Antitumor Effect of Glandora rosmarinifolia (Boraginaceae) Essential Oil through Inhibition of the Activity of the Topo II Enzyme in Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Anticancer Activity of G. rosmarinifolia Essential Oil
2.2. In Vitro Pro-Oxidant Activity of G. rosmarinifolia Essential Oil
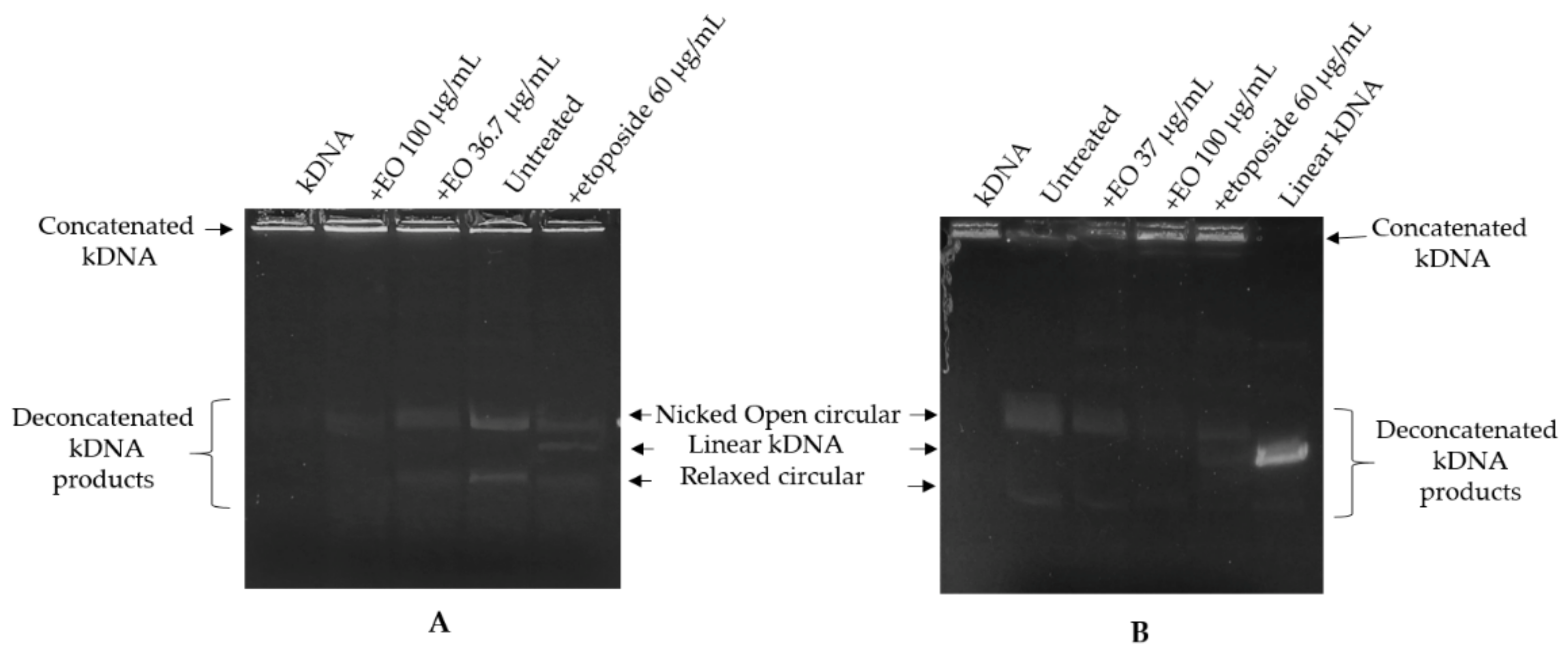
2.3. Kinetoplast DNA Decatenation Assay
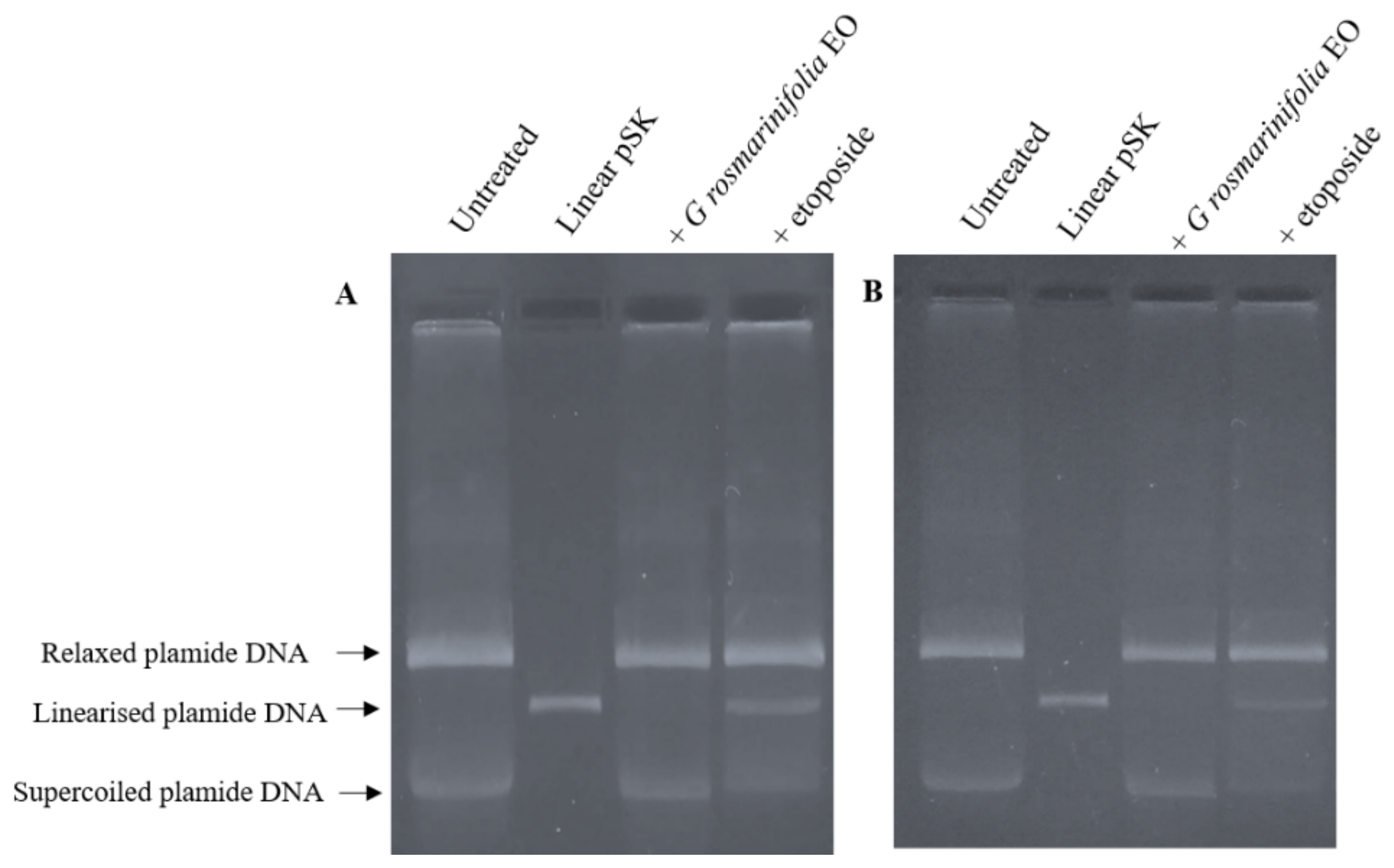
2.4. Plasmid DNA Linearization Assay
2.5. Cell-Cycle Analysis
2.6. Cytotoxic Effects of G. rosmarinifolia EO in Combination with Etoposide and Doxorubicin
3. Materials and Methods
3.1. Essential Oil
3.2. Cell Lines and Culture Conditions
3.3. Cell Growth Inhibition Assays
3.4. Pro-Oxidant Activity
3.5. Preparation of Nuclear Extracts
3.6. Kinetoplast DNA Decatenation Assay
3.7. Plasmid DNA Linearization Assay
3.8. Cell-Cycle Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Nouzha, H.; Souhila, B.; Manel, M.; Yasmine, O.; Lamraoui, A. Screening for antibacterial activity of some essential oils and evaluation of their synergistic effec. Int. J. Biosci. 2018, 12, 292–301. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 15, 268–291. [Google Scholar] [CrossRef]
- Sakkas, H.; Gousia, P.; Economou, V.; Sakkas, V.; Petsios, S.; Papadopoulou, C. In vitro antimicrobial activity of five essential oils on multidrug resistant Gram-negative clinical isolates. J. Intercult. Ethnopharmacol. 2016, 5, 212–218. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poma, P.; Labbozzetta, M.; Zito, P.; Alduina, R.; Ramarosandratana, A.V.; Bruno, M.; Rosselli, S.; Sajeva, M.; Notarbartolo, M. Essential oil composition of Alluaudia procera and in vitro biological activity on two drug-resistant models. Molecules 2019, 24, 2871. [Google Scholar] [CrossRef] [Green Version]
- Poma, P.; Labbozzetta, M.; Notarbartolo, M.; Bruno, M.; Maggio, A.; Rosselli, S.; Sajeva, M.; Zito, P. Chemical composition, in vitro antitumor and prooxidant activities of Glandora rosmarinifolia (Boraginaceae) essential oil. PLoS ONE 2018, 13, e0196947. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.; Labbozzetta, M.; Notarbartolo, M.; Sajeva, M.; Poma, P. Essential oil of Cyphostemma juttae (Vitaceae): Chemical compositionand antitumor mechanismin triple negative breast cancer cells. PLoS ONE 2019, 14, e0214594. