A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway
Abstract
:1. Introduction
2. Results
2.1. APP, Aβ, and p-tau/tau Levels Were Increased in Stably Transfected SH-SY5Y Cells
2.2. PSD-95 Was Significantly Downregulated in APPsw-Positive Cells at Different Time Points
2.3. BG45 Increased APPsw-Positive Cell Viability and Decreased the Expression of Class I HDACs (HDAC1 and 2) in APPsw-Positive Cells
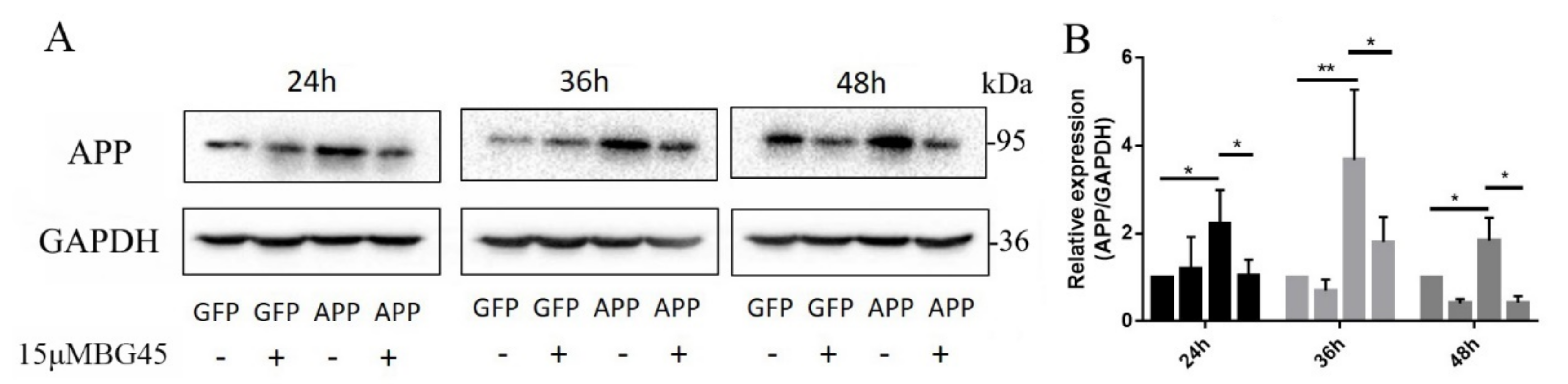
2.4. BG45 Significantly Decreased the Expression of the AD-Related Protein APP in APPsw-Positive Cells
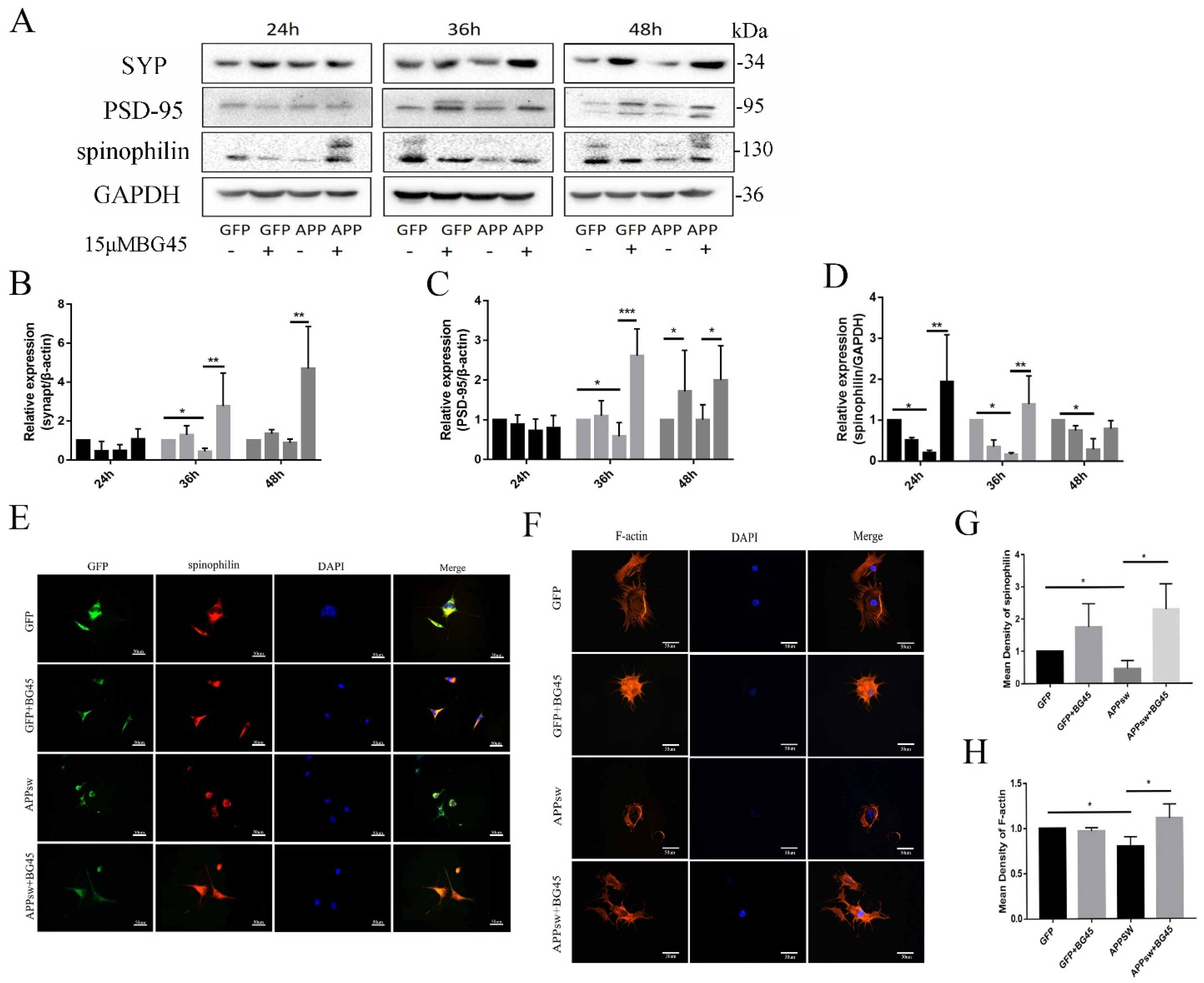
2.5. BG45 Significantly Increased the Expression of SYP, PSD-95, Spinophilin, and F-Actin
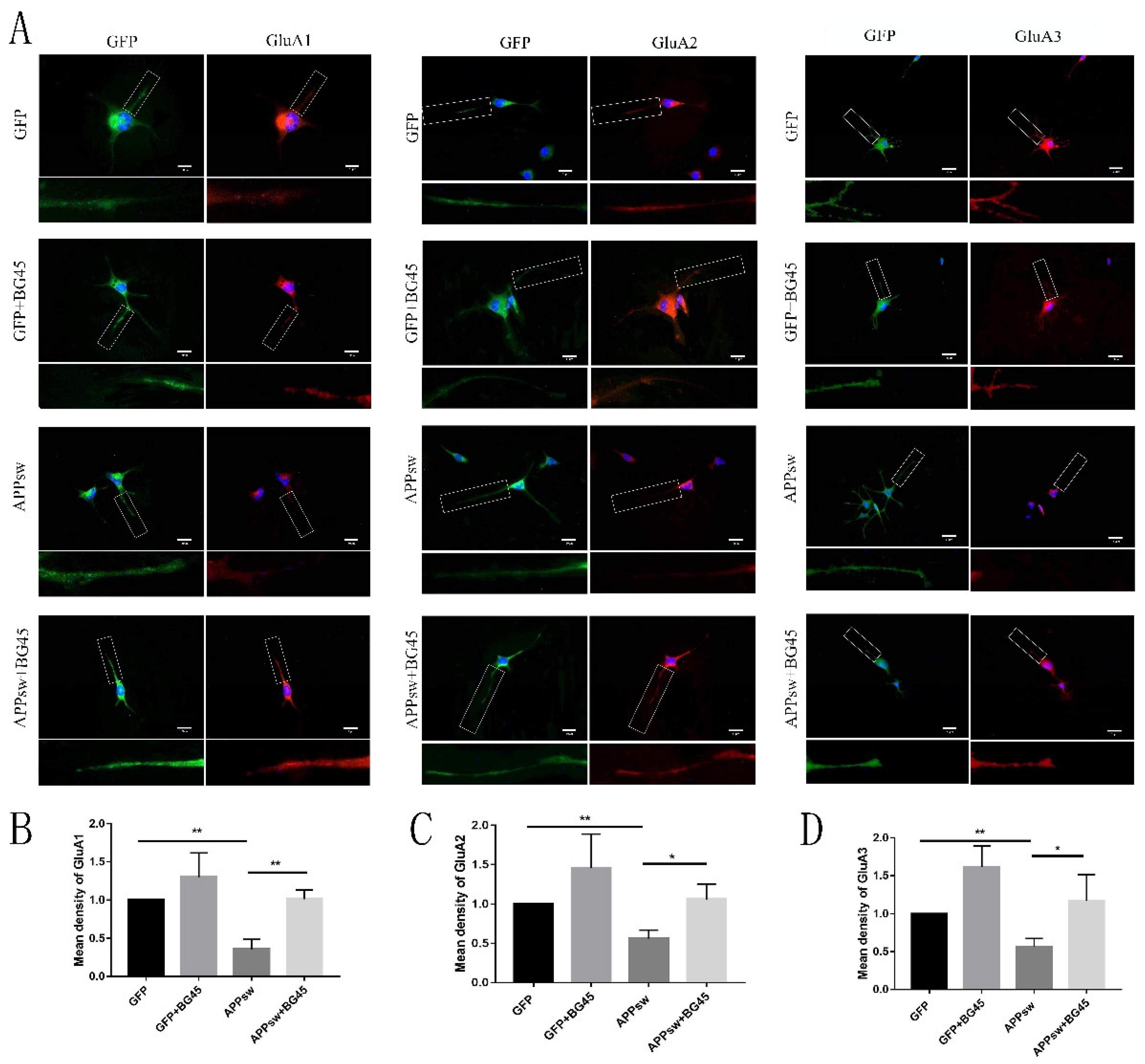
2.6. BG45 Increased the Cell Surface Levels of the Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptor Subunits GluA1 and GluA2
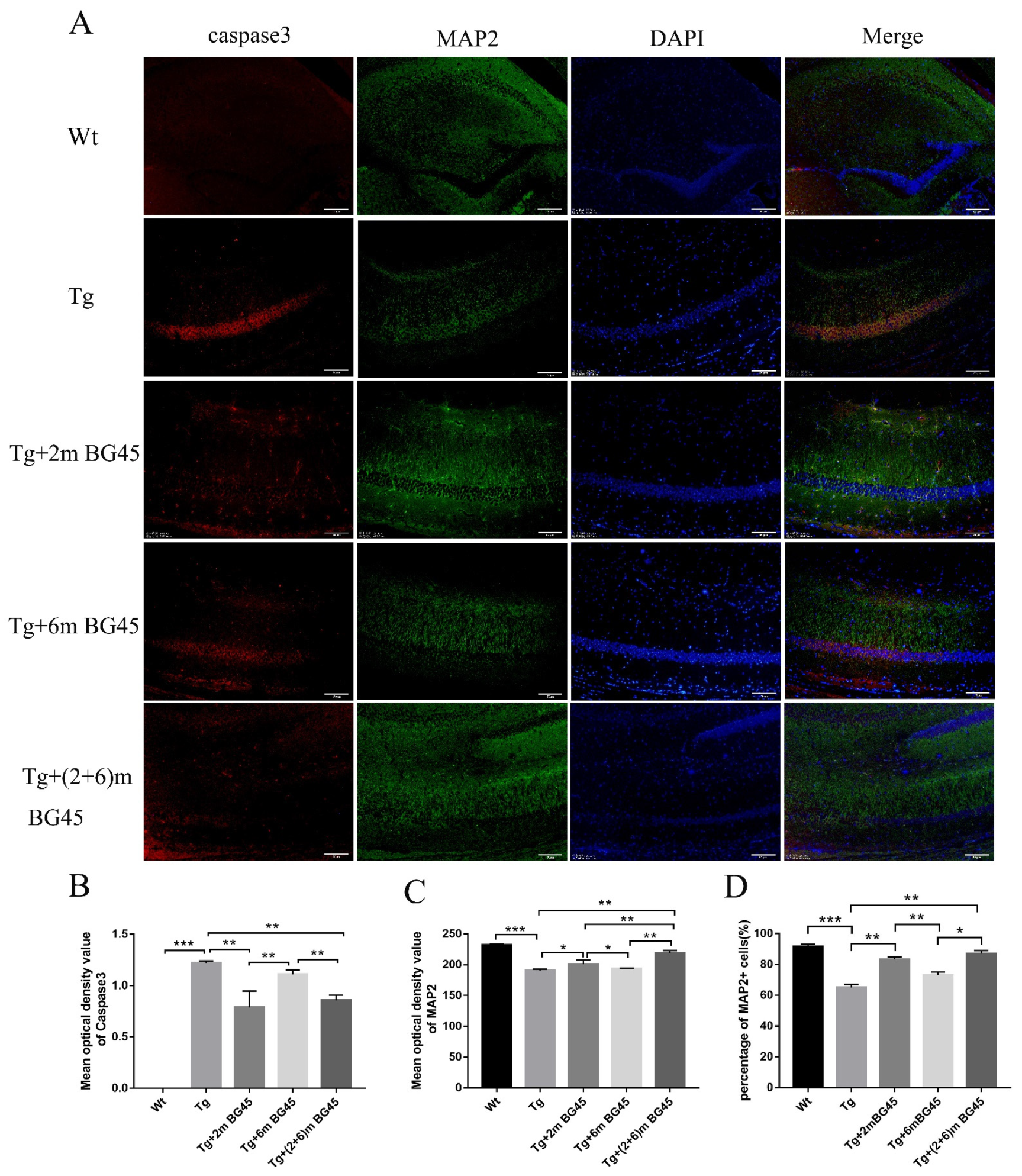
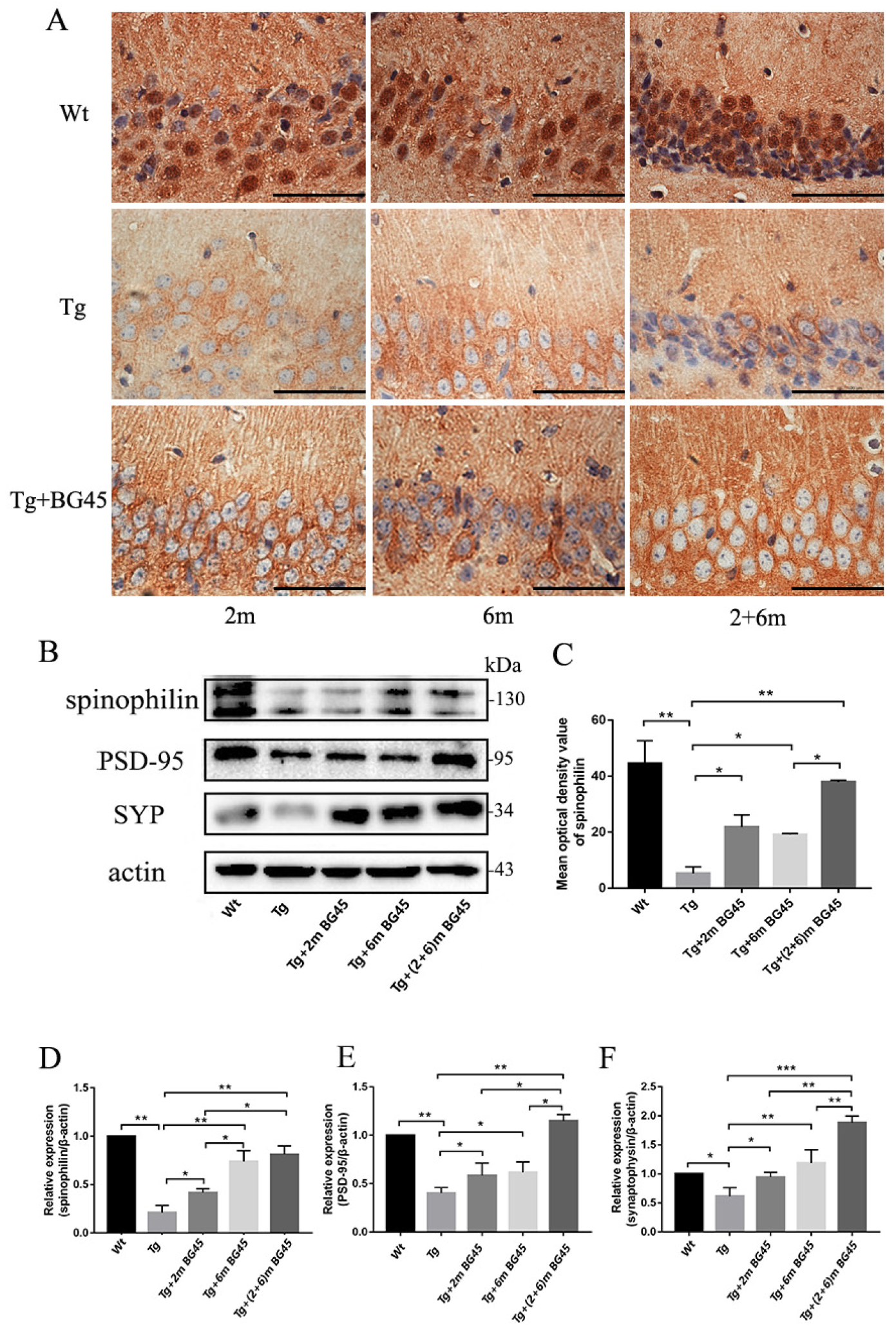
2.7. BG45 Alleviated Neuronal Loss in the Hippocampus of APP/PS1 Transgenic Mice
2.8. BG45 Decreased Aβ Deposition and the Level of the Phosphorylated tau Protein in the Hippocampus of APP/PS1 Transgenic Mice
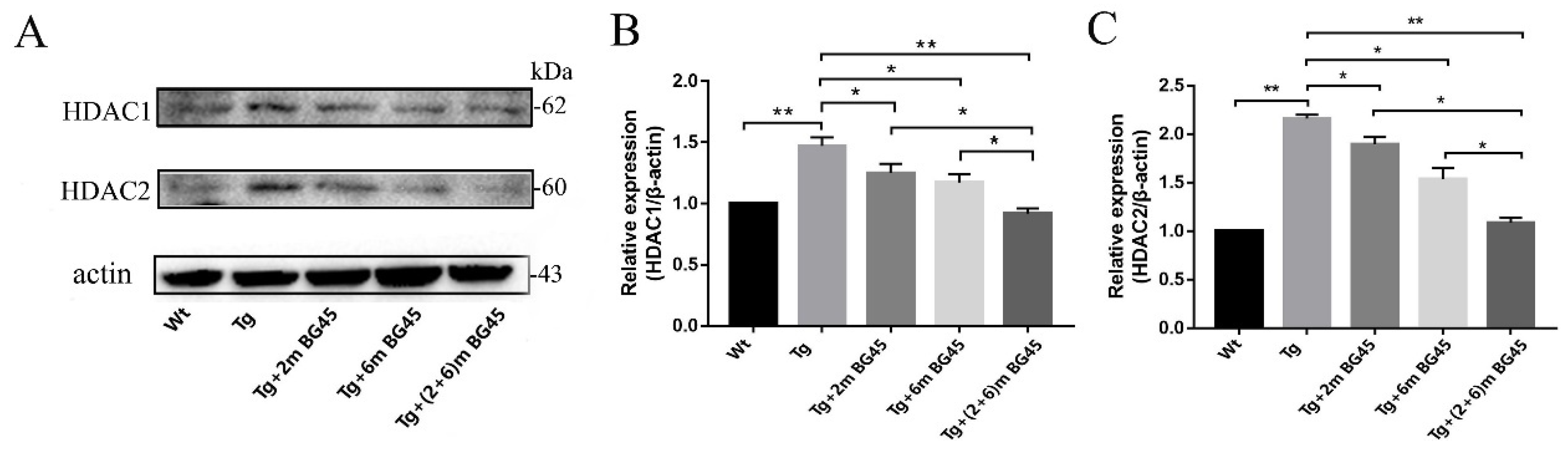
2.9. BG45 Inhibited HDAC1 and HDAC2 Protein Expression in the Hippocampal Neurons of APP/PS1 Mice
2.10. BG45 Increased the Expression of Synapse-Associated Proteins in the Hippocampal Neurons of APP/PS1 Mice
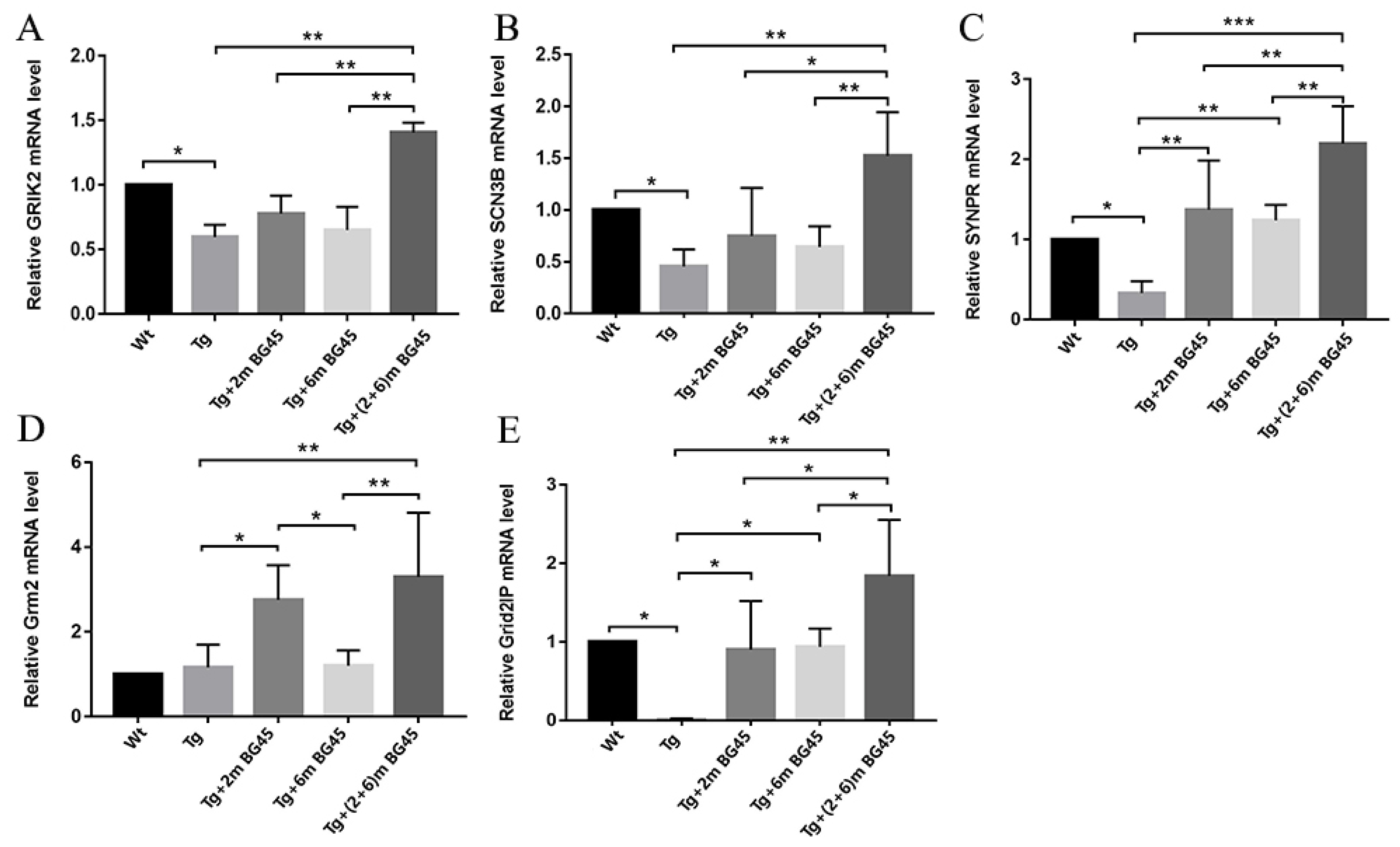
2.11. Effects of BG45 on the mRNA Levels of Synapse-Related Genes in the Hippocampal Neurons of APP/PS1 Mice
2.12. Effects of BG45 on the Levels of AMPARs and Related Genes in Hippocampal Neurons of APP/PS1 Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Differentiation, and Transfection
4.2. Drug Preparation
4.3. CCK-8 Assay
4.4. Animals and Treatment
4.5. Western Blot Assay
4.6. Immunohistochemistry
4.7. Labeling Cytoskeletal F-Actin
4.8. Live Cell Surface Immunostaining
4.9. Reverse Transcription PCR (RT-PCR)
