The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test
Abstract
:1. Introduction
2. Results
2.1. The Impact of Cholinergic Ligands on the Memory and Learning Processes in Mice in the PA Test
2.2. The Impact of CB2 Receptor Ligands on the Memory and Learning Processes in Mice in the PA Test
2.2.1. The Impact of the CB2-Receptor Agonist JWH 133 on the Memory and Learning Processes in Mice in the PA Test
2.2.2. The Impact of the CB2-Receptor Antagonist AM 630 on the Memory and Learning Processes in Mice in the PA Test
2.3. The Influence of the Administration of CB2 Receptor Ligands on the Memory-Related Responses Provoked by Nicotine in the PA Test in Mice
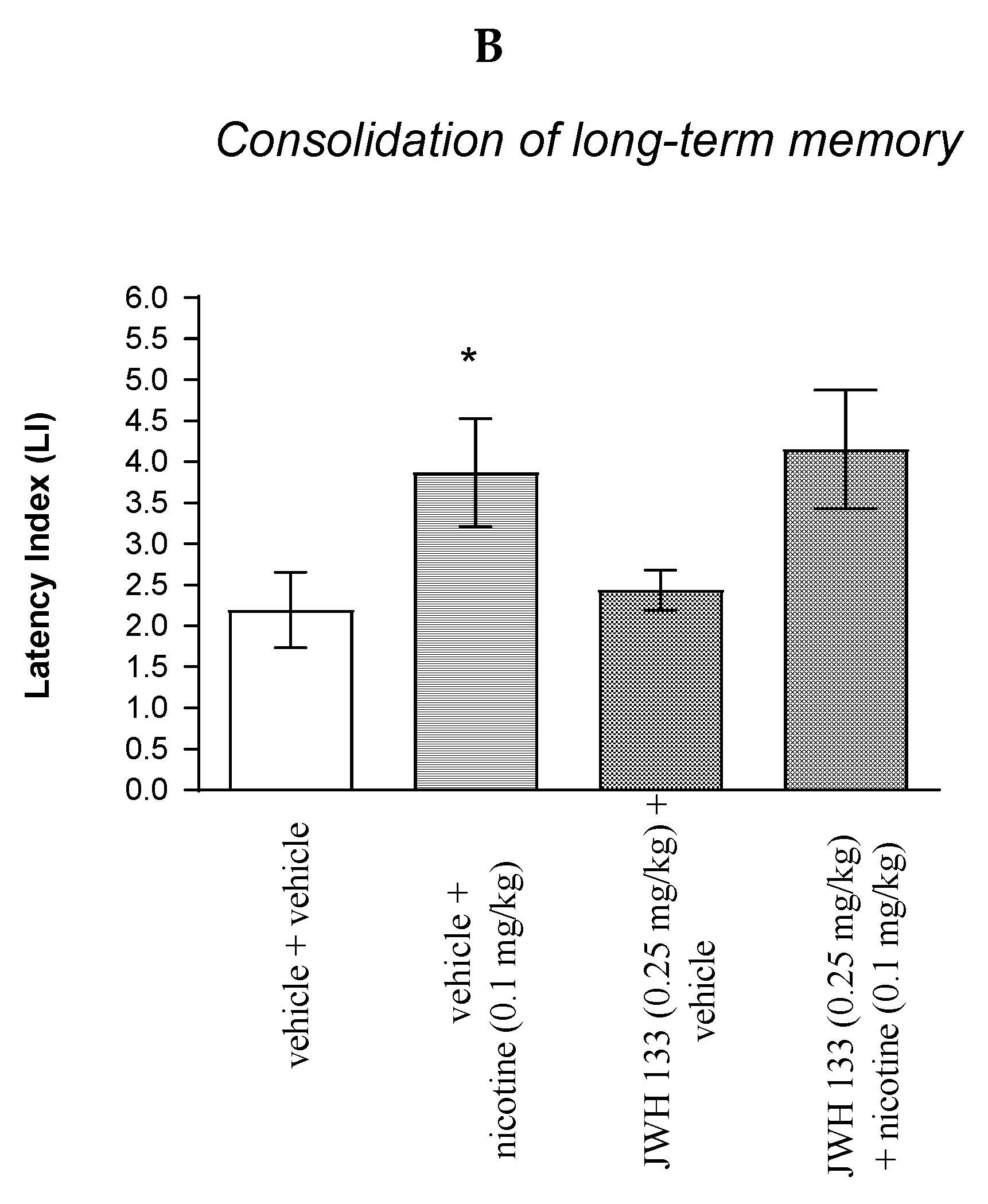
2.3.1. The Impact of the CB2 Receptor Agonist JWH 133 on the Memory and Learning Processes Provoked by Non-Effective Dose of Nicotine in Mice in the PA Test
2.3.2. The Impact of the CB2 Receptor Antagonist AM 630 on the Memory and Learning Processes Provoked by a Non-Effective Dose of Nicotine in Mice in the PA Test
2.3.3. The Impact of the CB2 Receptor Agonist JWH 133 on the Memory and Learning Processes Provoked by Effective (Pro-Cognitive) Dose of Nicotine in Mice in the PA Test
2.3.4. The Impact of the CB2-Receptor Antagonist AM 630 on the Memory and Learning Processes Induced by Effective (Pro-Cognitive) Dose of Nicotine in Mice in the PA Test
2.4. The Influence of the Administration of CB2-Receptor Ligands on the Memory-Related Responses Provoked by Scopolamine in the PA Test in Mice
2.4.1. The Impact of the CB2-Receptor Agonist JWH 133 on the Memory and Learning Processes Induced by Effective (Amnestic) Dose of Scopolamine in Mice in the PA Test
2.4.2. The Impact of the CB2-Receptor Antagonist AM 630 on the Memory and Learning Processes Induced by Effective (Amnestic) Dose of Scopolamine in Mice in the PA Test
3. Discussion
3.1. The Impact of Cholinergic Receptor Ligands on the Memory Acquisition and Consolidation in PA Test in Mice
3.2. The Impact of CB2-Receptor Ligands on Memory Acquisition and Consolidation in the PA Test in Mice
3.3. The Impact of CB2-Receptor Ligands on the Memory Acquisition and Consolidation Responses Induced by Cholinergic Receptor Ligands in the PA Test in Mice
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Experimental Procedures
4.4. Treatment
- the non-effective and effective dose of nicotine (0.05 mg/kg and 0.1 mg/kg, respectively)
- the effective dose of scopolamine (1 mg/kg)
- non-effective dose of JWH 133(0.25 mg/kg) and AM 630 (0.25 mg/kg).
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, J.X.; Du, X.X.; Zhao, L.; Tian, Q.; Zhu, L.Q.; Wang, S.H.; Wang, J.Z. Temporal correlation of the memory deficit with Alzheimer-like lesions induced by activation of glycogen synthase kinase-3. J. Neurochem. 2008, 106, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Zlatar, Z.Z.; Muniz, M.; Galasko, D. Subjective Cognitive Decline Correlates with Depression Symptoms and Not with Concurrent Objective Cognition in a Clinic-Based Sample of Older Adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2018, 73, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Sobow, T. Combination treatments in Alzheimer’s disease: Risks and benefits. Exp. Rev. Neurother. 2010, 10, 693–702. [Google Scholar] [CrossRef]
- Zubenko, G.S. Neurobiology of major depression in Alzheimer’s disease. Int. Psychogeriatr. 2000, 10, 129–141. [Google Scholar] [CrossRef]