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; McCubrey, J.A.; Ramarosandratana, A.V.; Sajeva, M.; Zito, P.; Notarbartolo, M. Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals 2019, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- López, L.L.I.; Flores, N.D.S.; Silva Belmares, S.Y.; Sáenz Galindo, A. Naphthoquinones: Biological properties and synthesis of lawsone and derivatives-a structured review. Vitae 2014, 21, 248–258. [Google Scholar]
- Wu, L.; Zhang, C.; Li, W. Synthesis and antiproliferative evaluation of 13-aryl-13H-benzo[g]benzothiazolo [2,3-b]quinazoline-5,14-diones. Bioorg. Med. Chem. Lett. 2014, 24, 1462. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Zhang, G.; Hager, A.; Moos, M.; Irmer, A.; Bargou, R.; Chatterjeel, M. Anti-tumoral activities of dioncoquinones B and C and related naphthoquinones gained from total synthesis or isolation from plants. Eur. J. Med. Chem. 2011, 46, 5778. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Bettega, K.; Pedrosa, R.C.; Buc Calderon, P.; Verrax, J. Biological evaluation of donor-acceptor aminonaphthoquinones as antitumor agents. Eur. J. Med. Chem. 2010, 45, 6052. [Google Scholar] [CrossRef]
- Kumar, B.S.; Ravi, K.; Verma, A.K.; Fatima, K.; Hasanain, M.; Singh, A.; Sarkar, J.; Luqman, S.; Chanda, D.; Negi, A.S. Synthesis of pharmacologically important naphthoquinones and anticancer activity of 2-benzyllawsone through DNA topoisomerase-II inhibition. Bioorg. Med. Chem. 2017, 25, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Haneda, M.; Iwata, S.; Yoshino, M. Effect of hydroxy substituent on the pro-oxidant action of naphthoquinone compounds. Toxicol. Vitr. 2010, 24, 905–909. [Google Scholar] [CrossRef]
- Kumagai, Y.; Shinkai, Y.; Miura, T.; Cho, A.K. The chemical biology of naphthoquinones and its environmental implications. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, M.T.; Ljungman, M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol. Appl. Pharmacol. 2005, 209, 1–9. [Google Scholar] [CrossRef]
