Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
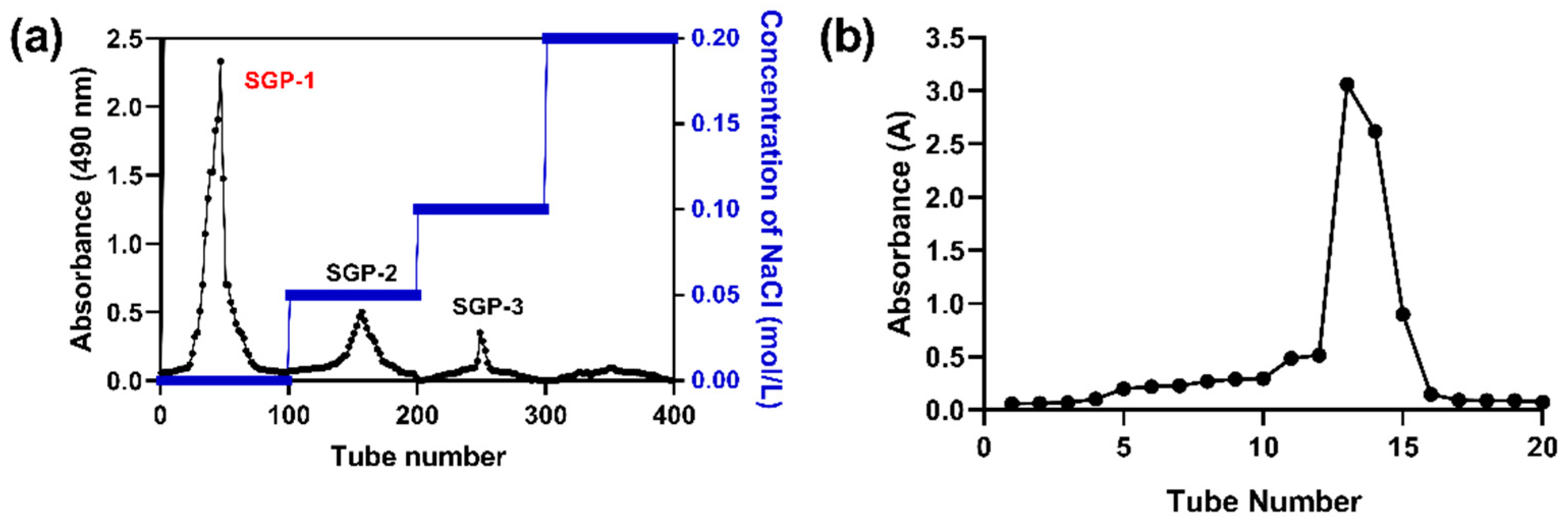
2.2. Isolation and Purification of SGP-1-1
2.3. Determination of SGP-1-1 Total Sugar, Protein, and Reducing Sugar Content
2.4. Structural Characterization of SGP-1-1
2.4.1. Molecular Weight (Mw)-Determination Analysis
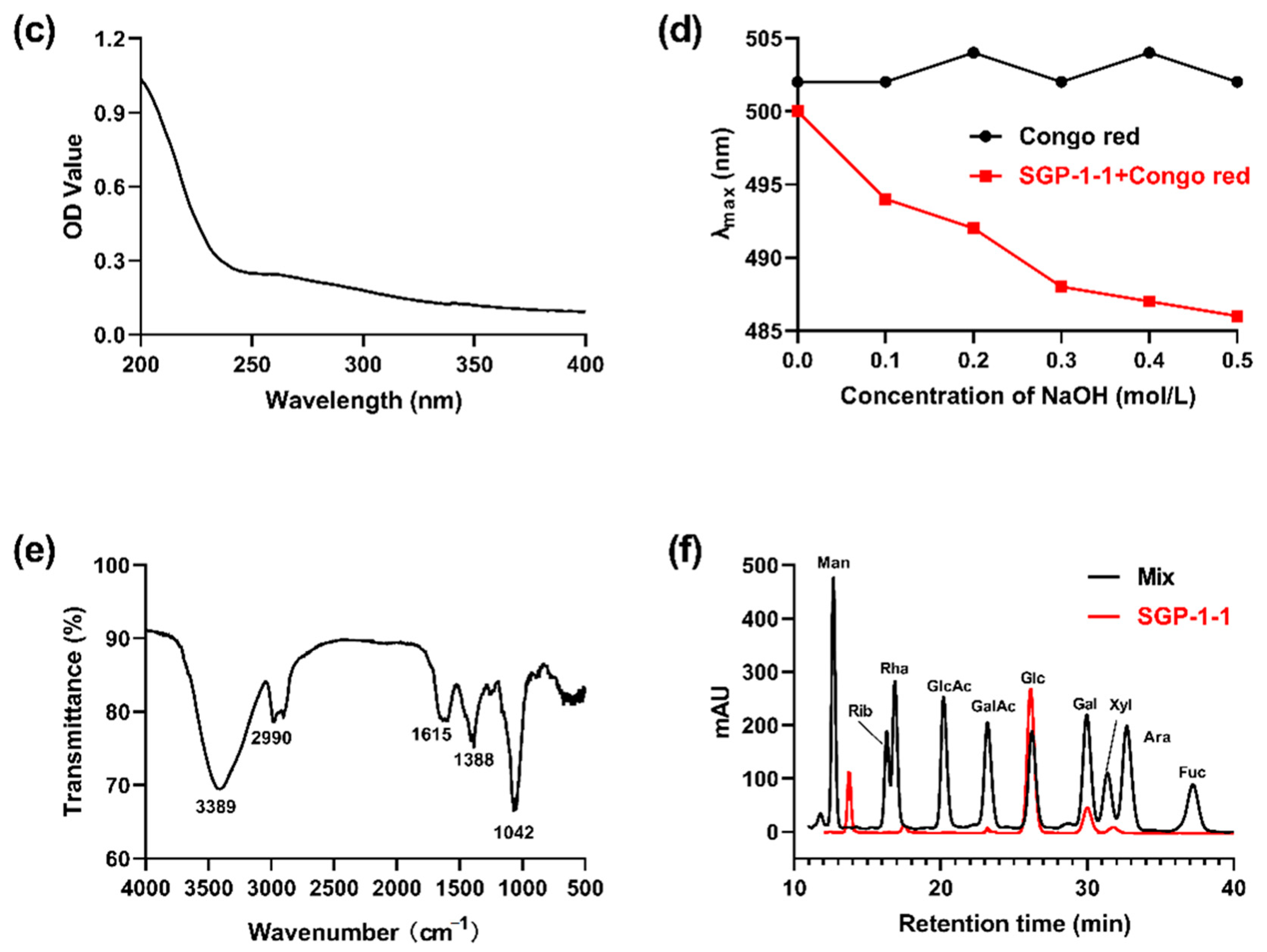
2.4.2. Monosaccharide-Composition Analysis
2.4.3. Ultraviolet (UV) and Fourier Transform Infrared (FT-IR)-Spectra Analysis
2.4.4. Nuclear Magnetic Resonance (NMR)-Spectroscopy Analysis
2.4.5. Congo-Red Test
2.4.6. Morphology Analysis
2.4.7. Methylation Analysis
2.5. In Vitro Antioxidant Capacity
2.6. In Vitro Hypoglycemic Activity
2.6.1. In Vitro α-Glucosidase- and α-Amylase-Inhibitory-Activity Assay
2.6.2. Construction of an Insulin Resistance (IR)-HepG2 Model
2.6.3. Assay of Hexokinase (HK), Pyruvate Kinase (PK) Activities, Triglyceride (TG), and Glycogen Content in HepG2 Cells
2.7. Statistical Analysis
3. Results and Discussion
3.1. Total Polysaccharide, Reducing Sugar, and Protein Content of SGP-1-1
3.2. Structural Characterization of SGP-1-1
3.2.1. Mw and Monosaccharides Composition of SGP-1-1
3.2.2. Spectroscopic Characteristics of SGP-1-1
3.2.3. Methylation Analysis
3.2.4. NMR Spectra of SGP-1-1
3.2.5. Congo-Red Test of SGP-1-1
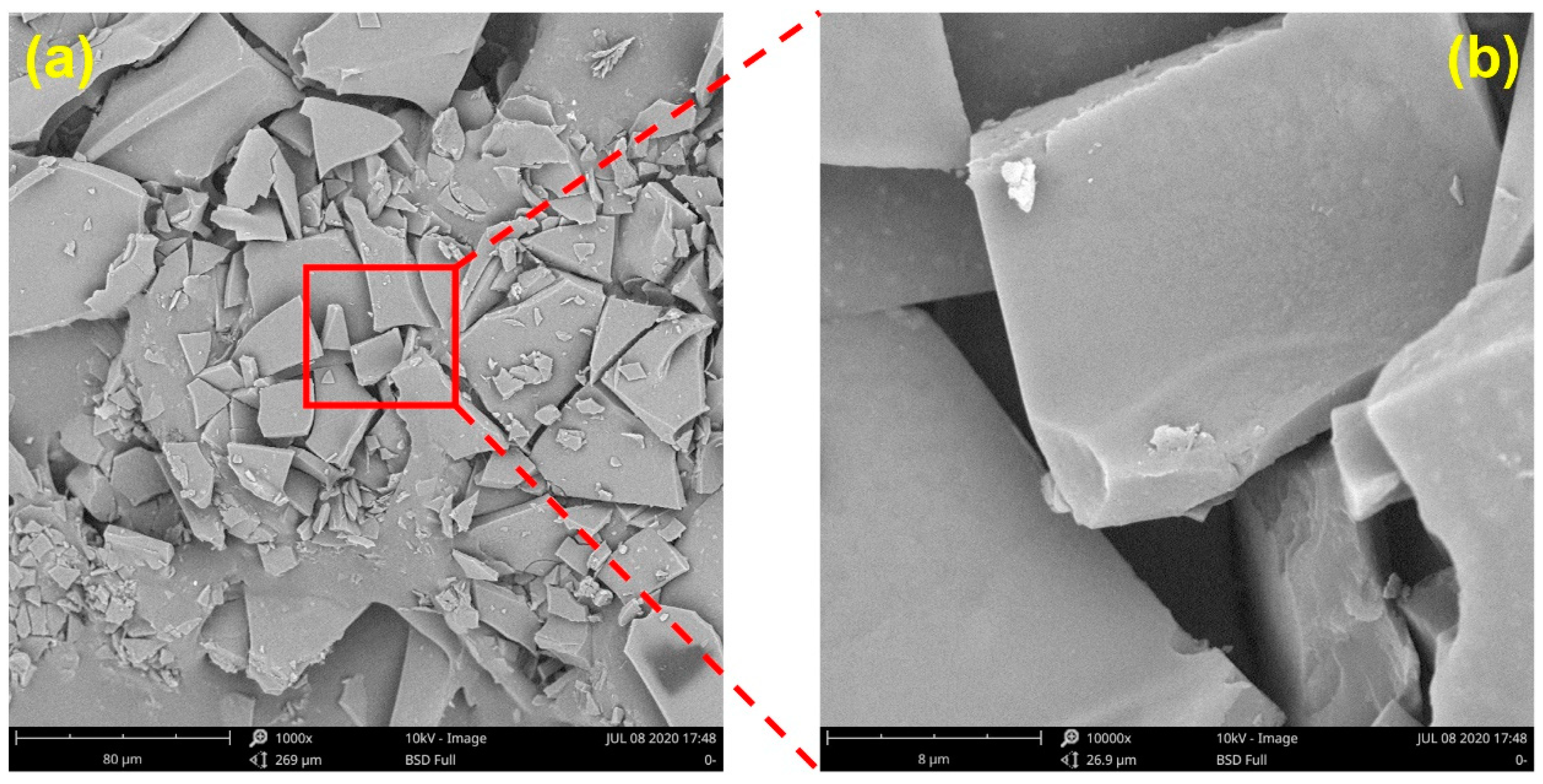
3.2.6. Microstructure of SGP-1-1
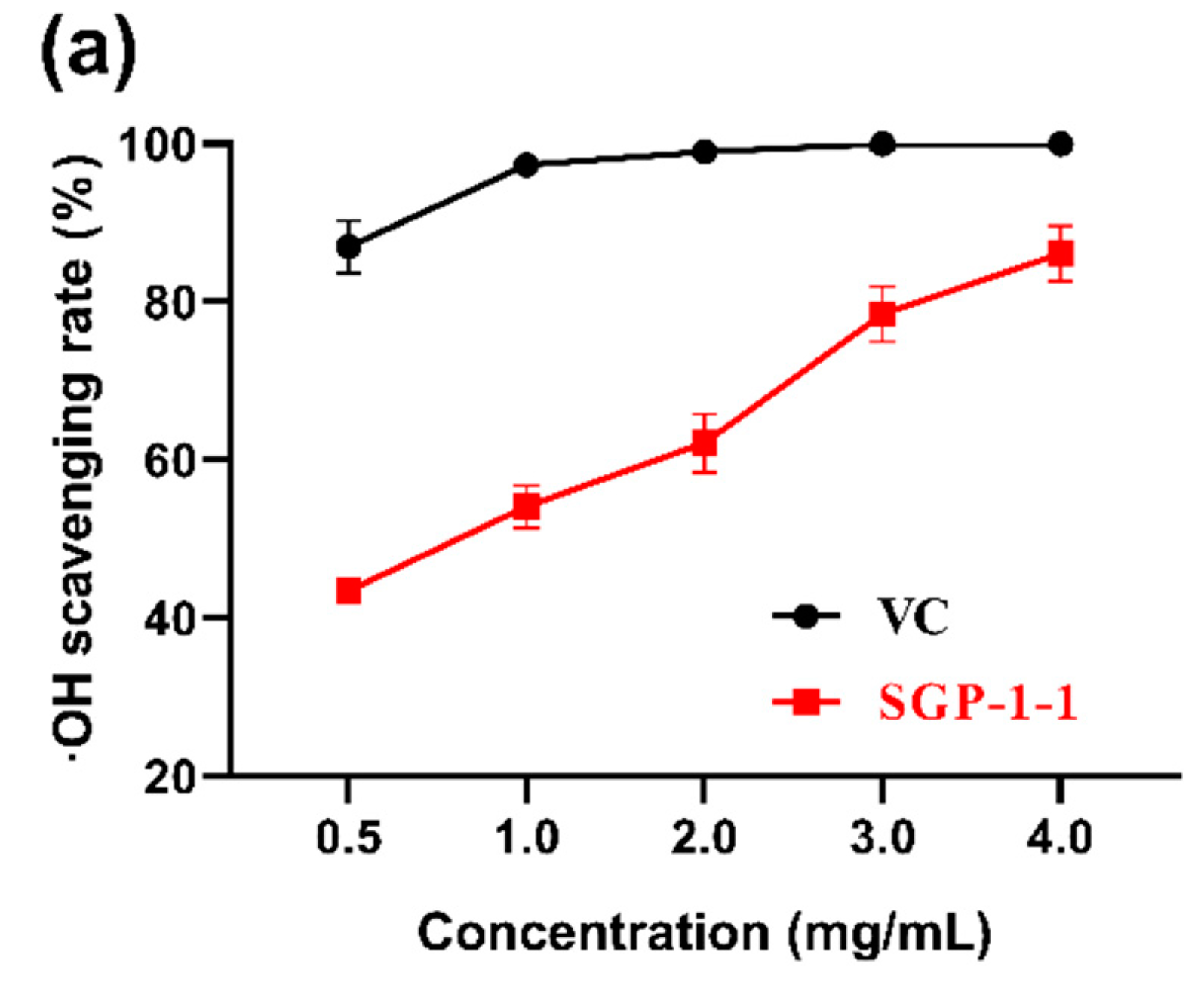
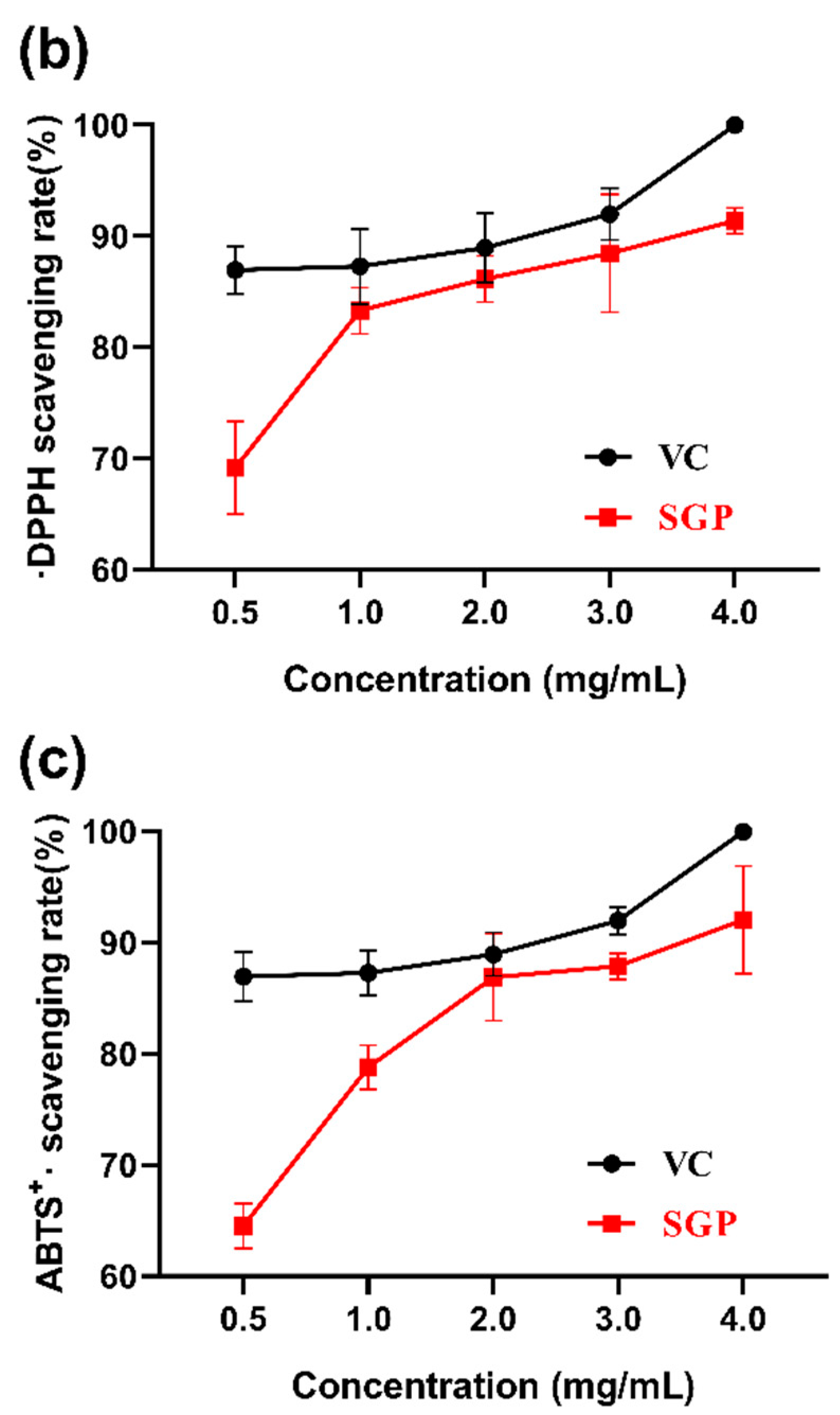
3.3. Antioxidant Activities In Vitro
3.4. Hypoglycemic Activities of SGP-1-1
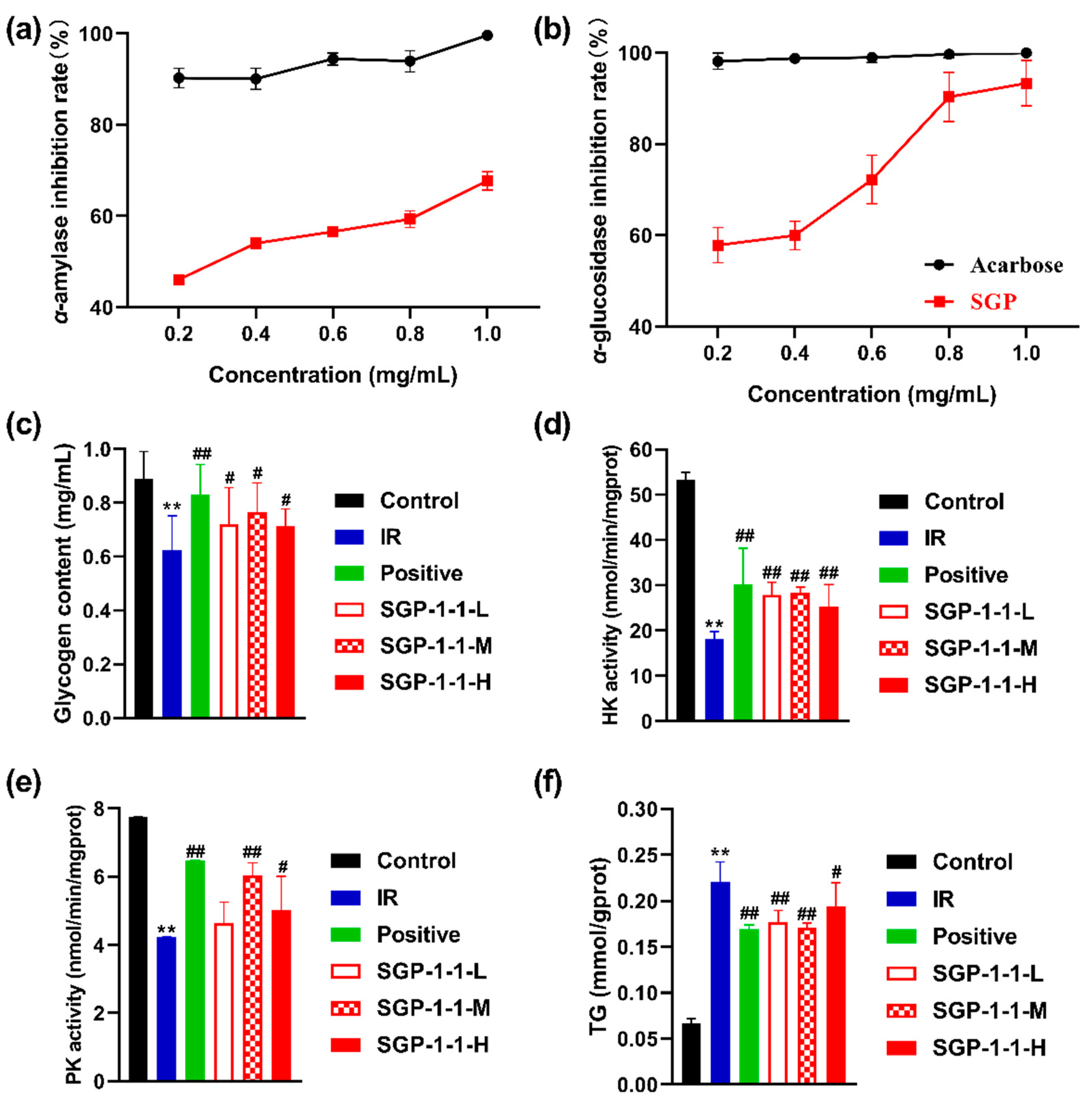