4.10. Real-Time Quantitative PCR (Real-Time PCR)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Kowalska, A. The Beta-Amyloid Cascade Hypothesis: A Sequence of Events Leading to Neurodegeneration in Alzheimer’s Disease. Neurol. I Neurochir. Pol. 2004, 38, 405. [Google Scholar]
- Thal, D.R.; Walter, J.; Saido, T.C.; Fandrich, M. Neuropathology and Biochemistry of Abeta and Its Aggregates in Alzheimer’s Disease. Acta Neuropathol. 2015, 129, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Katzmarski, N.; Ziegler-Waldkirch, S.; Scheffler, N.; Witt, C.; Abou-Ajram, C.; Nuscher, B.; Prinz, M.; Haass, C.; Meyer-Luehmann, M. Aβ Oligomers Trigger and Accelerate Aβ Seeding. Brain Pathol. 2019, 30, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Felice, F.G.; Wu, D.; Lambert, M.P.; Fernandez, S.J.; Velasco, P.T.; Lacor, P.N.; Bigio, E.H.; Jerecic, J.; Acton, P.J.; Shughrue, P.J.; et al. Alzheimer’s Disease-Type Neuronal Tau Hyperphosphorylation Induced by a Beta Oligomers. Neurobiol. Aging 2008, 29, 1334–1347. [Google Scholar] [CrossRef] [Green Version]
- Hillen, H. The Beta Amyloid Dysfunction (Bad) Hypothesis for Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1154. [Google Scholar] [CrossRef] [Green Version]
- Crimins, J.L.; Pooler, A.; Polydoro, M.; Luebke, J.I.; Spires-Jones, T.L. The Intersection of Amyloid Beta and Tau in Glutamatergic Synaptic Dysfunction and Collapse in Alzheimer’s Disease. Ageing Res. Rev. 2013, 12, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- Lyon, J. Alzheimer Outlook Far from Bleak. JAMA 2017, 317, 896. [Google Scholar] [CrossRef]
- Townsend, M.; Shankar, G.M.; Mehta, T.; Walsh, D.M.; Selkoe, D.J. Effects of Secreted Oligomers of Amyloid Beta-Protein on Hippocampal Synaptic Plasticity: A Potent Role for Trimers. J. Physiol. 2006, 572 Pt 2, 477–492. [Google Scholar] [CrossRef]
