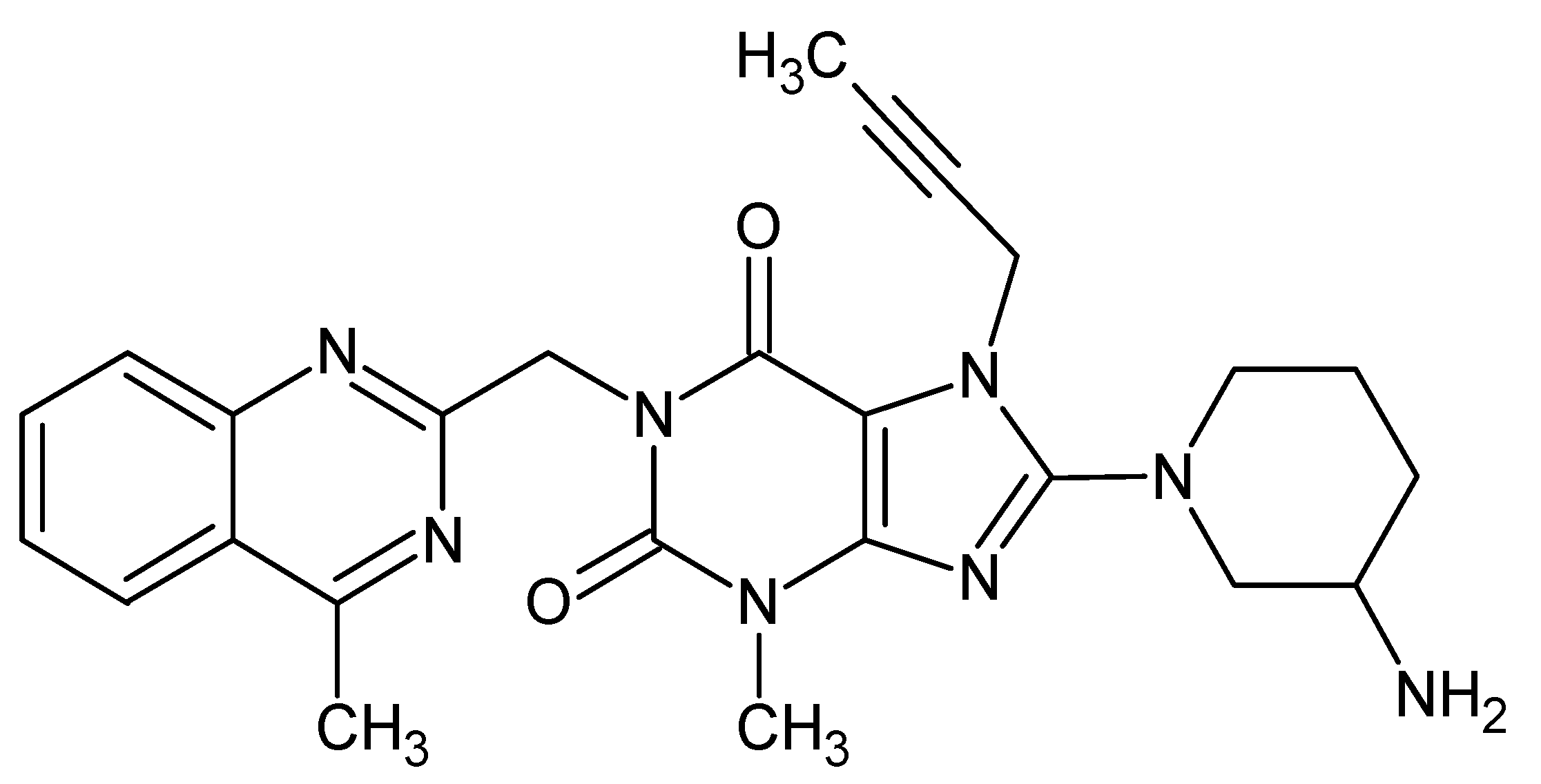
2.2. FT-IR Experiments
FT-IR characteristics obtained for LINA and four excipients, i.e., LAC, MAN, MGS and PVP, are shown in
Table 1 and
Figure 4 and
Figure 5. From the spectra obtained, it was clearly seen that LINA changed slightly after stressing by high temperature/high humidity. Especially, the peaks at 1697 cm
−1 and 1506 cm
−1 were affected (
Figure 4).
