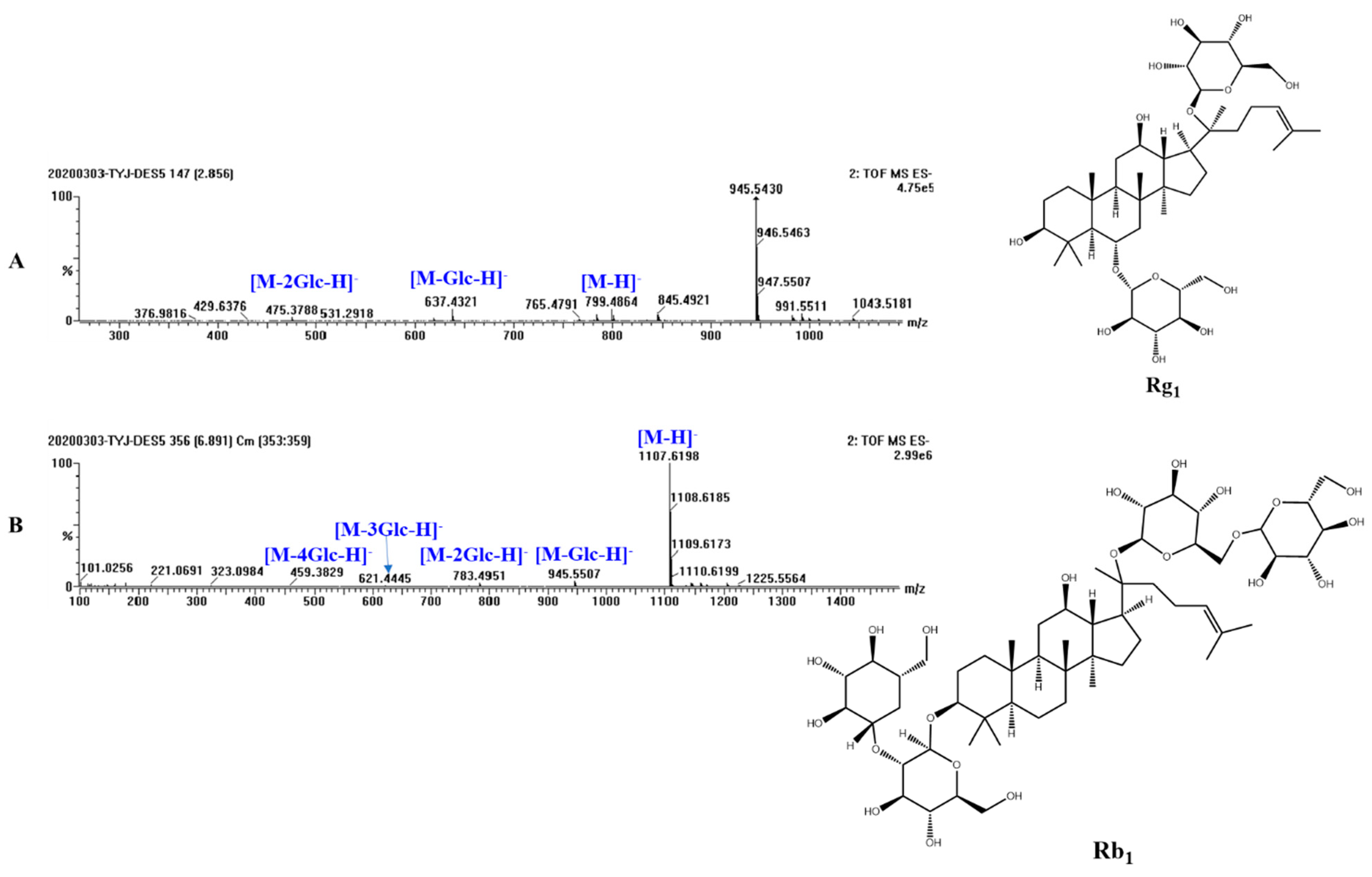
2.6. Qualitative Analysis of Extracted Ginsenosides by UPLC-Q-TOF-MS Analysis
2.7. Quantification of Extracted Ginsenosides by HPLC–DAD
2.8. Investigation and Optimization of Extraction Conditions
2.9. Method Validation
3. Results and Discussion
3.1. The Selection of Conventional Solvent and Active Compounds
3.2. Synthesis of DESs
3.3. Screening of DESs for Extraction of Ginsenosides
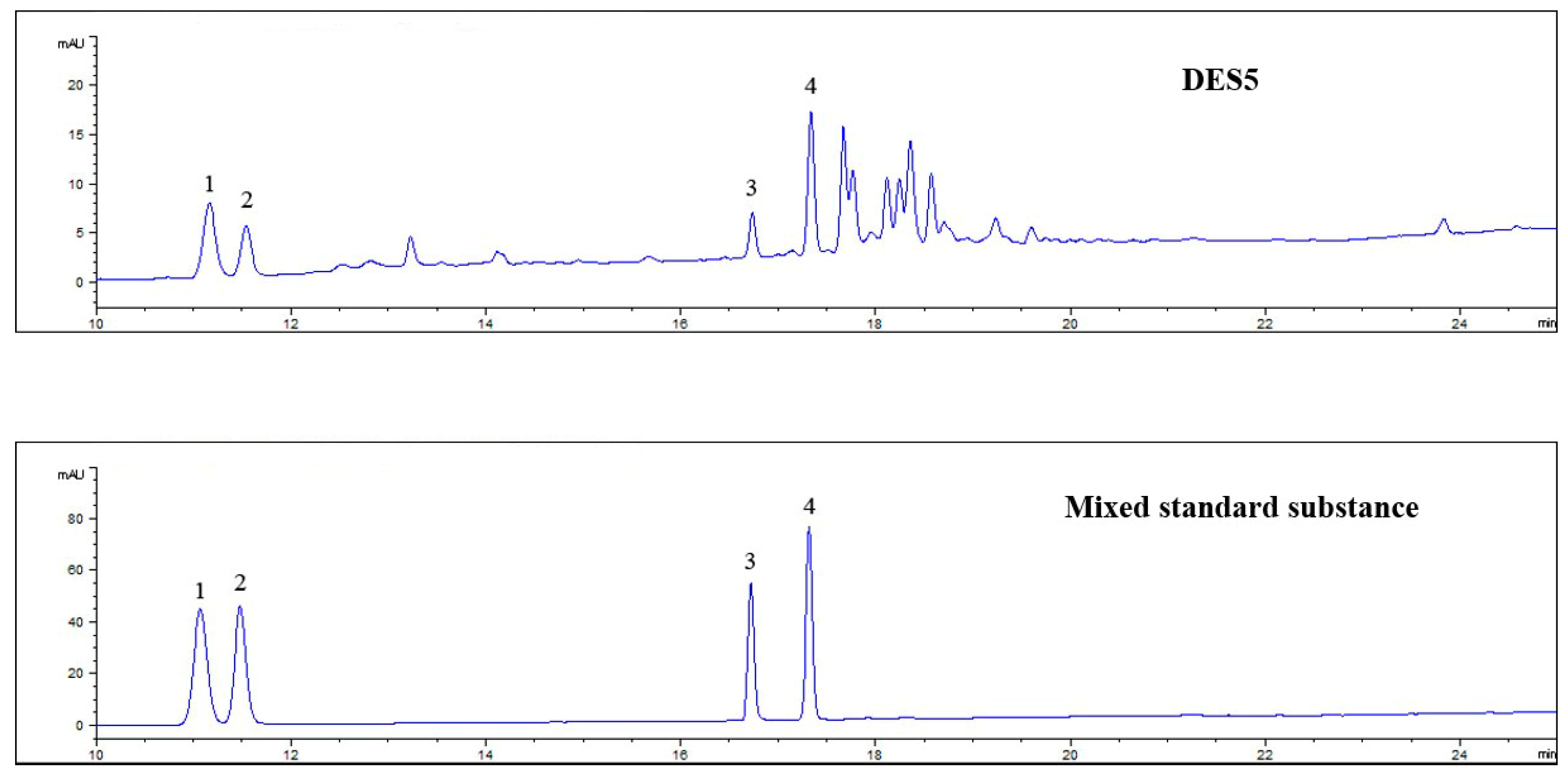
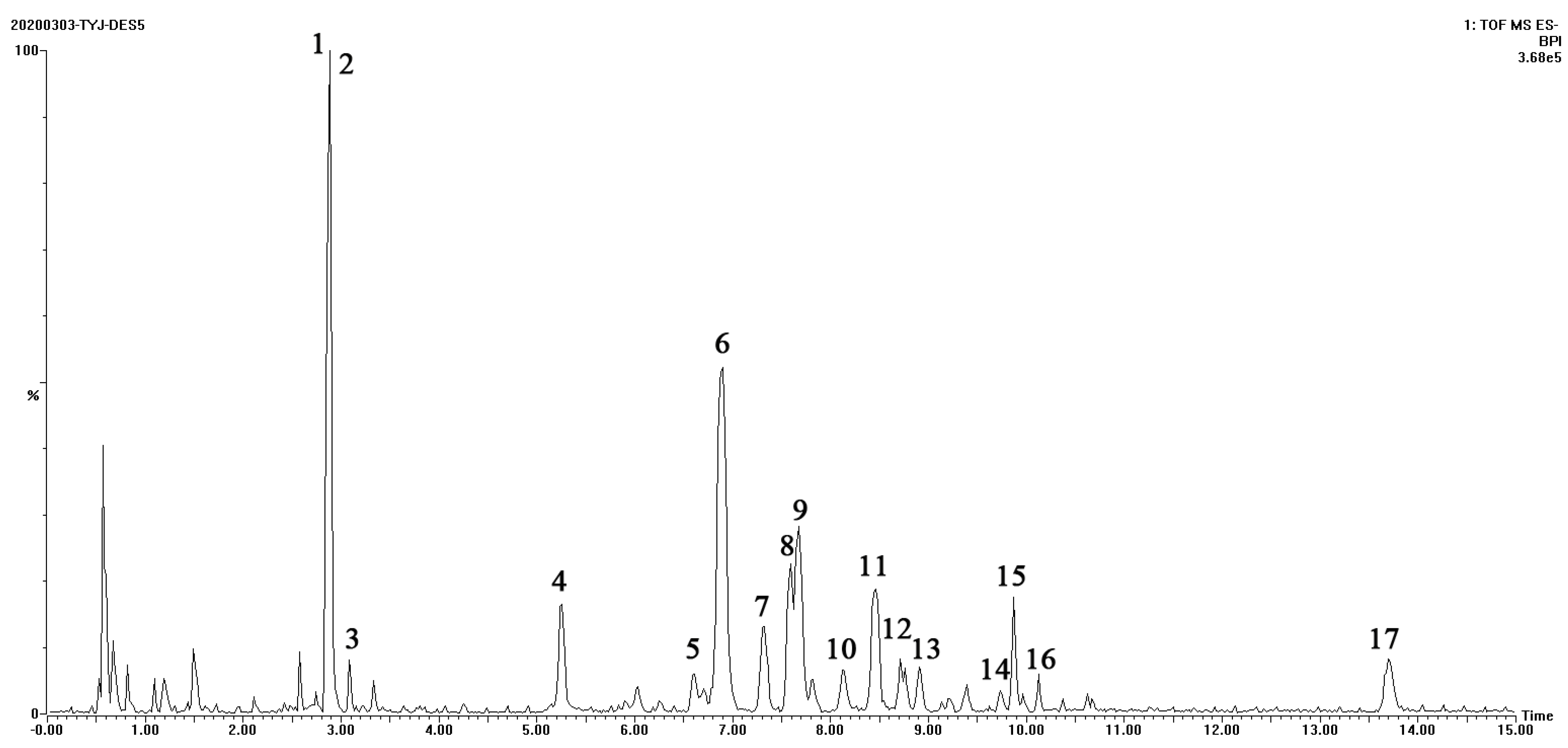
3.4. Qualitative Analysis of Extracted Ginsenosides
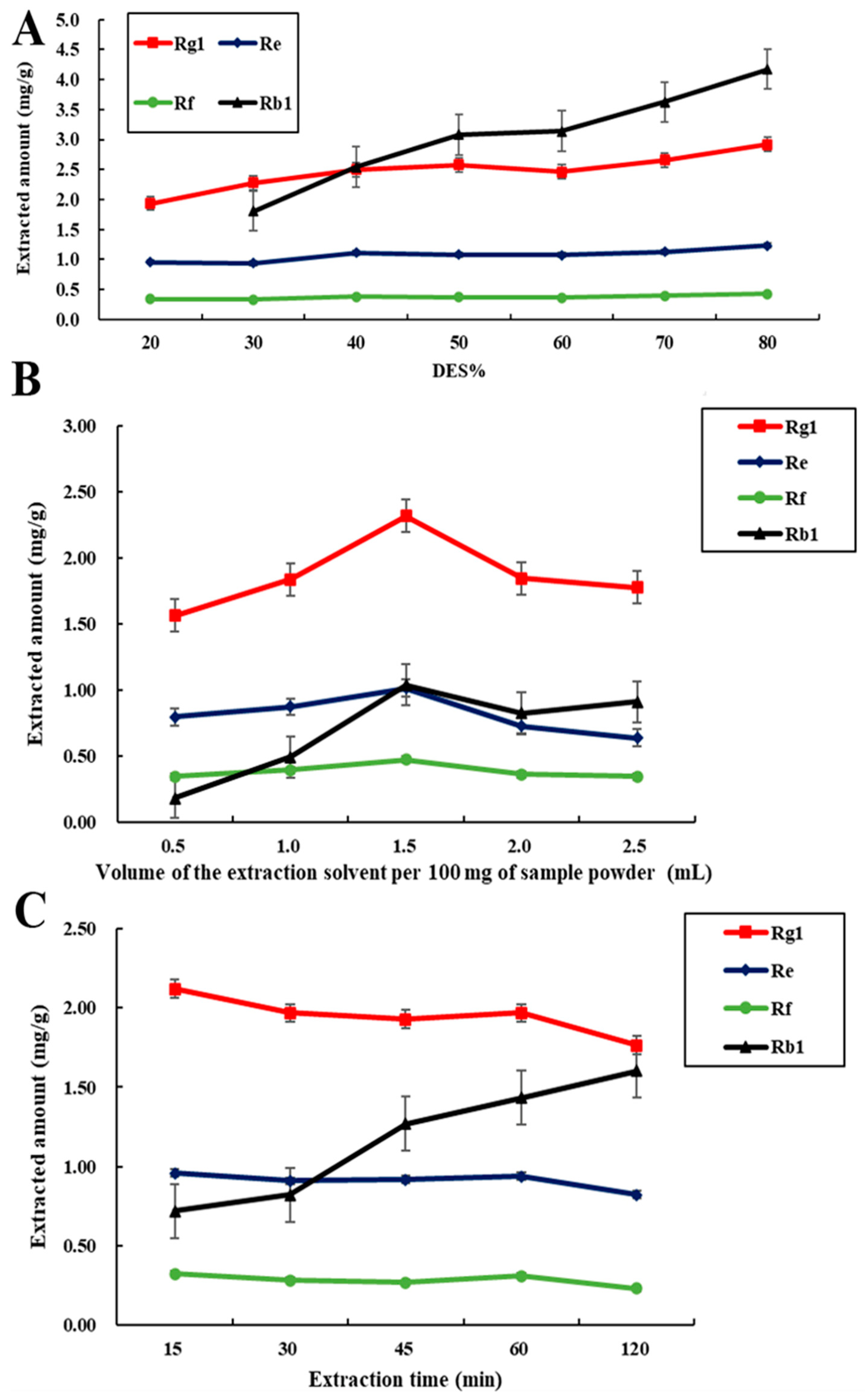
3.5. Investigation and Optimization of Extraction Conditions
3.6. Method Validation
3.7. Comparison of the DES-UAE Method with Other Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng. Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Wu, L.; Hu, M.; Dong, W.; Xu, M.; Zhang, P. Glycyrrhizic acid: A promising carrier material for anticancer therapy. Biomed. Pharm. 2017, 95, 670–678. [Google Scholar] [CrossRef]
- Liu, C.; Ma, R.; Wang, L.; Zhu, R.; Liu, H.; Guo, Y.; Zhao, B.; Zhao, S.; Tang, J.; Li, Y.; et al. Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J. Ethnopharmacol. 2017, 198, 351–362. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, J.; Wang, Y. Discrimination and identification of Q-markers based on ‘Spider-web’ mode for quality control of traditional Chinese medicine. Phytomedicine 2018, 44, 98–102. [Google Scholar] [CrossRef]