- Ji, Y.B.; Qu, Z.Y.; Zou, X. Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp. Toxicol. Pathol. 2011, 63, 69–78. [Google Scholar] [CrossRef]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Zhang, R.; Qu, X.R.; Chen, Y.P.; Ma, X.Y.; Sui, D.Y. Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondriadependent pathway. Eur. J. Pharmacol. 2010, 645, 14–22. [Google Scholar] [CrossRef]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Qu, X.R.; Jiang, Y.F.; Sui, D.Y. Juglone, from Juglans mandshruica Maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 through a reactive oxygen species-dependent mechanism. Food Chem. Toxicol. 2012, 50, 590–596. [Google Scholar] [CrossRef]
- Kaewbumrung, S.; Panichayupakaranant, P. Antibacterial activity of plumbagin derivative rich Plumbago indica root extracts and chemical stability. Nat. Prod. Res. 2014, 28, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wong, Y.F.; Ge, L.; Zhang, Z.F.; Liu, Y.; Liu, L.; Zhou, H. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear Factor κB activation. J. Pharmacol. Exp. Ther. 2010, 335, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. BioMed Res. Int. 2020, 2020, 6940953. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Wang, T.; Tang, T. Advances in tumor suppression by plumbagin and it mechanism. Tumor 2014, 34, 957–962. [Google Scholar] [CrossRef]
- Anaissi-Afonso, L.; Oramas-Royo, S.; Ayra-Plasencia, J.; Martín-Rodríguez, P.; García-Luis, J.; Lorenzo-Castrillejo, I.; Fernández-Pérez, L.; Estévez-Braun, A.; Machín, F. Lawsone, Juglone, and β-Lapachone Derivatives with Enhanced Mitochondrial-Based Toxicity. ACS Chem. Biol. 2018, 17, 1950–1957. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New Horizons for Old Drugs and Drug Leads. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef]
- Mu, Z.; Guo, J.; Zhan, D.; Xu, Y.; Zhou, M.; Guo, Y.; Hou, Y.; Gao, X.; Han, X.; Geng, L. Therapeutic Effects of Shikonin on Skin Diseases: A Review. Am. J. Chin. Med. 2021, 49, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, S.H.; Kim, Y.M.; Kim, S.H.; Yoo, E.S.; Woo, J.S.; Jung, G.H.; Jung, S.H.; Kim, B.S.; Jung, J.Y. Shikonin inhibits proliferation of melanoma cells by MAPK pathway-mediated induction of apoptosis. Biosci. Rep. 2021, 29, BSR20203834. [Google Scholar] [CrossRef]