As far as excipients are concerned, LAC, MAN and MGS did not show changes under stress conditions. However, slight changes were observed in the spectrum of PVP, especially in the region 1600–1500 cm
−1 (
Figure 5).
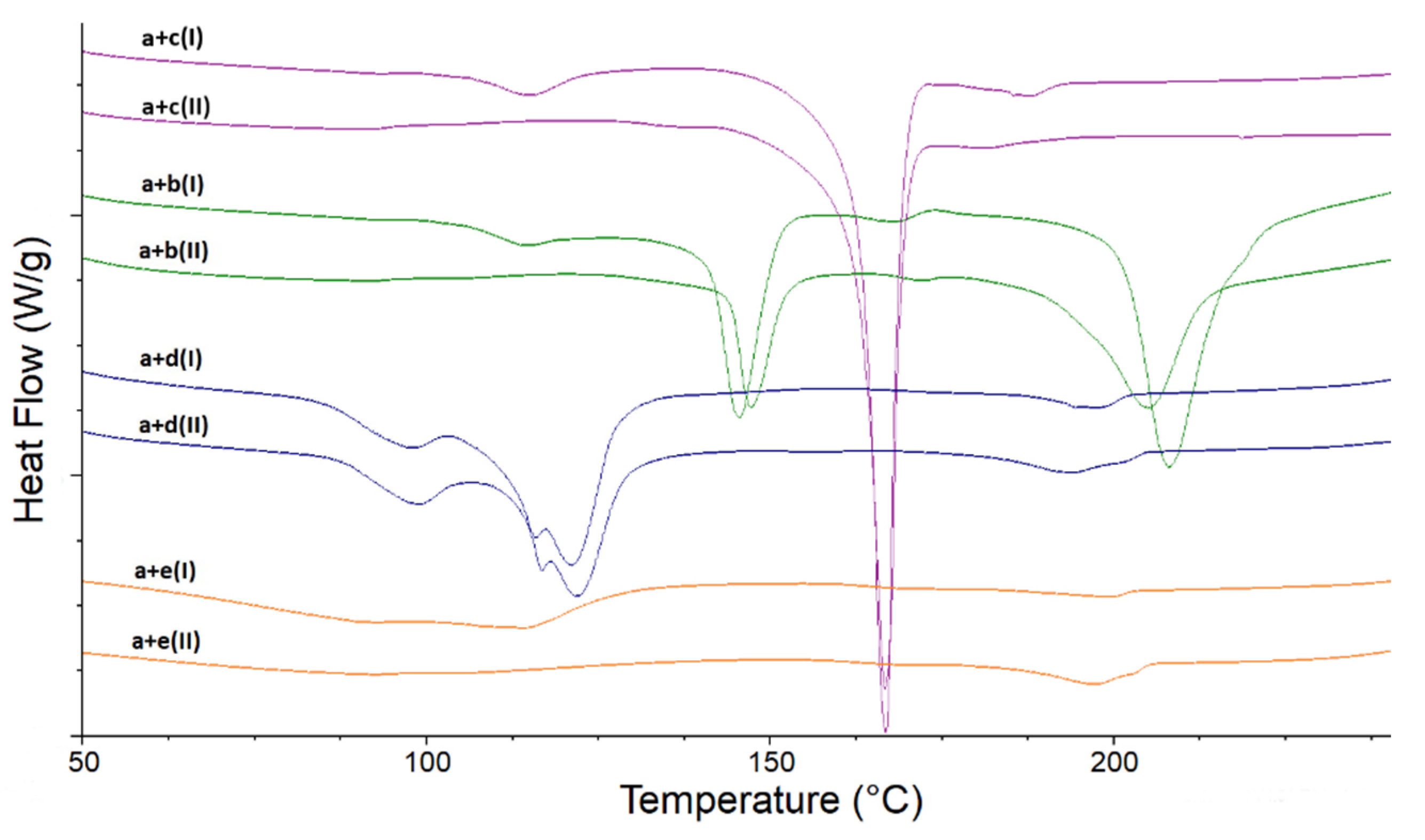
As far as the binary mixtures of LINA with excipients are concerned, some interactions were observed without stressing, whereas other changes occurred only under high-temperature/high-humidity conditions (
Figure 6). The stressed mixture of LINA and LAC showed potential interactions such as decreasing of characteristic peaks of LINA at 3331 cm
−1 and 3285 cm
−1 due to primary amine group (N–H stretching). In addition, broadening the peaks at 2944 cm
−1 (C–H stretching), 1654 cm
−1 (C=O stretching) and 1506 cm
−1 (C=C stretching), as well as changing the shape of the peaks of LINA in the region 1450–1100 cm
−1 (C=C stretching and C–N stretching vibrations) was observed. Bearing in mind the above results, especially the broadening the peak at 1654 cm
−1, a new vibration due to imine formation (1690–1640 cm
−1) could be supposed and the possibility of chemical interaction via Maillard’s reaction between the amine group of LINA and LAC could be suggested. In the literature, similar interactions with LAC were reported for other drugs from gliptins, i.e., for sitagliptin containing a primary amine group and vildagliptin containing a secondary amine group [
11,
12].