- Bohme, G.A.; Laville, M.; Ledent, C.; Parmentier, M.; Imperato, A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience 2000, 95, 5–7. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Biala, G. CB1 receptors in the formation of the different phases of memory-related processes in the inhibitory avoidance test in mice. Behav. Brain Res. 2016, 301, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Boguszewska-Czubara, A.; Slomka, T.; Budzynska, B.; Biala, G. Correlations between the memory-related behavior and the level of oxidative stress biomarkers in the mice brain, provoked by an acute administration of CB receptor ligands. Neural Plast. 2016, 2016, 9815092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos. Trans. R. Soc. Lond B Biol. Sci. 2012, 367, 3254–3263. [Google Scholar] [CrossRef] [Green Version]
- Morena, M.; Campolongo, P. The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014, 112, 30–43. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Navaeian, M.; Nasehi, M. Inflluence of three-day morphine-treatment upon impairment of memory consolidation induced by cannabinoid infused into the dorsal hippocampus in rats. Neurosci. Res. 2011, 69, 51–59. [Google Scholar] [CrossRef]
- Schurman, L.D.; Lu, D.; Kendall, D.A.; Howlett, A.C.; Lichtman, A.H. Molecular mechanism and cannabinoid pharmacology. Handb. Exp. Pharmacol. 2020, 258, 323–353. [Google Scholar] [PubMed]
- Kruk-Slomka, M.; Budzynska, B.; Biala, G. Involvement of cholinergic receptors in the different stages of memory measured in the modified elevated plus maze test in mice. Pharmacol. Rep. 2012, 64, 1066–1080. [Google Scholar] [CrossRef]
- Alhowail, A. Molecular insights into the benefits of nicotine on memory and cognition (Review). Mol. Med. Rep. 2021, 23, 398. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.S.; Robles, O.; Kakoyannis, A.N.; Sherr, J.D.; Avila, M.T.; Blaxton, T.A.; Thaker, G.K. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology 2004, 174, 334–340. [Google Scholar] [CrossRef]
- Wylie, K.P.; Rojas, D.C.; Tanabe, J.; Martin, L.F.; Tregellas, J.R. Nicotine Increases Brain Functional Network Efficiency. Neuroimage 2012, 63, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Beer, A.L. Chapter 27-Nicotine and Cognition: Effects of Nicotine on Attention and Memory Systems in Humans. In Neuropathology of Drug Addictions and Substance Misuse. Volume 1: Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids; Academic Press: Cambridge, MA, USA, 2016; pp. 282–290. [Google Scholar]
- Rusted, J.M.; Trawley, S.; Heath, J.; Kettle, G.; Walker, H. Nicotine improves memory for delayed intentions. Psychopharmacology 2005, 182, 355–365. [Google Scholar] [CrossRef]
- White, H.K.; Levi, E.D. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology 2004, 171, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Blokland, A. Scopolamine-induced deficits in cognitive performance: A review of animal studies. Scopolamine Rev. 2005, 1, 1–76. [Google Scholar]
- Hodges, D.B., Jr.; Lindner, M.D.; Hogan, J.B.; Jones, K.M.; Markus, E.J. Scopolamine induced deficits in a battery of rat cognitive tests: Comparisons of sensitivity and specificity. Behav. Pharmacol. 2009, 20, 237–251. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Budzynska, B.; Biala, G.; Boguszewska-Czubara, A. Scopolamine-induced memory impairment is alleviated by xanthotoxin: Role of acetylcholinesterase and oxidative stress processes. ACS Chem. Neurosci. 2018, 9, 1184–1194. [Google Scholar] [CrossRef]