- Ligor, M.; Ratiu, I.A.; Kielbasa, A.; Al-Suod, H.; Buszewski, B. Extraction approaches used for the determination of biologically active compounds (cyclitols, polyphenols and saponins) isolated from plant material. Electrophoresis 2018, 39, 1860–1874. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, Z.; Yang, L.; Liu, H. Development and applications of deep eutectic solvent derived functional materials in chromatographic separation. J. Sep. Sci. 2021, 44, 1098–1121. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis—an alternative to ionic liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar] [CrossRef] [Green Version]
- He, A.; Dong, B.; Feng, X.; Yao, S. Extraction of bioactive ginseng saponins using aqueous two-phase systems of ionic liquids and salts. Sep. Purif. Technol. 2018, 196, 270–280. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Toledo Hijo, A.A.C.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, Z.; Domínguez de María, P. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Hou, Y.; Yao, C.; Wu, W. Deep Eutectic Solvents: Green Solvents for Separation Applications. Acta Phys.-Chim. Sin. 2018, 34, 873–885. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep eutectic solvents in separations: Methods of preparation, polarity, and applications in extractions and capillary electrochromatography. J. Chromatogr. A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Li, P.; Zhao, P.; Liu, W.; Jiang, Y.; Wang, W.; Bao, L.; Jin, Y.; Li, X. Determination of common ginsenosides in Kang’ai injection by aqueous two-phase extraction with deep eutectic solvents and HPLC-UV/DAD. Microchem. J. 2018, 137, 302–308. [Google Scholar] [CrossRef]