When the non-stressed mixture of LINA and MAN was studied, the peak of LINA at 1506 cm
−1 visibly changed its shape. In addition, the peaks of LINA at 1436 cm
−1 and MAN at 1450 cm
−1 were observed as overlapped. After high-temperature/high-humidity stressing, these overlapped peaks changed their shapes and deteriorated to lower wavenumbers, suggesting a new sort of interaction. However, the peaks due to the –OH groups of MAN did not change visibly. Thus, the possibility of hydrogen bond formation between the amine group of LINA and –OH groups of MAN cannot be suggested, contrary to our previous results obtained for sitagliptin [
11] (
Figure 6).
The spectrum of the non-stressed mixture of LINA and MGS showed a lack of peaks at 3331 cm
−1, 3285 cm
−1 and 2944 cm
−1 due to N–H stretching and C–H stretching of LINA. When the mixture was treated with high temperature/high humidity, one more change occurred, i.e., the lack of the peak of LINA of at 1506 cm
−1. Thus, the interactions via hydrogen bonding between the amine group of LINA and the carboxylic group of MGS could be suggested. What is more, the characteristic peak of MGS at 1572 cm
−1 (–COOH group) was also changed in the stressed mixture (
Figure 6).
As far as the mixture of LINA with PVP is concerned, the spectrum of the non-stressed mixture showed a lack of peaks at 3331 cm
−1, 3285 cm
−1 and 2944 cm
−1 due to N–H stretching and C–H stretching of LINA, and overlapping of the peaks of LINA at 1697 cm
−1 and PVP at 1652 cm
−1. When the mixture treated with high temperature/high humidity was examined, these overlapped peaks were significantly broadened up to 1613 cm
−1. In addition, the spectrum of the stressed mixture shows changes at 1291 cm
−1 and 1287 cm
−1 (the peaks of LINA due to C–N stretching) and at 1291 cm
−1 (the peak of PVP due to C–O stretching) (
Figure 6). Thus, it could be suggested that the NH, CN and CO groups of LINA and the CO groups of PVP could be involved in some interactions. Previously, the changes concerning the peak due to secondary amine of vildagliptin due to interactions with PVP were observed [
12]. What is more, the possibility of hydrogen bonding via the oxygen atom in PVP for many drug molecules was described in the literature [
15].
2.4. Multivariate Statistical Calculations
To improve the interpretation of DSC curves, as well as FT-IR and NIR spectra, two unsupervised multivariate statistical methods were used, principal component analysis (PCA) and hierarchical cluster analysis (HCA) [
16]. PCA is based on the dimensionality reduction of huge data sets. This increases the interpretability of data under study with minimal loss of information. On the other hand, HCA shows the tendency of samples to form clusters, that is to create groups of samples with similar characteristics. The findings obtained during multivariate assessment of thermal (DSC) and spectroscopic (FT-IR, NIR) data for LINA, excipients and their binary physical mixtures, stored under ambient and stressed conditions, are summarized in
Table 2.
In general, these data reveal that interpretation of DSC and FT-IR data leads to similar conclusions, regardless of the fact that DSC curves reflect such phase transitions that occurred in substances when heating (the characteristic temperatures and heats of transitions), whereas FT-IR spectra reflect the chemical structure of substances under study (the chemical bonds and functional groups).
Findings obtained using NIR spectra differ slightly from those obtained using DSC and FT-IR measurements. Probably, this is due to the fact that NIR spectra are based on molecular overtone and combination vibrations, hence, the absorption bands are typically quite small and very broad. This leads to complex spectra, in which it is difficult to attribute a specific band to a specific chemical compound.