- Rezvani, A.H.; Cauley, M.; Sexton, H.; Xiao, Y.; Brown, M.L.; Paige, M.A.; McDowell, B.E.; Kellar, K.J.; Levin, E.D. Sazetidine-A, a selective a4b2 nicotinic acetylcholine receptor ligand: Effects on dizocilpine and scopolamine-induced attentional impairments in female SpragueDawley rats. Psychopharmacology 2011, 215, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Hefco, V.; Yamada, K.; Hefco, A.; Hritcu, L.; Tiron, A.; Olariu, A.; Nabeshima, T. Effects of nicotine on memory impairment induced by blockade of muscarinic, nicotinic and dopamine D2 receptors in rats. Eur. J. Pharmacol. 2003, 474, 227–232. [Google Scholar] [CrossRef]
- Levin, E.D.; Rezvani, A.H. Development of nicotinic drug therapy for cognitive disorders. Eur. J. Pharmacol. 2000, 393, 141–146. [Google Scholar] [CrossRef]
- Matta, J.A.; Gu, S.; Davini, W.B.; Bredt, D.S. Nicotinic acetylcholine receptor redux: Discovery of accessories opens therapeutic vistas. Science 2021, 373, eabg6539. [Google Scholar] [CrossRef]
- Han, G.; An, L.; Yang, B.; Si, L.; Zhang, T. Nicotine-induced impairments of spatial cognition and long-term potentiation in adolescent male rats. Hum. Exp. Toxicol. 2014, 33, 203–213. [Google Scholar] [CrossRef]
- Tanda, G.; Goldberg, S.R. Cannabinoids: Reward dependence and underlying neurochemical mechanisms-a review a recent preclinical data. Psychopharmacology 2003, 169, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.D.; Jones, A.K.; Brown, L.A.; Sattelle, D.B. Nicotinic acetylcholine receptor signalling: Roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol. Rev. 2009, 61, 39–61. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, P.J.; Levin, E.D. Effects of dopaminergic drugs on working and reference memory in rats. Pharmacol. Biochem. Behav. 1993, 45, 765–776. [Google Scholar] [CrossRef]
- Handler, A.; Graham, T.G.W.; Cohn, R.; Morantte, I.; Siliciano, A.F.; Zeng, J.; Li, Y.; Ruta, V. Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell 2019, 178, 60–75.e19. [Google Scholar] [CrossRef]
- Rezvani, A.H.; Levin, E.D. Cognitive effects of nicotine. Biol. Psychiatry 2001, 49, 258–267. [Google Scholar] [CrossRef]
- Sambeth, A.; Riedel, W.J.; Smits, L.T.; Blokland, A. Cholinergic drugs affect novel object recognition in rats: Relation with hippocampal EEG? Eur. J. Pharmacol. 2007, 572, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Seifhosseini, S.; Jahanshahi, M.; Moghimi, A.; Aazami, N.-S. The Effect of Scopolamine on Avoidance Memory and Hippocampal Neurons in Male Wistar Rats. BCN 2011, 3, 9–15. [Google Scholar]
- Spinelli, S.; Ballard, T.; Feldon, J.; Higgins, G.A.; Pryce, C.R. Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology 2006, 51, 238–250. [Google Scholar] [CrossRef]
- Azami, N.S.; Jahanshahi, M.; Babapour, V. The role of CA1 α-adrenoceptor on scopolamine induced memory impairment in male rats. Physiol. Pharmacol. 2010, 14, 66–77. [Google Scholar]
- Blake, M.G.; Boccia, M.M.; Krawczyk, M.C.; Baratti, C.M. Scopolamine prevents retrograde memory interference between two different learning tasks. Physiol. Behav. 2011, 102, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Power, A.E.; Vazdarjanova, A.; McGaugh, J.L. Muscarinic cholinergic influences in memory consolidation. Neurobiol. Learn. Mem. 2003, 80, 178–193. [Google Scholar] [CrossRef]