3.4.1. In Vitro Inhibition of α-Glucosidase and α-Amylase Activities
3.4.2. Glycogen Content of IR-HepG2 Cells
3.4.3. Hexokinase (HK) and Pyruvate Kinase (PK) Activities of IR-HepG2 Cells
3.4.4. TG Content of IR-HepG2 Cells
3.5. Relationship between Structure and Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amjadi, S.; Abbasi, M.M.; Shokouhi, B.; Ghorbani, M.; Hamishehkar, H. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. J. Funct. Foods 2019, 59, 119–128. [Google Scholar] [CrossRef]
- Chao, E.C.; Henry, R.R. Sglt2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, J.; Shang, H.; Guo, Y.; Chen, S. Extraction, purification, hypoglycemic and antioxidant activities of red clover (Trifolium pratense L.) polysaccharides. Int. J. Biol. Macromol. 2020, 148, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.-B.; Li, Z.-R.; Wu, J.; Ou, Z.-R.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Physicochemical properties of polysaccharide fractions from sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-C.; Zhu, Y.-M.; Zhu, Z.-Y.; Xue, W.; Liu, C.-Y.; Sun, H.-Q.; Yue, Y. Chemical structure and effects of antioxidation and against alpha-glucosidase of natural polysaccharide from glycyrrhiza inflata batalin. Int. J. Biol. Macromol. 2020, 155, 560–571. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Chen, F.; Cui, D.; Wang, M. Advances in the in vitro digestion and fermentation of polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 4970–4982. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, M.; Yao, W.; Yang, W.; Chen, F. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of Siraitia grosvenorii polysaccharides. Int. J. Food Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Chennaiah, A.; Dahiya, A.; Dubbu, S.; Vankar, Y.D. A Stereoselective Synthesis of an Imino Glycal: Application in the Synthesis of (−)-1- Epi -Adenophorine and a Homoiminosugar. Eur. J. Org. Chem. 2018, 2018, 6574–6581. [Google Scholar] [CrossRef]
- Chennaiah, A.; Bhowmick, S.; Vankar, Y.D. Conversion of glycals into vicinal-1,2-diazides and 1,2-(or 2,1)-azidoacetates using hypervalent iodine reagents and Me3SiN3. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar. RSC Adv. 2017, 7, 41755–41762. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Lin, L.-M.; Sui, F.; Wang, Z.-M.; Huo, H.-R.; Dai, L.; Jiang, T.-L. Chemistry and pharmacology of Siraitia grosvenorii: A review. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2020, 303, 125400–125409. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-M.; Pan, L.-C.; Zhang, L.-J.; Yin, Y.; Zhu, Z.-Y.; Sun, H.-Q.; Liu, C.-Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Cui, D.; Guo, Y.; Wang, M.; Wang, Z.; Huang, Z.; Yang, W.; Chen, F.; Chen, X. A novel polysaccharide obtained from siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the tlr4-nf-kappa b pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Chemistry, F.S.J.A. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zor, T.; Seliger, Z. Linearization of the bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Gusakov, A.V.; Kondratyeva, E.G.; Sinitsyn, A.P. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int. J. Anal. Chem. 2011, 2011, 283658. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef]