- Roy, D.S.; Arons, A.; Mitchell, T.I.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S. Memory Retrieval by Activating Engram Cells in Mouse Models of Early Alzheimer’s Disease. Nature 2016, 531, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, Y.I.; Fa, M.; Ashraf, H.; Zhang, H.; Staniszewski, A.; Latchman, D.S.; Arancio, O. Dysregulation of Histone Acetylation in the App/Ps1 Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2009, 18, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, Y.; Shimizu, H.; Takagi, E.; Kato, M.; Yamagata, H.; Mikuni, M.; Shirao, T. Histone Deacetylase Mediates the Decrease in Drebrin Cluster Density Induced by Amyloid Beta Oligomers. Neurochem. Int. 2014, 76, 114–121. [Google Scholar] [CrossRef]
- Singh, P.; Thakur, M.K. Histone Deacetylase 2 Inhibition Attenuates Downregulation of Hippocampal Plasticity Gene Expression During Aging. Mol. Neurobiol. 2018, 55, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Chang, C.W.; Yang, B.H.; Gelovani, J.G.; Liu, R.S. Evaluation of Class Iia Histone Deacetylases Expression and in Vivo Epigenetic Imaging in a Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 8633. [Google Scholar] [CrossRef] [PubMed]
- Polis, B.; Srikanth, K.D.; Gurevich, V.; Bloch, N.; Gil-Henn, H.; Samson, A.O. Arginase Inhibition Supports Survival and Differentiation of Neuronal Precursors in Adult Alzheimer’s Disease Mice. Int. J. Mol. Sci. 2020, 21, 1133. [Google Scholar] [CrossRef] [Green Version]
- Rumbaugh, G.; Sillivan, S.E.; Ozkan, E.D.; Rojas, C.S.; Hubbs, C.R.; Aceti, M.; Kilgore, M.; Kudugunti, S.; Puthanveettil, S.V.; Sweatt, J.D.; et al. Pharmacological Selectivity within Class I Histone Deacetylases Predicts Effects on Synaptic Function and Memory Rescue. Neuropsychopharmacology 2015, 40, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.S.; Zhang, R.; Wang, G.; Zhang, Y.F. The Development Prospection of Hdac Inhibitors as a Potential Therapeutic Direction in Alzheimer’s Disease. Transl. Neurodegener. 2017, 6, 19. [Google Scholar] [CrossRef]
- Polis, B.; Karasik, D.; Samson, A.O. Alzheimer’s Disease as a Chronic Maladaptive Polyamine Stress Response. Aging-US 2021, 13, 10770–10795. [Google Scholar] [CrossRef]