- Gomes, C.L.; de Albuquerque Wanderley Sales, V.; Gomes de Melo, C.; Ferreira da Silva, R.M.; Vicente Nishimura, R.H.; Rolim, L.A.; Rolim Neto, P.J. Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry 2021, 186, 112713. [Google Scholar] [CrossRef]
- Wang, H.; Mao, Y.; Chen, A.Y.; Zhou, N.; LaVoie, E.J.; Liu, L.F. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry 2001, 40, 3316–3323. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Yamashita, Y.; Arima, Y.; Nagashima, M.; Nakano, H. Induction of topoisomerase II-mediated DNA cleavage by the plant naphthoquinones plumbagin and shikonin. Antimicrob. Agents Chemother. 1992, 36, 2589–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Chen, Y.; Duan, W.; Zhang, C.; Zhu, H.; Ding, J. SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int. J. Cancer 2006, 1, 1184–1193. [Google Scholar] [CrossRef]
- Denny, W.A. Emerging DNA topisomerase inhibitors as anticancer drugs. Expert Opin. Emerg. Drugs 2004, 9, 105–133. [Google Scholar] [CrossRef]
- Smart, D.J.; Halicka, H.D.; Schmuck, G.; Traganos, F.; Darzynkiewicz, Z.; Williams, G.M. Assessment of DNA double-strand breaks and gammaH2AX induced by the topoisomerase II poisons etoposide and mitoxantrone. Mutat. Res. 2008, 10, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 28, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcy, N.D.; Gabrielli, B. Topoisomerase II Inhibitors and Poisons, and the Influence of Cell Cycle Checkpoints. Curr. Med. Chem. 2017, 24, 1504–1519. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Cervello, M.; Poma, P.; Dusonchet, L.; Meli, M.; D’Alessandro, N. Expression of the IAPs in multidrug resistant tumor cells. Oncol. Rep. 2004, 11, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.B.; Sorensen, B.S.; Demant, E.J.; Sehested, M.; Jensen, P.S.; Vindelov, L.; Hansen, H.H. Antagonistic effect of aclarubicin on the cytotoxixity of etoposide and 40-(9-acridinylamino) methanesulfon-m-anisidide in human small cell lung cancer cell lines and on topoisomerase II-mediated DNA cleavage. Cancer Res. 1990, 50, 3311–3316. [Google Scholar]
- Mathur, R.; Chandna, S.; Kapoor, P.N.; Dwarakanath, B.S. Peptidyl prolyl isomerase, Pin1 is a potential target for enhancing the therapeutic efficacy of etoposide. Curr. Cancer Drug Targets 2011, 11, 380–392. [Google Scholar] [CrossRef] [PubMed]