- Liu, X.; Liu, D.; Chen, Y.; Zhong, R.; Gao, L.; Yang, C.; Ai, C.; El-Seedi, H.R.; Zhao, C. Physicochemical characterization of a polysaccharide from agrocybe aegirita and its anti-ageing activity. Carbohydr. Polym. 2020, 236, 116056. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Cui, Y.; Liu, H.; Dong, C.; Sun, Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym. 2020, 235, 115969–115975. [Google Scholar] [CrossRef]
- Xiao, H.; Fu, X.; Cao, C.; Li, C.; Chen, C.; Huang, Q. Sulfated modification, characterization, antioxidant and hypoglycemic activities of polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 121, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, X.; Ma, M.; Long, T.; Xiao, C.; Zhang, J.; Liu, J.; Zhao, L. Immunoenhancing glucuronoxylomannan from tremella aurantialba bandoni et zang and its low-molecular-weight fractions by radical depolymerization: Properties, structures and effects on macrophages. Carbohydr. Polym. 2020, 238, 116184–116193. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Y.; Gao, Y.; Niu, X.; Wang, L.; Li, X.; Chen, H.; Yu, Z.; Yang, Y. Seperation, characterization and inhibition on alpha-glucosidase, alpha-amylase and glycation of a polysaccharide from blackcurrant fruits. LWT-Food Sci. Technol. 2018, 93, 16–23. [Google Scholar] [CrossRef]