- Li, P.; Ye, J.; Zhang, Y.; Wang, Z.; Ren, S.; Li, X.; Jin, Y. Centrifugation free and air-assisted liquid-liquid microextraction based on deep eutectic solvent for determination of rare ginsenosides in Kang’ai injection. Microchem. J. 2018, 142, 313–320. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, S.; Xing, J.; Zheng, Z.; Pi, Z.; Song, F.; Liu, Z. Enhanced one-step sample pretreatment method for extraction of ginsenosides from rat plasma using tailor-made deep eutectic mixture solvents. Anal. Methods 2019, 11, 1035–1042. [Google Scholar] [CrossRef]
- Ji, Y.; Meng, Z.; Zhao, J.; Zhao, H.; Zhao, L. Eco-friendly ultrasonic assisted liquid-liquid microextraction method based on hydrophobic deep eutectic solvent for the determination of sulfonamides in fruit juices. J. Chromatogr. A 2020, 1609, 460520. [Google Scholar] [CrossRef]
- Alanon, M.E.; Ivanovic, M.; Pimentel-Mora, S.; Borras-Linares, I.; Arraez-Roman, D.; Segura-Carretero, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109646. [Google Scholar] [CrossRef]
- Wang, J.; Jing, W.; Tian, H.; Liu, M.; Yan, H.; Bi, W.; Chen, D.D.Y. Investigation of Deep Eutectic Solvent-Based Microwave-Assisted Extraction and Efficient Recovery of Natural Products. ACS Sustain. Chem. Eng. 2020, 8, 12080–12088. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents as Green and Efficient Media for the Extraction of Artemisinin from Artemisia annua Leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Patil, J.; Ghodke, S.; Jain, R.; Dandekar, P. Extraction of Vitamin D from Button Mushroom (Agaricus bisporus) Using Deep Eutectic Solvent and Ultrasonication. ACS Sustain. Chem. Eng. 2018, 6, 10578–10586. [Google Scholar] [CrossRef]