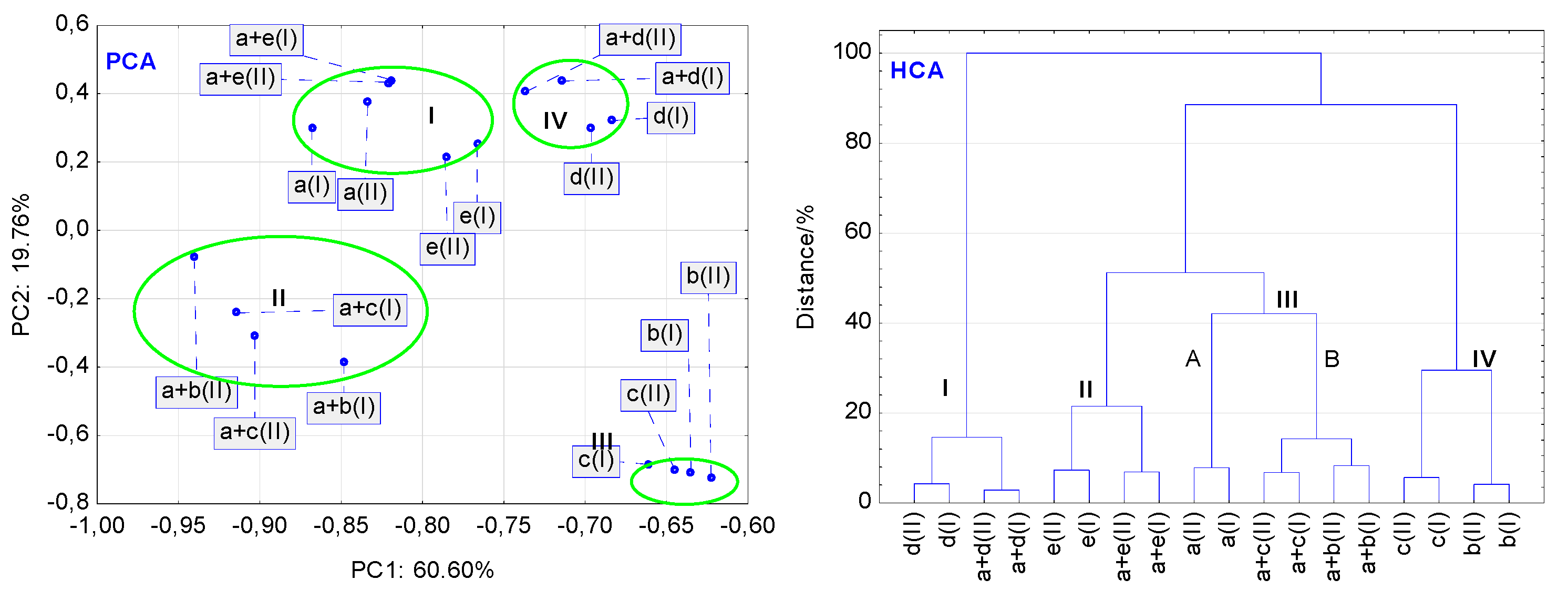
In general, PCA calculations for the data acquired from the DSC curves revealed that stressed conditions have a higher impact on the stability of binary mixtures than that on the stability of LINA and pharmaceutical excipients alone. However, some differences occurred among respective compounds and mixtures. As shown in
Figure 10 (PCA), the non-stressed and stressed samples of MAN, LAC and MGS are grouped into three separate clusters I, II and III, respectively, at very similar values of the first two principal components (PC1 and PC2). This implies that high temperature and high humidity did not affect the thermal stability of these excipients. In contrast to this, the stressed conditions strongly affected the thermal stability of pure LINA and PVP. Both compounds and their stressed counterparts are localized in cluster IV, at very different PC1 and PC2 values. Such localization of these samples confirms that DSC curves of the stressed and non-stressed samples are not the same. They display physical or chemical changes that occurred in a sample under the influence of high temperature and high humidity. Additionally,
Figure 10 (PCA) shows that, besides excipients, all binary mixtures of LINA, i.e., with MAN, LAC, MGS and PVP (non-stressed and stressed as well), are also placed in clusters I, II, III and IV, respectively. However, excluding the mixture of LINA with PVP (cluster IV), the remaining samples are localized at slightly different values of PC1 or PC2 as compared with the excipients alone. Similarly, as in the case of PVP alone, high temperature and high humidity affect, to the greatest extent, the stability of the mixture of LINA with PVP. Thus, such organization of the PCA clusters proves that excluding PVP, the rest of excipients have a slight effect on the thermal stability of LINA, also taking into account the stressed conditions.