- Agrawal, R.; Tyagi, E.; Saxena, G.; Nath, C. Cholinergic influence on memory stages: A study on scopolamine amnesic mice. Indian J. Pharmacol. 2009, 41, 192–196. [Google Scholar] [PubMed]
- Mirza, N.R.; Stolerman, I.P. The role of nicotinic and muscarinic acetylcholine receptors in attention. Psychopharmacology 2000, 148, 243–250. [Google Scholar] [CrossRef]
- Widy-Tyszkiewicz, E. Long Term Administration of Hypericum perforatum Improves Spatial Learning and Memory in the Water Maze. Biol. Pharm. Bull. 2002, 25, 1289–1294. [Google Scholar] [CrossRef] [Green Version]
- Buccafusco, J.J. The revival of scopolamine reversal for the assessment of cognition-enhancing drugs. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 329–341. [Google Scholar]
- Kim, Y.H.; Lee, Y.; Kim, D.; Jung, M.W.; Lee, C.J. Scopolamine-induced learning impairment reversed by physostigmine in zebrafish. Neurosci. Res. 2010, 67, 156–161. [Google Scholar] [CrossRef]
- Naik, R.S.; Hartmann, J.; Kiewert, C.; Duysen, E.G.; Lockridge, O.; Klein, J. Effects of rivastigmine and donepezil on brain acetylcholine levels in acetylcholinesterase-deficient mice. J. Pharm. Pharm. Sci. 2009, 12, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodi, G.; Ahmadi, S.; Pourmotabbed, A.; Oryan, S.; Zarrindast, M.R. Inhibitory avoidance memory deficit induced by scopolamine: Interaction of cholinergic and glutamatergic systems in the ventral tegmental area. Neurobiol. Learn. Mem. 2010, 94, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Pakpour, B.; Ahmadi, S.; Nayer-Nouri, T.; Oryan, S.; Zarrindast, M.R. Inhibitory avoidance memory deficit induced by scopolamine: Interaction with glutamatergic system in the nucleus accumbens. Behav. Pharmacol. 2010, 21, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.; Packard, M.G. The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiol. Learn. Mem. 2015, 125, 1–14. [Google Scholar] [CrossRef]
- Maroso, M.; Szabo, G.G.; Kim, H.K.; Alexander, A.; Bui, A.D.; Lee, S.-H.; Lutz, B.; Soltesz, I. Cannabinoid Control of Learning and Memory through HCN Channels. Neuron 2016, 89, 1059–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, G.; Davies, S.N. Cannabinoid Function in Learning, Memory and Plasticity. Cannabinoids 2005, 168, 445–477. [Google Scholar]
- Koppel, J.; Davies, P. Targetting the endocannabinoid system in Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Croxford, J.L. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs 2003, 17, 179–202. [Google Scholar] [CrossRef]
- Van der Stelt, M.; Di Marzo, V. Cannabinoid receptors and their role in neuroprotection. Neuromol. Med. 2005, 7, 37–50. [Google Scholar] [CrossRef]
- Abush, H.; Akirav, I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 2010, 20, 1126–1138. [Google Scholar] [CrossRef]
- Wolff, M.C.; Leander, J.D. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur. J. Pharmacol. 2003, 477, 213–217. [Google Scholar] [CrossRef]