- Ivanovic, M.; Islamcevic Razborsek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chemistry 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng. Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Analysis of Ginsenoside Content (Panax ginseng) from Different Regions. Molecules 2019, 24, 3491. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Choi, B.R.; Kim, Y.C.; Choi, D.J.; Lee, Y.S.; Kim, G.S.; Baek, N.I.; Kim, S.Y.; Lee, D.Y. Comprehensive Profiling and Quantification of Ginsenosides in the Root, Stem, Leaf, and Berry of Panax ginseng by UPLC-QTOF/MS. Molecules 2017, 22, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCrehan, W.A.; White, C.M. Simplified ultrasonically- and microwave-assisted solvent extractions for the determination of ginsenosides in powdered Panax ginseng rhizomes using liquid chromatography with UV absorbance or electrospray mass spectrometric detection. Anal. Bioanal. Chem. 2013, 405, 4511–4522. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Qiu, Z.D.; Qu, X.Y.; Deng, A.P.; Yuan, Y.; Huang, L.Q.; Lai, C.J. Discoursing on Soxhlet extraction of ginseng using association analysis and scanning electron microscopy. J. Pharm. Anal. 2018, 8, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Zhang, Y.; Li, S.P.; Yue, H.; Chen, C.B.; Liu, S.Y. Multicomponent assessment and ginsenoside conversions of Panax quinquefolium L. roots before