- Luo, Q.-L.; Tang, Z.-H.; Zhang, X.-F.; Zhong, Y.-H.; Yao, S.-Z.; Wang, L.-S.; Lin, C.-W.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, Y.; Yang, Y.; Zhang, H.; Ding, C.; Hu, C.; Zhou, L.; Zhang, Z.; Yuan, S.; Chen, Y.; et al. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019, 133, 127–136. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M. Purification and structural characterization of polysaccharides isolated from Auricularia cornea var. Li. Carbohydr. Polym. 2020, 230, 115680–115689. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Zhang, X.; Huang, H. Structure characterization of a novel polysaccharide from hericium erinaceus fruiting bodies and its immunomodulatory activities. Food Funct. 2018, 9, 294–306. [Google Scholar] [CrossRef]
- Wen, Y.; Peng, D.; Li, C.; Hu, X.; Bi, S.; Song, L.; Peng, B.; Zhu, J.; Chen, Y.; Yu, R. A new polysaccharide isolated from morchella importuna fruiting bodies and its immunoregulatory mechanism. Int. J. Biol. Macromol. 2019, 137, 8–19. [Google Scholar] [CrossRef]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from passiflora edulis sims peel under different degradation methods. Carbohydr. Polym. 2019, 218, 46–52. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ma, X.; Ren, H.; Fan, W.; Leng, F.; Yang, M.; Wang, X. Extraction, purification, and bioactivities analyses of polysaccharides from glycyrrhiza uralensis. Ind. Crop. Prod. 2018, 122, 596–608. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Xu, X.; Liu, A. Optimization of polysaccharide extraction process from grifola frondosa and its antioxidant and anti-tumor research. J. Food Meas. Charact. 2019, 13, 144–153. [Google Scholar] [CrossRef]