- Janczura, K.J.; Volmar, C.H.; Sartor, G.C.; Rao, S.J.; Ricciardi, N.R.; Lambert, G.; Brothers, S.P.; Wahlestedt, C. Inhibition of Hdac3 Reverses Alzheimer’s Disease-Related Pathologies in Vitro and in the 3xtg-Ad Mouse Model. Proc. Natl. Acad. Sci. USA 2018, 115, E11148–E11157. [Google Scholar] [CrossRef] [Green Version]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of Class 1 Histone Deacetylases Reverse Contextual Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, S.; Yu, L.; Jin, J.; Ye, X.; Liu, Y.; Xu, Y. Hdac3 Negatively Regulates Spatial Memory in a Mouse Model of Alzheimer’s Disease. Aging Cell 2017, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Ricobaraza, A.; Cuadrado-Tejedor, M.; Perez-Mediavilla, A.; Frechilla, D.; del Rio, J.; Garcia-Osta, A. Phenylbutyrate Ameliorates Cognitive Deficit and Reduces Tau Pathology in an Alzheimer’s Disease Mouse Model. Neuropsychopharmacology 2009, 34, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Lovell, M.A.; Ehmann, W.D.; Butler, S.M.; Markesbery, W.R. Elevated Thiobarbituric Acid-Reactive Substances and Antioxidant Enzyme Activity in the Brain in Alzheimer’s Disease. Neurology 1995, 45, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Emerit, J.; Edeas, M.; Bricaire, F. Neurodegenerative Diseases and Oxidative Stress. Biomed. Pharmacother. 2004, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.J.; Zhang, D.D.; Li, P.; Wei, C.Y.; Cao, Y.P. Nicorandil Inhibits Oxidative Stress and Amyloid-Β Precursor Protein Processing in Sh-Sy5y Cells Overexpressing Appsw. Int. J. Clin. Exp. Med. 2015, 8, 1966–1975. [Google Scholar]
- Han, Y.; Chen, L.; Guo, Y.; Wang, C.; Zhang, C.; Kong, L.; Ma, H. Class I Hdac Inhibitor Improves Synaptic Proteins and Repairs Cytoskeleton through Regulating Synapse-Related Genes in Vitro and in Vivo. Front. Aging Neurosci. 2020, 12, 619866. [Google Scholar] [CrossRef]