and after steaming by HPLC-MS(n). J. Ginseng. Res. 2019, 43, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, L.; Zhang, Z.; Guo, Y.; Liu, S. Profiling and multivariate statistical analysis of Panax ginseng based on ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 141–150. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Qiu, H.; Ju, Z.; Shi, Y.; Wang, Z.; Yang, L. Localization of constituents for determining the age and parts of ginseng through ultraperfomance liquid chromatography quadrupole/time of flight-mass spectrometry combined with desorption electrospray ionization mass spectrometry imaging. J. Pharm. Biomed. Anal. 2021, 193, 113722. [Google Scholar] [CrossRef]
- Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia[M] Peking; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Zhao, Z.; Zhao, J.; Liang, N.; Zhao, L. Deep eutectic solvent-based magnetic colloidal gel assisted magnetic solid-phase extraction: A simple and rapid method for the determination of sex hormones in cosmetic skin care toners. Chemosphere 2020, 255, 127004. [Google Scholar] [CrossRef]
- Mansinhos, I.; Goncalves, S.; Rodriguez-Solana, R.; Ordonez-Diaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef]
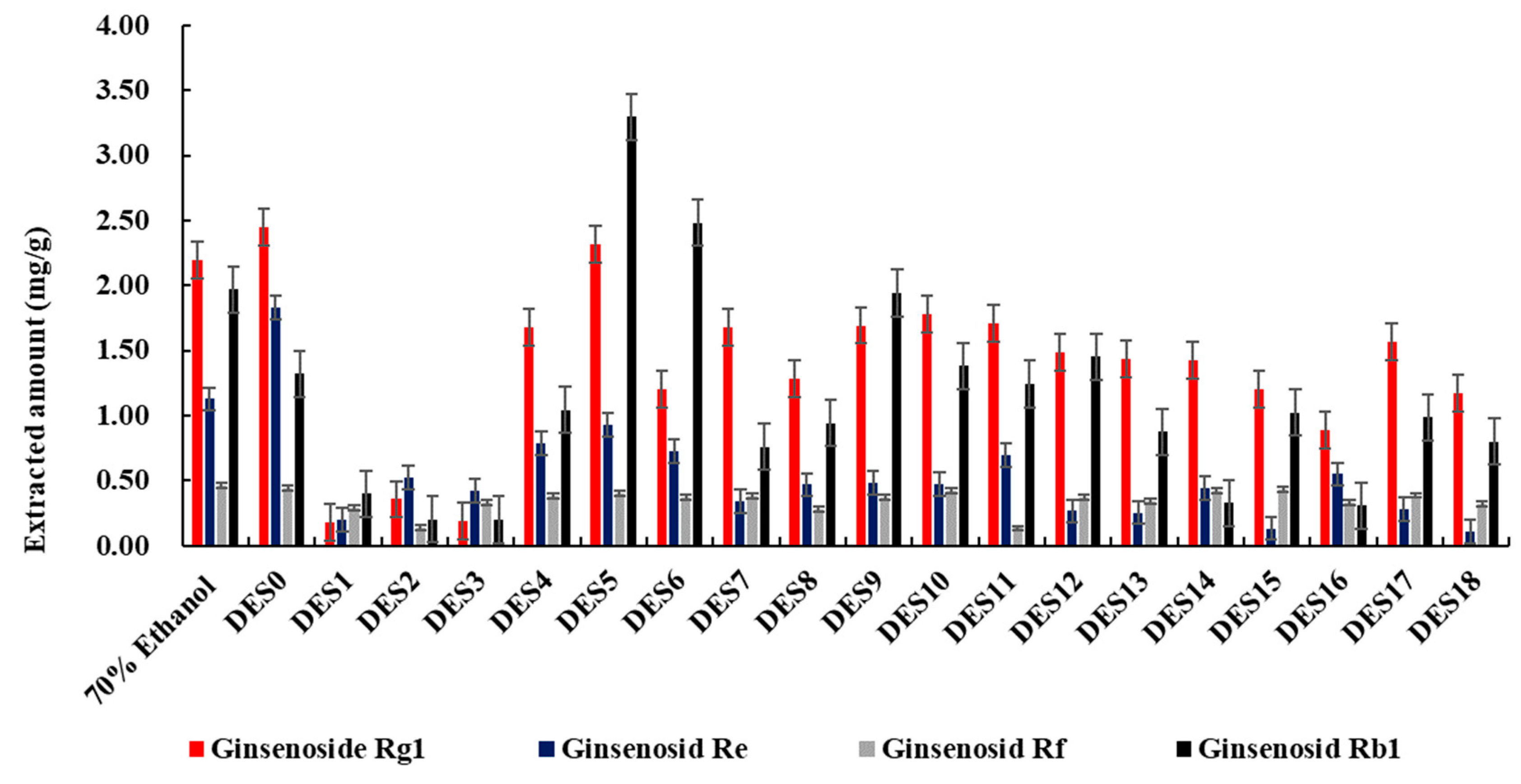
| Number | Deep Eutectic Solvents | Mole Ratio |
|---|---|---|
| 1 | Choline chloride: lactic acid | 1:4 |
| 2 | L-proline: lactic acid | 1:4 |
| 3 | Betaine: lactic acid | 1:4 |
| 4 | Betaine: urea | 1:2 |
| 5 | Choline chloride: urea | 1:2 |
| 6 | L-proline: D-(+)-maltose | 2:1 |
| 7 | Betaine: D-(+)-maltose | 1:1 |