- Salehi, E.; Emam-Djomeh, Z.; Askari, G.; Fathi, M. Opuntia ficus indica fruit gum: Extraction, characterization, antioxidant activity and functional properties. Carbohydr. Polym. 2019, 206, 565–572. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Y.; Song, H.; Zhou, H.; Zhong, F.; Hu, H.; Feng, Y. Extraction optimization, characterization and antioxidant activity of polysaccharide from gentiana scabra bge. Int. J. Biol. Macromol. 2016, 93, 369–380. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, P.; Zhou, H.; Li, Y. Extraction, characterization and in vitro antioxidant activity of polysaccharides from carex meyeriana kunth using different methods. Int. J. Biol. Macromol. 2018, 120, 2155–2164. [Google Scholar] [CrossRef]
- Teng, C.; Qin, P.; Shi, Z.; Zhang, W.; Yang, X.; Yao, Y.; Ren, G. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocoll. 2021, 113, 106392–106402. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, M.; Yao, X.; Yu, L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohydr. Polym. 2020, 236, 116020–116029. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Q.; Li, Y.; Li, L.; Lan, W.; He, J.; Li, H.; Xiong, Y.; Qin, W. Extraction, characterization and antioxidant activities of polysaccharides of chuanminshen violaceum. Int. J. Biol. Macromol. 2016, 86, 224–232. [Google Scholar] [CrossRef]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef]