- Guan, J.S.; Haggarty, S.J.; Giacometti, E.; Dannenberg, J.H.; Joseph, N.; Gao, J.; Nieland, T.J.; Zhou, Y.; Wang, X.; Mazitschek, R.; et al. Hdac2 Negatively Regulates Memory Formation and Synaptic Plasticity. Nature 2009, 459, 55–60. [Google Scholar] [CrossRef]
- Ganai, S.A.; Banday, S.; Farooq, Z.; Altaf, M. Modulating Epigenetic Hat Activity for Reinstating Acetylation Homeostasis: A Promising Therapeutic Strategy for Neurological Disorders. Pharmacol. Ther. 2016, 166, 106–122. [Google Scholar] [CrossRef]
- Sinclair, L.I.; Tayler, H.M.; Love, S. Synaptic Protein Levels Altered in Vascular Dementia. Neuropathol. Appl. Neurobiol. 2015, 41, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Tada, T.; Sheng, M. Molecular Mechanisms of Dendritic Spine Morphogenesis. Curr. Opin. Neurobiol. 2006, 16, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Futter, M.; Uematsu, K.; Bullock, S.A.; Kim, Y.; Hemmings, H.C.; Nishi, A.; Greengard, P.; Nairn, A.C. Phosphorylation of Spinophilin by Erk and Cyclin-Dependent Pk 5 (Cdk5). Proc. Natl. Acad. Sci. USA 2005, 102, 3489–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, S.D.; Futter, M.; Snyder, G.L.; Allen, P.B.; Nairn, A.C.; Greengard, P.; Hsieh-Wilson, L.C. Spinophilin Is Phosphorylated by Ca2+/Calmodulin-Dependent Protein Kinase Ii Resulting in Regulation of Its Binding to F-Actin. J. Neurochem. 2004, 90, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. Molecular and Cellular Basis of Neurodegeneration in Alzheimer’s Disease. Mol. Cells 2017, 40, 613–620. [Google Scholar] [PubMed] [Green Version]
- Zhang, W.; Bai, M.; Xi, Y.; Hao, J.; Liu, L.; Mao, N.; Su, C.; Miao, J.; Li, Z. Early Memory Deficits Precede Plaque Deposition in Appswe/Ps1de9 Mice: Involvement of Oxidative Stress and Cholinergic Dysfunction. Free Radic. Biol. Med. 2012, 52, 1443–1452. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.; Xu, L.; Zheng, N.; He, X.; Xu, F. Imaging and Spectral Characteristics of Amyloid Plaque Autofluorescence in Brain Slices from the App/Ps1 Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2019, 35, 1126–1137. [Google Scholar] [CrossRef]
