Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Copper-64 Radiolabelling
2.1.1. Copper-64 Radiolabelling with Cyclen-Based S-Rich Chelators
2.1.2. Copper-64 Radiolabelling with Non-Cyclen-Based S-Rich Chelators
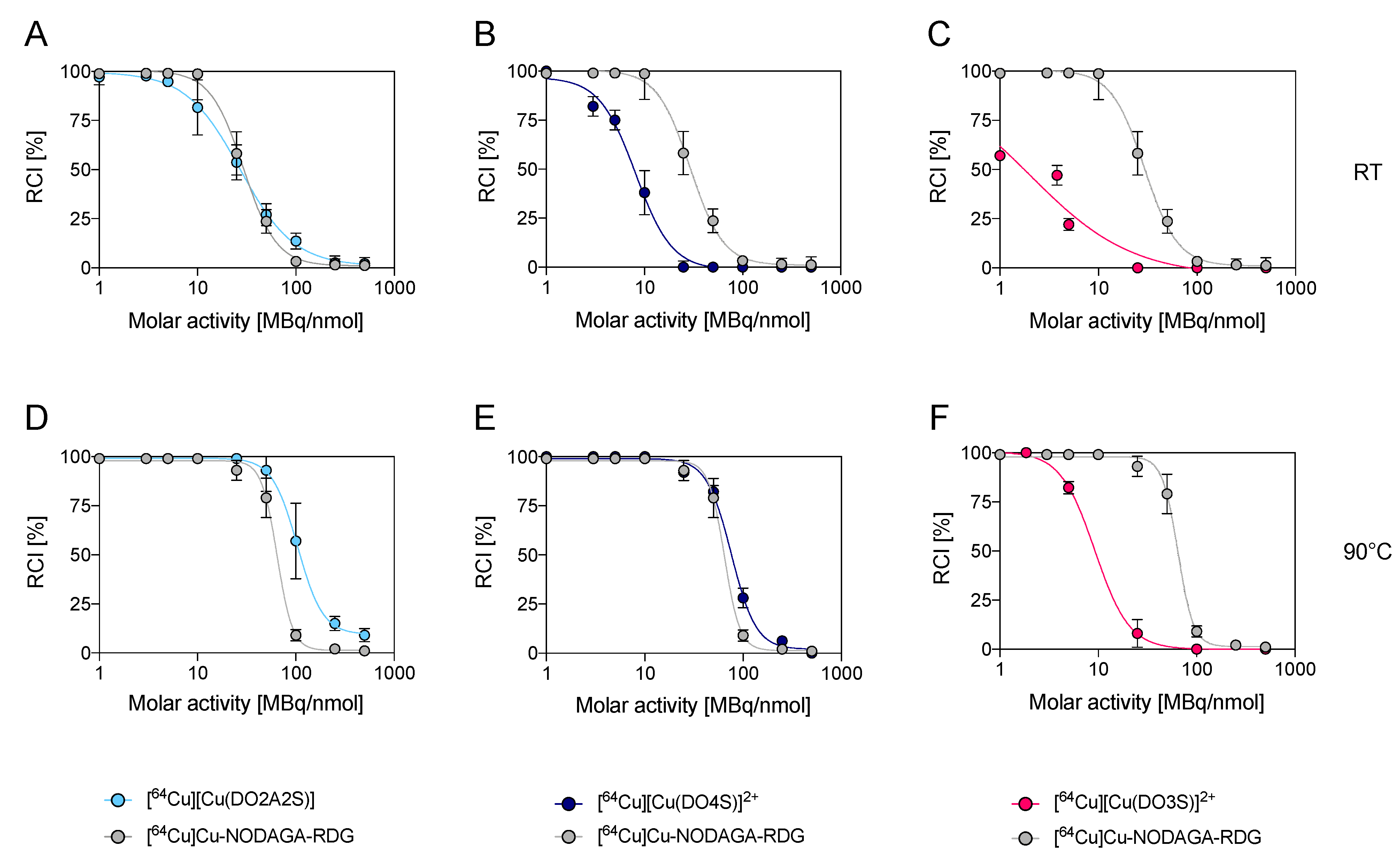
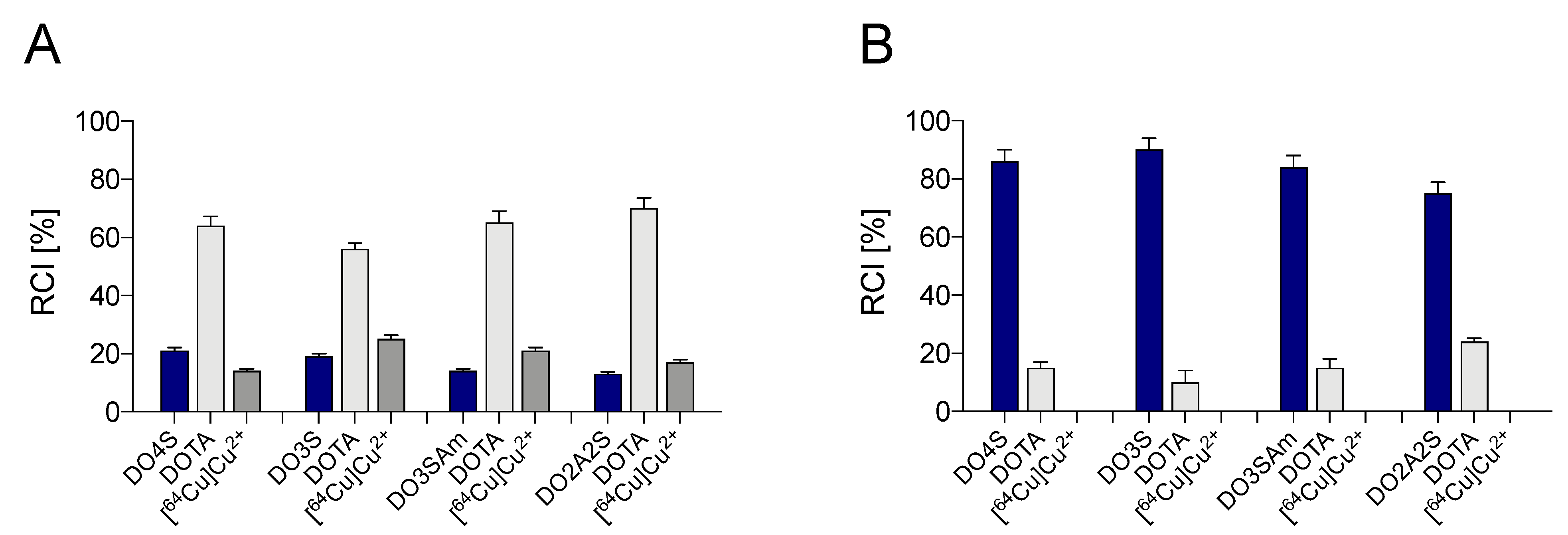