| 8 | Choline chloride: D-(+)-maltose | 2:1 |
| 9 | Betaine: ethylene glycol | 1:4 |
| 10 | Betaine:1,4-butanediol | 1:4 |
| 11 | Choline chloride: 1,2-propanediol | 1:4 |
| 12 | Betaine:1,2-propanediol | 1:4 |
| 13 | Choline chloride: D-sorbitol | 1:1 |
| 14 | Glycerol: Choline chloride: D-sorbitol | 1:0.5:0.5 |
| 15 | Glycerol: Choline chloride: D-(+)-maltose | 5:4:1 |
| 16 | Glycerol: L-proline: D-(+)-maltose | 5:4:1 |
| 17 | Glycerol: Betaine: D-(+)-maltose | 5:1:1 |
| 18 | Glycerol: Choline chloride: D-(+)-glucose | 5:1:1 |
| Number | Retention Time | Identity | Molecular Formula | Molecule Weight | [M−H]− Measured Value | Mass Fragment |
|---|---|---|---|---|---|---|
| m/z | m/z | |||||
| 1 | 2.87 | Ginsenoside Re | C48H82O18 | 946.5501 | 945.5577 | 783.4982, 637.4429, 475.3860 |
| 2 | 2.87 | Ginsenoside Rg1 | C42H72O14 | 800.4922 | 799.4974 | 637.4429, 475.3853 |
| 3 | 3.08 | Acetyl-Ginsenoside Rg1 | C44H74O15 | 842.5028 | 841.5055 | 637.4424, 475.3689, 179.0534, 161.0432 |
| 4 | 5.24 | Ginsenoside Rf | C42H72O14 | 800.4922 | 799.4948 | 637.4409, 475.3872, 391.3035 |
| 5 | 6.59 | Ginsenoside Rg2 | C42H72O13 | 784.4973 | 783.4976 | 637.4370, 475.3929, 391.2038 |
| 6 | 6.88 | Ginsenoside Rb1 | C54H92O23 | 1108.6029 | 1107.6194 | 945.5510, 783.4947, 621.4453, 459.3857 |
| 7 | 7.31 | M-Ginsenoside Rb1 | C57H94O26 | 1194.6033 | 1193.6294 | 1107.6102, 945.5532, 783.4930, 621.4282, 459.3890 |
| 8 | 7.65 | Ginsenoside Ro | C48H76O19 | 956.4981 | 955.5157 | 793.4459, 455.3476 |
| 9 | 7.65 | Ginsenoside Rc | C53H90O22 | 1078.5924 | 1077.6031 | 945.5517, 783.4988, 621.4410, 459.3914 |