- Yamakawa, H.; Cheng, J.; Penney, J.; Gao, F.; Rueda, R.; Wang, J.; Yamakawa, S.; Kritskiy, O.; Gjoneska, E.; Tsai, L.H. The Transcription Factor Sp3 Cooperates with Hdac2 to Regulate Synaptic Function and Plasticity in Neurons. Cell Rep. 2017, 20, 1319–1334. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, C.; Marie, H. Hippocampal Synaptic Plasticity in Alzheimer’s Disease: What Have We Learned So Far from Transgenic Models? Rev. Neurosci. 2011, 22, 373–402. [Google Scholar] [CrossRef]
- Megill, A.; Tran, T.; Eldred, K.; Lee, N.J.; Wong, P.C.; Hoe, H.S.; Kirkwood, A.; Lee, H.K. Defective Age-Dependent Metaplasticity in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015, 35, 11346–11357. [Google Scholar] [CrossRef] [Green Version]
- Diering, G.H.; Huganir, R.L. The Ampa Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, R.P.; Ikonomovic, M.D.; Sheffield, R.; Rubin, R.T.; Armstrong, D.M. Reduction of Ampa-Selective Glutamate Receptor Subunits in the Entorhinal Cortex of Patients with Alzheimer’s Disease Pathology: A Biochemical Study. Brain Res. 1995, 678, 161–167. [Google Scholar] [CrossRef]
- Hanley, J.G. Ampa Receptor Trafficking Pathways and Links to Dendritic Spine Morphogenesis. Cell Adhes. Migr. 2008, 2, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwatsubo, T.; Boehm, J.; Sisodia, S.S.; Sato, C.; Hsieh, P.C.H.; Malinow, R.; Tomita, T. Ampar Removal Underlies Abeta-Induced Synaptic Depression and Dendritic Spine Loss. Neuron 2006, 52, 831. [Google Scholar]
- Rui, Y.; Gu, J.; Yu, K.; Hartzell, H.C.; Zheng, J.Q. Inhibition of Ampa Receptor Trafficking at Hippocampal Synapses by Beta-Amyloid Oligomers: The Mitochondrial Contribution. Mol. Brain 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.Q.; Santini, F.; Breese, R.; Ross, D.; Zhang, X.D.; Stone, D.J.; Ferrer, M.; Townsend, M.; Wolfe, A.L.; Seager, M.A.; et al. Inhibition of Calcineurin-Mediated Endocytosis and Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (Ampa) Receptors Prevents Amyloid Beta