| IC50 (Mean ± SE) µg/mL | Resistance Factor (RF) | |||
|---|---|---|---|---|
| HL-60 | HL-60R | hTERT RPE-1 | ||
| G. rosmarinifolia EO | 36.75 ± 0.2 | 37.0 ± 0.7 | >50.0 | 1 |
| etoposide | 0.05 ± 2.9 | 7.4 ± 0.6 | NT | 148.0 |
| Cell Lines and Treatments | Cell Viability (%) |
|---|---|
| HL-60 | |
| NAC 1 mM | 100.0± 0.3 |
| NAC 2 mM | 81.5 ± 6.4 |
| essential oil of G. rosmarinifolia 36.7 µg/mL | 49.5 ± 5.3 * |
| NAC 1 mM + essential oil of G. rosmarinifolia 36.7 µg/mL | 81.5 ± 3.9 |
| NAC 2 mM + essential oil of G. rosmarinifolia 36.7 µg/mL | 80.0 ± 2.8 |
| HL-60R | |
| NAC 1 mM | 100.0 ± 0.3 |
| NAC 2 mM | 100.0 ± 2.1 |
| essential oil of G. rosmarinifolia 37 µg/mL | 47.5 ± 4.3 * |
| NAC 1 mM + essential oil of G. rosmarinifolia 37 µg/mL | 76.5 ± 0.3 * |
| NAC 2 mM + essential oil of G. rosmarinifolia 37 µg/mL | 97.0 ± 1.4 |
| G0/G1 | S | G2/M | |
|---|---|---|---|
| HL-60 | 45.0 ± 0.47 | 35.0 ± 0.94 | 18.3 ± 1.0 |
| + etoposide | 8.1± 1.65 *** | 19.1 ± 0.41 *** | 70.1 ± 0.52 *** |
| + G. rosmarinifolia EO | 69.0 ±1.88 *** | 14.8 ± 0.88 *** | 12.6 ± 0.17 * |
| HL-60R | 60.4 ± 0.67 | 22.4 ± 1.14 | 15.9 ± 0.52 |
| + etoposide | 7.4 ± 0.29 *** | 12.3 ± 0.61 *** | 79.5 ± 0.71 *** |
| + G. rosmarinifolia EO | 73.6 ± 0.76 *** | 13.0 ± 0.47 ** | 13.1 ± 0.52 |
| Treatments | Cell Growth Inhibition, % | Expected(%) |
|---|---|---|
| HL-60 | ||
| essential oil 20 µg/mL | 13.0 ± 2.1 | |
| essential oil 30 µg/mL | 31.2 ± 5.6 * | |
| etoposide 0.005 µg/mL | 15.0 ± 0.9 | |
| etoposide 0.01 µg/mL | 22.0 ± 0.9 * | |
| doxorubicin 0.002 µg/mL | 8.0 ± 2.1 | |
| doxorubicin 0.005 µg/mL | 21.0 ± 0.3 | |
| essential oil 20 µg/mL + etoposide 0.005µg/mL | 38.0 ± 0.7 ** | 26.0 ± 0.5 * b |
| essential oil 20 µg/mL + etoposide 0.01 µg/mL | 55.0 ± 0.4 ** | 32.0 ± 0.2 ** a |
| essential oil 30 µg/mL + etoposide 0.005 µg/mL | 54.0 ± 0.9 ** | 43.0 ± 0.2 ** b |
| essential oil 30 µg/mL + etoposide 0.01 µg/mL | 60.0 ± 1.2 ** | 46.0 ± 1.4 ** a |
| essential oil 20 µg/mL + doxorubicin 0.002 µg/mL | 35.0 ± 0.1 ** | 20.0 ± 1.4 a |
| essential oil 20 µg/mL + doxorubicin 0.005 µg/mL | 80.0 ± 1.3 *** | 32.0 ± 1.4 * a |
| essential oil 30 µg/mL + doxorubicin 0.002 µg/mL | 55.0 ± 1.2 ** | 39.0 ± 0.7 ** a |
| essential oil 30 µg/mL + doxorubicin 0.005 µg/mL | 83.0 ± 1.5 *** | 47.0 ± 2.1 ** a |