- Skaper, S.D.; Di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. Lond B Biol. Sci. 2012, 367, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. The clinical toxicology perspective on the therapeutic use of cannabis and cannabinoids. Acta Med. Port. 2019, 32, 87–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, C.; Nunez, E.; Tolón, R.; Carrier, E.; Rảbano, A.; Hillard, C.; Romero, J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque–associated glia in Alzheimer’s disease brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. J. Neurol. 2009, 132, 3152–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Valdepeñas, L.; Benito, C.; Tolon, R.M.; Martinez-Orgado, J.A.; Romero, J. The endocannabinoid system and amyloid-related diseases. Exp. Neurol. 2010, 224, 66–73. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, C.L.; Chen, M.; Manivannan, A.; Cabay, L.; Pertwee, R.G.; Coutts, A.; Forrester, J.V. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH 133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007, 82, 532–541. [Google Scholar] [CrossRef]
- Andó, R.D.; Bíró, J.; Csölle, C.; Ledent, C.; Sperlágh, B. The inhibitory action of exo- and endocannabinoids on [3H]GABA release are mediated by both CB1 and CB2 receptors in the mouse hippocampus. Neurochem. Int. 2012, 60, 145–152. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; García-Bueno, B.; Zoppi, S.; Leza, J.C.; Manzanares, J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA-A receptors. Br. J. Pharmacol. 2012, 165, 951–964. [Google Scholar] [CrossRef] [Green Version]
- Morgan, N.H.; Stanford, I.M.; Woodhall, G.L. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 2009, 57, 356–368. [Google Scholar] [CrossRef]
- Bolognini, D.; Cascio, M.G.; Parolaro, D.; Pertwee, R.G. AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor. Br. J. Pharmacol. 2012, 165, 2561–2574. [Google Scholar] [CrossRef] [Green Version]
- García-Gutiérrez, M.S.; Ortega-Álvaro, A.; Busquets-García, M.; Pérez-Ortiz, J.M.; Caltana, L.; Ricatti, M.J.; Brusco, A.; Maldonado, R.; Manzanares, J. Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors. Neuropharmacology 2013, 73, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Häring, M.; Marsicano, G.; Lutz, B.; Monory, K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 2007, 146, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Tolon, R.M.; Nunez, E.; Pazos, M.R.; Benito, C.; Castillo, A.I.; Martinez-Orgado, J.A.; Romero, J. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. Rev. 2009, 1283, 148–154. [Google Scholar] [CrossRef]
- Balerio, G.N.; Aso, E.; Berrendero, F.; Murtra, P.; Maldonado, R. Delta 9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur. J. Neurosci. 2004, 20, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Balerio, G.N.; Aso, E.; Maldonado, R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology 2006, 184, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Castañé, A.; Berrendero, F.; Maldonado, R. The role of the cannabinoid system in nicotine addiction. Pharmacol. Biochem. Behav. 2005, 81, 381–386. [Google Scholar] [CrossRef]
- Valjent, E.; Mitchell, J.M.; Besson, M.J.; Caboche, J.; Maldonado, R.; Le Fur, G.; Soubrie, P. Behavioral and biochemical evidence for interactions between delta-9-tetrahydrocannabinol and nicotine. Br. J. Pharmacol. 2002, 135, 564–578. [Google Scholar] [CrossRef] [Green Version]
- Masuoka, T.; Kamei, C. The role of nicotinic receptors in the amelioration of cholinesterase inhibitors in scopolamine-induced memory deficits. Psychopharmacology 2009, 206, 259–265. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Brunzell, D.H.; Caldarone, B.J. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport 2002, 13, 1097–1106. [Google Scholar] [CrossRef]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Cannabis addiction and the brain: A review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk-Slomka, M.; Biala, G. Cannabidiol attenuates MK-801-induced cognitive symptoms of schizophrenia in the passive avoidance test in mice. Molecules 2021, 26, 5977. [Google Scholar] [CrossRef] [PubMed]