| 10 | 8.12 | M-Ginsenoside Rc | C56H92O25 | 1164.5928 | 1163.6096 | 1077.5968, 945.5502, 783.4940, 621.4370, 459.4090 |
| 11 | 8.46 | Ginsenoside Rb2 | C53H90O22 | 1078.5924 | 1077.6072 | 945.5524, 783.4978, 621.4457 |
| 12 | 8.71 | Ginsenoside Rb3 | C53H90O22 | 1078.5924 | 1077.5988 | 945.5562, 783.5023, 621.4474 |
| 13 | 8.90 | M-Ginsenoside Rb2 | C56H92O25 | 1164.5928 | 1163.6073 | 1077.5972, 945.5626, 783.5019, 621.4164, 459.3085 |
| 14 | 9.75 | M-Ginsenoside Rb3 | C56H92O25 | 1164.5928 | 1163.6047 | 1077.5912, 945.5482, 783.4807, 621.4417 |
| 15 | 9.87 | Ginsenoside Rd | C48H82O18 | 946.5501 | 945.5523 | 783.4973, 621.4481, 459.4015 |
| 16 | 10.12 | M-Ginsenoside Rd | C51H84O21 | 1032.5505 | 1031.5533 | 945.5515, 783.5026, 621.4420, 459.3722 |
| 17 | 13.70 | Chikusetsusaponin Iva | C42H66O14 | 794.4453 | 793.4451 | 631.3763, 455.3561 |
| Analytes | Regression Equation | Linear Range (μg mL−1) | Correlation Coefficient (r) | LOD (μg mL−1) | LOQ (μg mL−1) | Precision (%) |
|---|---|---|---|---|---|---|
| Ginsenoside Rg1 | y = 1.3862x + 1.3295 | 25.3–404.0 | 0.9999 | 3.897 | 12.990 | 0.87 |
| Ginsenoside Re | y = 1.1635x + 1.9422 | 25.6–410.0 | 1.0000 | 3.345 | 11.150 | 3.78 |
| Ginsenoside Rf | y = 2.8179x + 4.6915 | 6.6–105.0 | 0.9999 | 1.602 | 5.339 | 0.96 |
| Ginsenoside Rb1 | y = 1.0523x + 0.2323 | 25.3–404.0 | 1.0000 | 1.524 | 5.081 | 2.70 |
| Analytes | Initial Content (μg) | Added Content (μg) | Found (μg) | Recovery (%) |
|---|---|---|---|---|
| Ginsenoside Rg1 | 251.3 | 152.6 | 416.4 | 108.2 |