| HL-60R | ||
| essential oil 20 µg/mL | 22.3 ± 2.1 * | |
| essential oil 30 µg/mL | 32.0 ± 1.6 * | |
| etoposide 2.5 µg/mL | 11.0 ± 0.9 | |
| etoposide 5 µg/mL | 33.0 ± 0.7 * | |
| doxorubicin 1 µg/mL | 12.0 ± 2.6 | |
| doxorubicin 5 µg/mL | 32.0 ± 1.4 * | |
| essential oil 20 µg/mL + etoposide 2.5µg/mL | 42.0 ± 0.7 ** | 31.0 ± 0.5 ** b |
| essential oil 20 µg/mL + etoposide 5 µg/mL | 65.0 ± 0.7 ** | 48.0 ± 0.7 ** a |
| essential oil 30 µg/mL + etoposide 2.5 µg/mL | 53.0 ± 0.6 ** | 40.0 ± 0.4 ** b |
| essential oil 30 µg/mL + etoposide 5 µg/mL | 77.0 ± 0.5 *** | 55.0 ± 0.8 ** a |
| essential oil 20 µg/mL + doxorubicin 1 µg/mL | 44.0 ± 2.2 * | 31.0 ± 0.6 * a |
| essential oil 20 µg/mL + doxorubicin 5 µg/mL | 59.0 ± 0.7 ** | 48.0 ± 0.9 ** b |
| essential oil 30 µg/mL + doxorubicin 1 µg/mL | 56.0 ± 0.5 ** | 40.0 ± 2.3 ** a |
| essential oil 30 µg/mL + doxorubicin 5 µg/mL | 67.0 ± 0.9 ** | 53.0 ± 1.7 ** a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labbozzetta, M.; Poma, P.; Occhipinti, C.; Sajeva, M.; Notarbartolo, M. Antitumor Effect of Glandora rosmarinifolia (Boraginaceae) Essential Oil through Inhibition of the Activity of the Topo II Enzyme in Acute Myeloid Leukemia. Molecules 2022, 27, 4203. https://doi.org/10.3390/molecules27134203
Labbozzetta M, Poma P, Occhipinti C, Sajeva M, Notarbartolo M. Antitumor Effect of Glandora rosmarinifolia (Boraginaceae) Essential Oil through Inhibition of the Activity of the Topo II Enzyme in Acute Myeloid Leukemia. Molecules. 2022; 27(13):4203. https://doi.org/10.3390/molecules27134203
Chicago/Turabian StyleLabbozzetta, Manuela, Paola Poma, Chiara Occhipinti, Maurizio Sajeva, and Monica Notarbartolo. 2022. "Antitumor Effect of Glandora rosmarinifolia (Boraginaceae) Essential Oil through Inhibition of the Activity of the Topo II Enzyme in Acute Myeloid Leukemia" Molecules 27, no. 13: 4203. https://doi.org/10.3390/molecules27134203
APA StyleLabbozzetta, M., Poma, P., Occhipinti, C., Sajeva, M., & Notarbartolo, M. (2022). Antitumor Effect of Glandora rosmarinifolia (Boraginaceae) Essential Oil through Inhibition of the Activity of the Topo II Enzyme in Acute Myeloid Leukemia. Molecules, 27(13), 4203. https://doi.org/10.3390/molecules27134203