To avoid misinterpretation of the thermal data, hierarchical cluster analysis (HCA) was also used for the analysis of the DSC curves as a second multivariate statistical method. In general, the findings of the HCA calculations are consistent with those obtained using PCA.
Figure 10 (HCA) shows that non-stressed and stressed samples of LINA, excipients alone (MAN, LAC, MGS and PVP), and LINA binary mixtures with these excipients are linked together in the same way, as was the case for the PCA score scatter plot (
Figure 10 (PCA)). MAN and LAC, and their mixtures with LINA, form clusters I and II, respectively. Cluster III created on the HCA tree diagram could be divided into two subclusters, IIIA and IIIB. The former cluster contains the non-stressed and stressed MGS and its mixture with LINA, whereas the latter is created by the non-stressed and stressed LINA and PVP alone, and their mixture. Thus, the HCA diagram reveals that the four excipients and their mixtures with LINA create four clusters (I-IV), taking into account similarities in their thermal properties.
The findings of the PCA calculations for the FT-IR data are quite similar to those obtained for the DSC data. High temperature and high humidity affected the thermal stability of mixtures to a greater extent than the stability of LINA and excipients alone.
Figure 11 (PCA) shows that similarly, as in the case of DSC data the non-stressed and stressed samples of LINA, PVP and the mixture of LINA with PVP are grouped together in a separate cluster, I. Distribution of LINA and PVP at different values of PC1 and PC2 implies that high temperature and high humidity affected their stability. Furthermore, the non-stressed and stressed samples of MGS and its mixture with LINA create cluster IV. Scattering of the samples within this cluster may suggest the impact of stressed conditions on the stability of this mixture. Particular attention should also be paid to the distribution of the LAC and MAN samples, and their mixtures with LINA. The stressed and non-stressed samples of LAC and MAN alone create a separate cluster III, whereas their mixtures with LINA are grouped in cluster II. This could be due to the fact that MAN is a sugar alcohol with six hydroxy groups ((2R,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol), whereas LAC is a sugar composed of galactose and glucose (β-D-galactopyranosyl-(1→4)-D-glucose) that has eight hydroxy groups per molecule. As the dominant absorption bands are assigned to hydroxy groups, both excipients have similar FT-IR spectra. Thus, they show a tendency to create a cluster of samples with similar characteristics. The scattering of samples within the cluster II implies that high temperature and high humidity strongly affected thermal stability, especially stability of the mixture of LINA with LAC. As mentioned in the section “FT-IR Experiments”, this may be due to chemical interaction via Maillard’s reaction between the amine group of LINA and LAC.
Overall, the FT-IR spectra revealed that stressed conditions may induce physical or chemical changes in LINA and PVP alone, and potential interactions in the mixtures of LINA with all excipients examined. i.e., LAC, MAN, MGS and PVP.
As in the case of DSC data, the findings for HCA calculations based on the FT-IR data are, in general, consistent with those obtained by PCA. As shown in
Figure 11 (HCA), the non-stressed and stressed samples of LINA, excipients alone (MAN, LAC, MGS and PVP), and LINA binary mixtures with excipients are grouped together in separate clusters on the lowest linkage levels, below 10% on the similarity axis. At higher linkage levels, the samples of MGS and PVP, and their mixtures with LINA create separate clusters, I and II, respectively. Furthermore, samples of MAN and LAC are grouped in a separate cluster, IV, whereas their mixtures with LINA are localized in the cluster IIIB. Hence, HCA calculations using FT-IR data may imply that stressed conditions could induce physical or chemical changes in LINA and PVP alone, and potential interactions in the mixtures of LINA with LAC, MAN and PVP.