Oligomer-Induced Synaptic Disruption. J. Biol. Chem. 2010, 285, 7619–7632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.J.; Gasperini, R.; Foa, L.; Small, D.H. Amyloid-Beta Decreases Cell-Surface Ampa Receptors by Increasing Intracellular Calcium and Phosphorylation of Glur2. J. Alzheimer’s Dis. 2010, 21, 655–666. [Google Scholar] [CrossRef] [Green Version]
- Na, H.; Gan, Q.; Mcparland, L.; Yang, J.B.; Yao, H.; Tian, H.; Zhang, Z.; Qiu, W.Q. Characterization of the effects of calcitonin gene-related peptide receptor antagonist for Alzheimer’s disease. Neuropharmacology 2020, 168, 108017. [Google Scholar] [CrossRef]
- Malerba, H.N.; Pereira, A.A.R.; Pierrobon, M.F.; Abrao, G.S.; Toricelli, M.; Akamine, E.H.; Buck, H.S.; Viel, T.A. Combined Neuroprotective Strategies Blocked Neurodegeneration and Improved Brain Function in Senescence-Accelerated Mice. Front Aging Neurosci. 2021, 13, 681498. [Google Scholar] [CrossRef]
- Sohrabi, M.; Pecoraro, H.L.; Combs, C.K. Gut Inflammation Induced by Dextran Sulfate Sodium Exacerbates Amyloid-β Plaque Deposition in the AppNL-G-F Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 79, 1235–1255. [Google Scholar] [CrossRef]
- Huang, L.; Lan, J.; Tang, J.; Kang, Y.; Feng, X.; Wu, L.; Peng, Y. L-3-n-Butylphthalide improves synaptic and dendritic spine plasticity and ameliorates neurite pathology in Alzheimer’s disease mouse model and cultured hippocampal neurons. Mol. Neurobiol. 2021, 58, 1260–1274. [Google Scholar] [CrossRef]
- Choi, G.E.; Lee, H.J.; Chae, C.W.; Cho, J.H.; Jung, Y.H.; Kim, J.S.; Kim, S.Y.; Lim, J.R.; Han, H.J. BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat. Commun. 2021, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Sarrouilhe, D.; di Tommaso, A.; Métayé, T.; Ladeveze, V. Spinophilin: From partners to functions. Biochimie 2006, 88, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Chen, S.T.; Chen, Y.J.; Shen, J.; Yao, W.B.; Gao, X.D.; Chen, S. Modulation of the Astrocyte-Neuron Lactate Shuttle System contributes to Neuroprotective action of Fibroblast Growth Factor 21. Theranostics 2020, 10, 8430–8445. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Yue, G.; Liu, Y.; Wang, Y.; Qiu, Y.; Li, Z.; Ma, P.; Liu, T.; Jiang, Y.; Liang, Y.; et al. Free Wanderer Powder regulates AMPA receptor homeostasis in chronic restraint stress-induced rat model of depression with liver-depression and spleen-deficiency syndrome. Aging 2020, 12, 19563–19584. [Google Scholar] [CrossRef]
- Maiti, A.; Qi, Q.; Peng, X.; Yan, L.; Takabe, K.; Hait, N.C. Class I histone deacetylase inhibitor suppresses vasculogenic mimicry by enhancing the expression of tumor suppressor and anti-angiogenesis genes in aggressive human TNBC cells. Int. J. Oncol. 2019, 55, 116–130. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Gao, X.; Tang, Q.; Huang, L.; Gao, S.; Yu, S.; Huang, J.; Li, J.; Zhou, D.; Zhang, Y.; et al. Nucleoporin 107 facilitates the nuclear export of Scn5a mRNA to regulate cardiac bioelectricity. J. Cell. Mol. Med. 2019, 23, 1448–1457. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Chen, L.; Liu, J.; Chen, J.; Wang, C.; Guo, Y.; Yu, X.; Zhang, C.; Chu, H.; Ma, H. A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway. Molecules 2022, 27, 4160. https://doi.org/10.3390/molecules27134160
Han Y, Chen L, Liu J, Chen J, Wang C, Guo Y, Yu X, Zhang C, Chu H, Ma H. A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway. Molecules. 2022; 27(13):4160. https://doi.org/10.3390/molecules27134160
Chicago/Turabian StyleHan, Ying, Le Chen, Jingyun Liu, Jie Chen, Chunyang Wang, Yu Guo, Xuebin Yu, Chenghong Zhang, Haiying Chu, and Haiying Ma. 2022. "A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway" Molecules 27, no. 13: 4160. https://doi.org/10.3390/molecules27134160
APA StyleHan, Y., Chen, L., Liu, J., Chen, J., Wang, C., Guo, Y., Yu, X., Zhang, C., Chu, H., & Ma, H. (2022). A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway. Molecules, 27(13), 4160. https://doi.org/10.3390/molecules27134160