2.2. Competition Assays
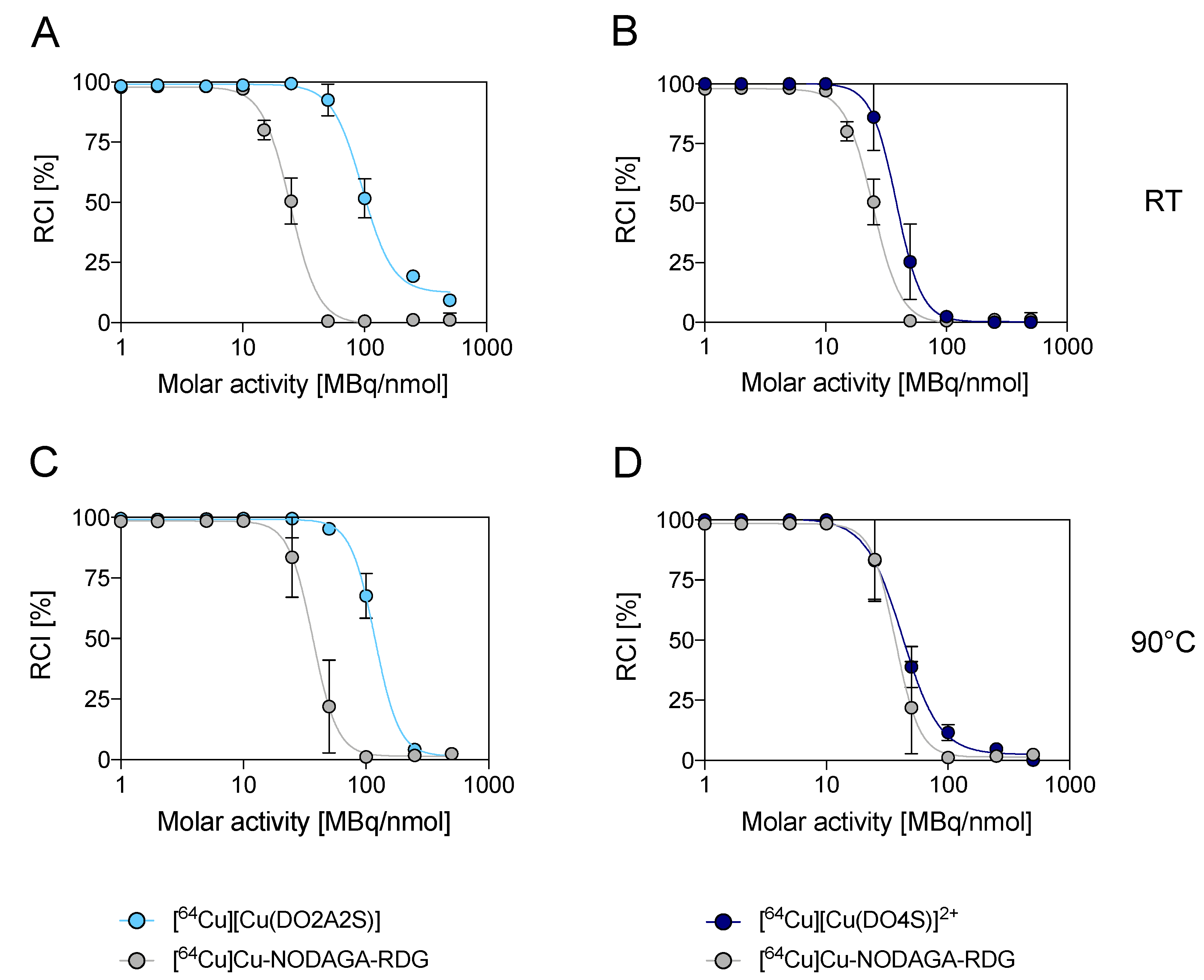
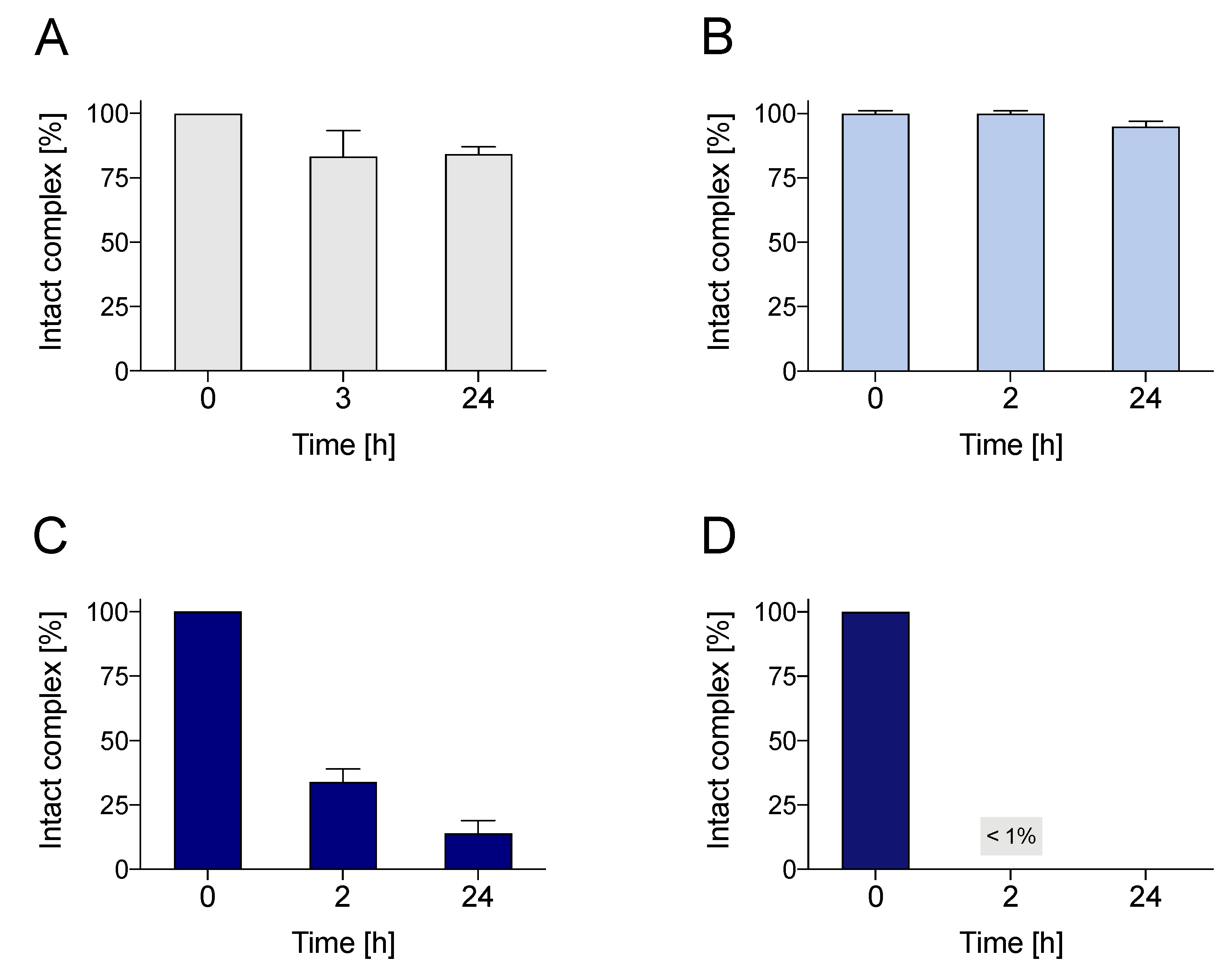
2.3. Stability Assays