The PCA score scatter plot for the data acquired from NIR spectra (
Figure 12 (PCA)) differs greatly from the PCA plots developed for the DSC and FT-IR data (
Figure 10 (PCA) and
Figure 11 (PCA)). As shown in
Figure 12 (PCA), there are eight small clusters, seven of them are localized at a narrow range of the PC1 values, i.e., between −0.85 and −1.0. Each of the clusters I, II, III, IV and VII comprise only two samples, that is the non-stressed and stressed MAN, LAC, LINA mixture with MAN, LINA mixture with LAC, and MGS, respectively. The values of PC1 or PC2 suggest that stressed conditions affect merely the stability of the LINA mixture with LAC, and MGS alone. As in the case of PCA plots developed for DSC and FT-IR data (
Figure 10 (PCA), cluster IV;
Figure 11 (PCA), cluster I), two neighboring clusters consisted of stressed and non-stressed samples of LINA and the LINA mixture with PVP (cluster V), and PVP alone (cluster VI). In the last cluster, the non-stressed LINA mixture with MGS is also placed, however, the reason for localization of this mixture in cluster VI is difficult to explain. On the other hand, the stressed LINA mixture with MGS is localized on the opposite side of the PCA score scatter plot (cluster VIII). This implies that high temperature and high humidity had a very strong influence on the stability of this mixture.
The HCA tree diagram developed for the NIR data is more complicated (
Figure 12 (HCA)) than the diagrams based on the DSC and FT-IR data (
Figure 10 (HCA) and
Figure 11 (HCA)). The mixture of LINA with PVP (cluster I) and the mixture of LINA with MGS, both stressed, and the mixture of LINA with PVP (cluster IIA) differ significantly from each other and from the remaining mixtures (
Figure 12 (HCA)). This suggests a strong effect of high temperature and high humidity on their stabilities. Moreover, the stressed mixtures of LINA with MAN and LINA with LAC grouped in cluster IIB differ completely from their counterparts localized in cluster IIIB. This also suggests a strong effect of stressed conditions on their stability. Unlike these samples, localization of the non-stressed and stressed LINA and excipients (LAC, MAN, MGS, PVP) in two-components subclusters (IIB, IIIA, and IIIB) created on the lowest linkage levels, below 8% on the distance axis, indicates that the stressed conditions did not have an impact on the stability on these compounds.
Concluding, the PCA score scatter plots and the HCA tree diagrams developed for the thermal and spectroscopic data allowed us to conclude that excipients, especially under stressed conditions, affect the stability of LINA in the mixtures.
Furthermore, to identify which absorption bands of the FT-IR spectra of LINA underwent significant changes due to mixing with excipients (LAC, MAN, MGS and PVP), and after high-temperature/high-humidity stressing, additional PCA calculations were performed using four matrices (A, B, C and D) described in detail in the section “Chemometrics”. This approach was previously used in the study of methylxanthine mixtures with hydroxypropyl methylcellulose [
17]. The findings of the PCA calculations for LINA and its mixture with excipients are graphically presented in
Figure 13 and the data obtained are compiled in
Table 3.
Overall, outcomes of these studies can be obtained by a comparison of the plots developed for PC1 and PC2 values, which were calculated for four successive matrices. As an example,
Figure 13 shows the results of PCA calculations using FT-IR data for LINA and its mixture with LAC. Comparing PC1 and PC2 lines calculated using the first matrix (
Figure 13, lines A, LINA and LAC alone) with those lines for PC1 and PC2 obtained for the second matrix (
Figure 13, lines B, LINA and its mixture with LAC), both under non-stressed conditions, the impact of the excipient (LAC) on the chemical stability of LINA can by assessed by taking into account the changes in absorption bands of LINA. Findings obtained after this comparison for all the excipients used in this study (LAC, MAN, MGS and PVP) are listed in
Table 3. On the other hand, comparing PC1 and PC2 lines calculated using the first matrix (
Figure 13, lines A, LINA and LAC alone under non-stressed conditions) with those lines for PC1 and PC2 obtained for the third matrix (
Figure 13, lines C, LINA and LAC alone under stressed conditions), the effect of high temperature and high humidity on the chemical stability of LINA can be evaluated (
Table 3). Additionally, detailed analysis of the PC1 and PC2 lines calculated using the fourth matrix (
Figure 13, lines D, LINA and its mixture with LAC under stressed conditions) with PC1 and PC2 lines obtained for each other matrix enable us to determine if there are additional differences that were not seen separately to the effects of the excipient (LAC) or stressed conditions on the drug substance (LINA).