| Ginsenoside Re | 181.1 | 97.8 | 278.4 | 99.5 |
| Ginsenoside Rf | 62.2 | 40.0 | 100.9 | 96.8 |
| Ginsenoside Rb1 | 237.4 | 120.7 | 352.1 | 95.0 |
| Method | Pretreatment Time (h) | Detection Time (min) | Extraction Amount (mg g−1) |
|---|---|---|---|
| Soxhlet extraction [37] | 24 | 100 | 7.51 |
| Ultrasonic extraction with 70% Ethanol | 2 | 40 | 8.49 |
| Ultrasonic extraction with deep eutectic solvent-0 [17] | 24 | 55 | 6.10 |
| Our optimized method | 2 | 40 | 11.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.; Li, L.; Fan, W.; Liu, L.; Wang, Z.; Yang, L. Development of Green and Efficient Extraction of Bioactive Ginsenosides from Panax ginseng with Deep Eutectic Solvents. Molecules 2022, 27, 4339. https://doi.org/10.3390/molecules27144339
Tu Y, Li L, Fan W, Liu L, Wang Z, Yang L. Development of Green and Efficient Extraction of Bioactive Ginsenosides from Panax ginseng with Deep Eutectic Solvents. Molecules. 2022; 27(14):4339. https://doi.org/10.3390/molecules27144339
Chicago/Turabian StyleTu, Yujia, Linnan Li, Wenxiang Fan, Longchan Liu, Zhengtao Wang, and Li Yang. 2022. "Development of Green and Efficient Extraction of Bioactive Ginsenosides from Panax ginseng with Deep Eutectic Solvents" Molecules 27, no. 14: 4339. https://doi.org/10.3390/molecules27144339
APA StyleTu, Y., Li, L., Fan, W., Liu, L., Wang, Z., & Yang, L. (2022). Development of Green and Efficient Extraction of Bioactive Ginsenosides from Panax ginseng with Deep Eutectic Solvents. Molecules, 27(14), 4339. https://doi.org/10.3390/molecules27144339