- Chimakurthy, J.; Talasila, M. Effects of curcumin on pentylenetetrazole-induced anxiety-like behaviors and associated changes in cognition and monoamine levels. Psychol. Neurosci. 2010, 3, 239–244. [Google Scholar] [CrossRef] [Green Version]











| Acquisition of Memory | |||||
|---|---|---|---|---|---|
| PA Test | Drug Administration | Interval | TL1 | Interval | TL2 |
| Long-term memory | Nicotine (0.05 or 0.1 mg/kg) | 30 min | + | 24 h | + |
| Scopolamine (1 mg/kg) | 30 min | + | 24 h | + | |
| JWH 133 (0.25; 0.5; 1 mg/kg) | 30 min | + | 24 h | + | |
| AM 630 (0.25; 0.5; 1 mg/kg) | 30 min | + | 24 h | + | |
| Vehicle (for the control group) | 30 min | + | 24 h | + | |
| Consolidation of Memory | |||||
|---|---|---|---|---|---|
| PA Test | TL1 | Interval | Drug Administration | Interval | TL2 |
| Long-term memory | + | 0 min | Nicotine (0.05 or 0.1 mg/kg) | 24 h | + |
| + | 0 min | Scopolamine (1 mg/kg) | 24 h | + | |
| + | 0 min | JWH 133 (0.25; 0.5; 1 mg/kg) | 24 h | + | |
| + | 0 min | AM 630 (0.25; 0.5; 1 mg/kg) | 24 h | + | |
| + | 0 min | Vehicle (for the control group) | 24 h | + | |
| Acquisition of Memory | |||||||
|---|---|---|---|---|---|---|---|
| PA Test | Drug Administration | Interval | Drug Administration | Interval | TL1 | Interval | TL2 |
| Long-term memory | JWH 133 (0.25 mg/kg) | 15 min | nicotine (0.05 mg/kg) or nicotine (0.1 mg/kg) or scopolamine (1 mg/kg) or vehicle | 15 min | + | 24 h | + |
| AM 630 (0.25 mg/kg) | 15 min | nicotine (0.05 mg/kg) or nicotine (0.1 mg/kg) or scopolamine (1 mg/kg) or vehicle | 15 min | + | 24 h | + | |
| vehicle (control group) | 15 min | nicotine (0.05 mg/kg) or nicotine (0.1 mg/kg) or scopolamine (1 mg/kg) or vehicle | 15 min | + | 24 h | + | |
| Consolidation of Memory | |||||||
|---|---|---|---|---|---|---|---|
| PA Test | TL1 | Interval | Drug Administration | Interval | Drug Administration | Interval | TL2 |
| Long-term memory | + | 0 min | JWH 133 (0.25 mg/kg) | 15 min | nicotine (0.05 mg/kg) or nicotine (0.1 mg/kg) or scopolamine (1 mg/kg) or vehicle | 24 h | + |
| + | 0 min | AM 630 (0.25 mg/kg) | 15 min | nicotine (0.05 mg/kg) or nicotine (0.1 mg/kg) or scopolamine (1 mg/kg) or vehicle | 24 h | + | |
| + | 0 min | vehicle (control group) | 15 min | nicotine (0.05 mg/kg) or nicotine (0.1 mg/kg) or scopolamine (1 mg/kg) or vehicle | 24 h | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk-Slomka, M.; Dzik, A.; Biala, G. The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test. Molecules 2022, 27, 4252. https://doi.org/10.3390/molecules27134252
Kruk-Slomka M, Dzik A, Biala G. The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test. Molecules. 2022; 27(13):4252. https://doi.org/10.3390/molecules27134252
Chicago/Turabian StyleKruk-Slomka, Marta, Agnieszka Dzik, and Grazyna Biala. 2022. "The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test" Molecules 27, no. 13: 4252. https://doi.org/10.3390/molecules27134252
APA StyleKruk-Slomka, M., Dzik, A., & Biala, G. (2022). The Influence of CB2-Receptor Ligands on the Memory-Related Responses in Connection with Cholinergic Pathways in Mice in the Passive Avoidance Test. Molecules, 27(13), 4252. https://doi.org/10.3390/molecules27134252