- Wang, C.; Santhanam, R.K.; Gao, X.; Chen, Z.; Chen, Y.; Wang, C.; Xu, L.; Chen, H. Preparation, characterization of polysaccharides fractions from Inonotus obliquus and their effects on alpha-amylase, alpha-glucosidase activity and H2O2 induced oxidative damage in hepatic L02 cells. J. Funct. Foods 2018, 48, 179–189. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Zhang, M.; Zhou, H.-L. Microwave-assisted extraction, purification, partial characterization, and bioactivity of polysaccharides from panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and alpha-glucosidase inhibitory activities of polysaccharides from mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, C.; Chen, Q.; Huang, Q.; Mondragon Perez, M.E.; Fu, X. Physicochemical characterization, potential antioxidant and hypoglycemic activity of polysaccharide from sargassum pallidum. Int. J. Biol. Macromol. 2019, 139, 1009–1017. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; Zhang, Z.; Yao, W.; Gao, X. Hypoglycemic, hypolipidemic and antioxidant effects of sarcandra glabra polysaccharide in type 2 diabetic mice. Food Funct. 2014, 5, 2850–2860. [Google Scholar] [CrossRef]
- Cao, C.; Huang, Q.; Zhang, B.; Li, C.; Fu, X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from sargassum pallidum by microwave-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 109, 357–368. [Google Scholar] [CrossRef]
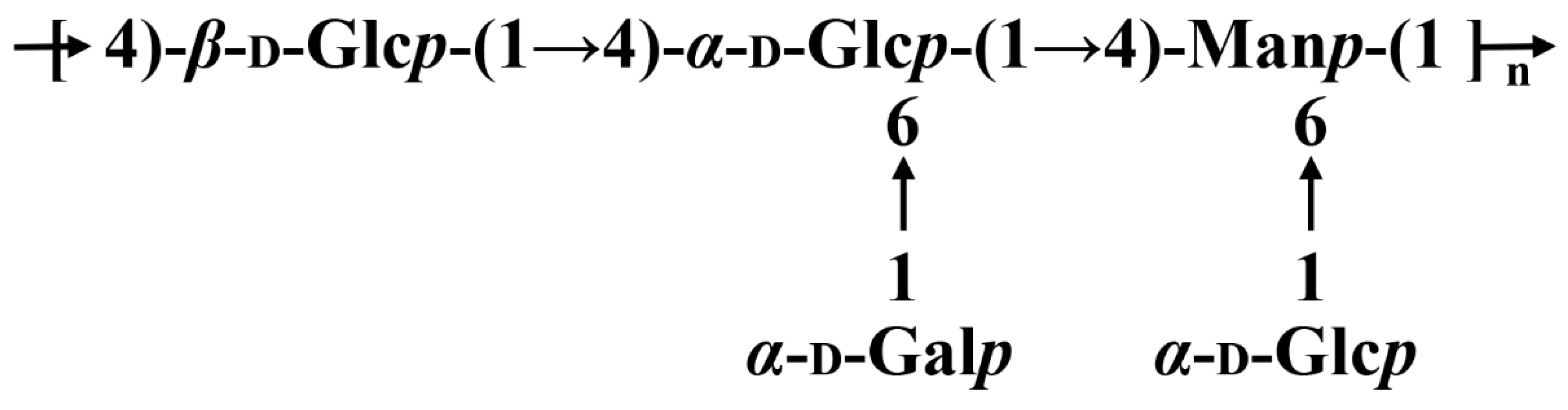

| Peak No. | Sugar Derivatives | Deduced Residues | Mol% | Mass Fragments (m/z) |
|---|---|---|---|---|
| 1 | 2,3,4,6-O-Me4-Glcp | T-Glcp | 18.4 | 55, 67, 79, 97, 110, 122, 136, 150, 164, 178, 192, 206 |
| 2 | 2,3,4,6-O-Me4-Manp | 1,4-linked-Manp | 30.4 | 60, 79, 98, 115, 129, 145, 169, 183, 197, 212, 226, 243 |
| 3 | 2,3,4-O-Me3-Galp | 1,6-linked-Galp | 10.5 | 60, 79, 98, 115, 145, 169, 187, 206, 226, 243, 270 |
| 4 | 2,3,4-O-Me3-Glcp | 1,6-linked-Glcp | 40.7 | 58, 69, 79, 88, 100, 114, 130, 145, 159, 169 |
| Sugar Residues | Chemical Shifts (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | ||
| A | →4)-α-D-Glcp→1 | 5.36 | 3.57 | 3.77 | 3.88 | 4.01 | 3.62 |
| 92.18 | 73.32 | 72.88 | 76.89 | 71.2 | 61.28 | ||
| B | →6)-α-D-Galp-(1→ | 5.17 | 3.48 | 3.18 | 3.49 | 3.65 | 3.91 |
| 92.09 | 71.4 | 74.12 | 71.2 | 73.1 | 65.4 | ||
| C | →4,6)-α-D-Manp-(1→ | 5.16 | 3.57 | 3.35 | 4.1 | 3.82 | 3.77 |
| 100.63 | 70.37 | 71.2 | 76.23 | 60.79 | 66.9 | ||
| D | β-D-Glcp-(1→ | 4.58 | 4.06 | 3.43 | 3.6 | 3.41 | 3.72 |
| 95.85 | 71.1 | 75.78 | 73.3 | 74.1 | 67.55 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Guo, Y.; Chen, X.; Cui, D.; Wang, M.; Yang, W.; Chen, F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules 2022, 27, 4192. https://doi.org/10.3390/molecules27134192
Gong P, Guo Y, Chen X, Cui D, Wang M, Yang W, Chen F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules. 2022; 27(13):4192. https://doi.org/10.3390/molecules27134192
Chicago/Turabian StyleGong, Pin, Yuxi Guo, Xuefeng Chen, Dandan Cui, Mengrao Wang, Wenjuan Yang, and Fuxin Chen. 2022. "Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii" Molecules 27, no. 13: 4192. https://doi.org/10.3390/molecules27134192
APA StyleGong, P., Guo, Y., Chen, X., Cui, D., Wang, M., Yang, W., & Chen, F. (2022). Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules, 27(13), 4192. https://doi.org/10.3390/molecules27134192