with Challenging Agents
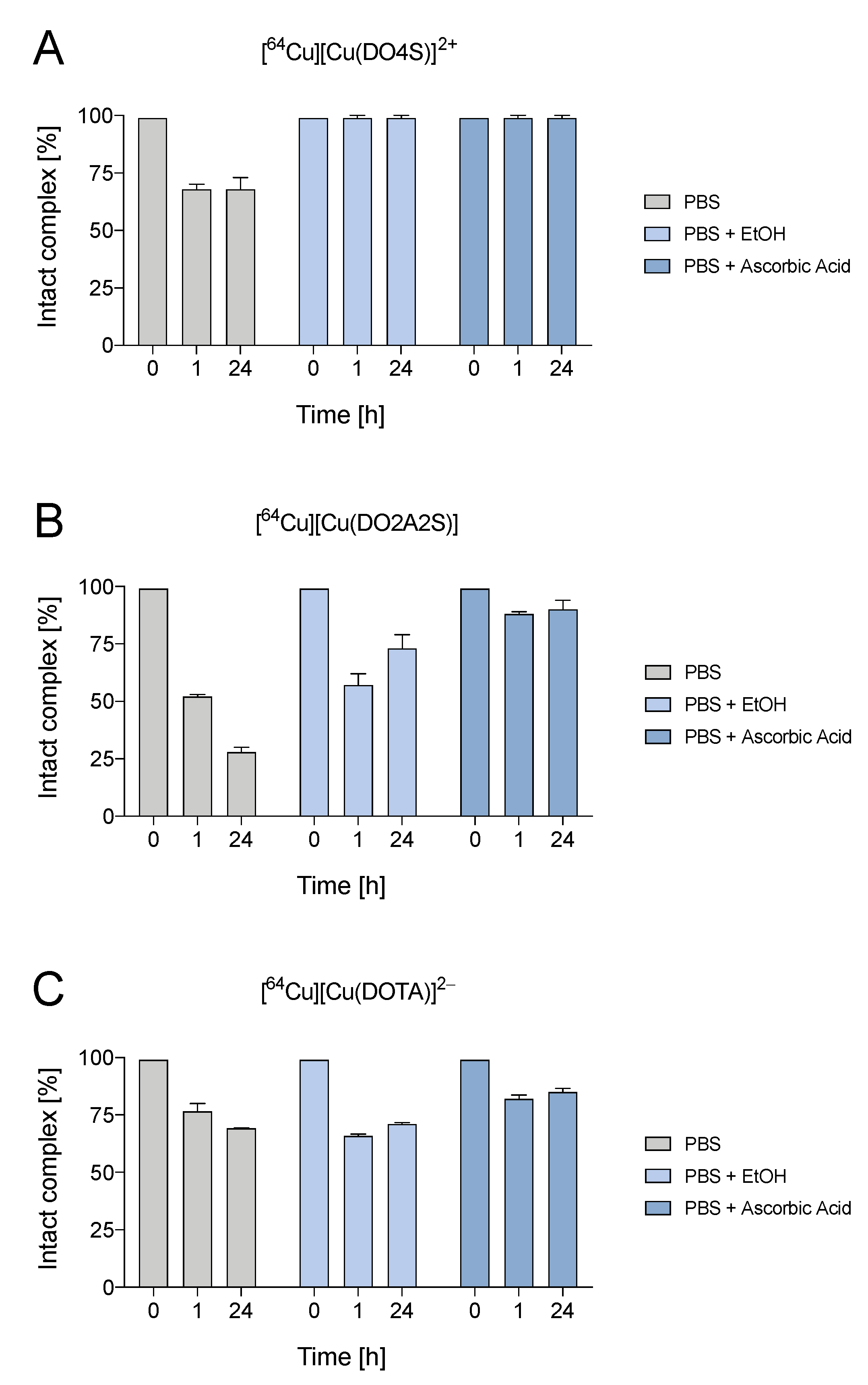
2.4. Stability in Phosphate-Buffered Saline
2.5. Human Serum Stability Assays
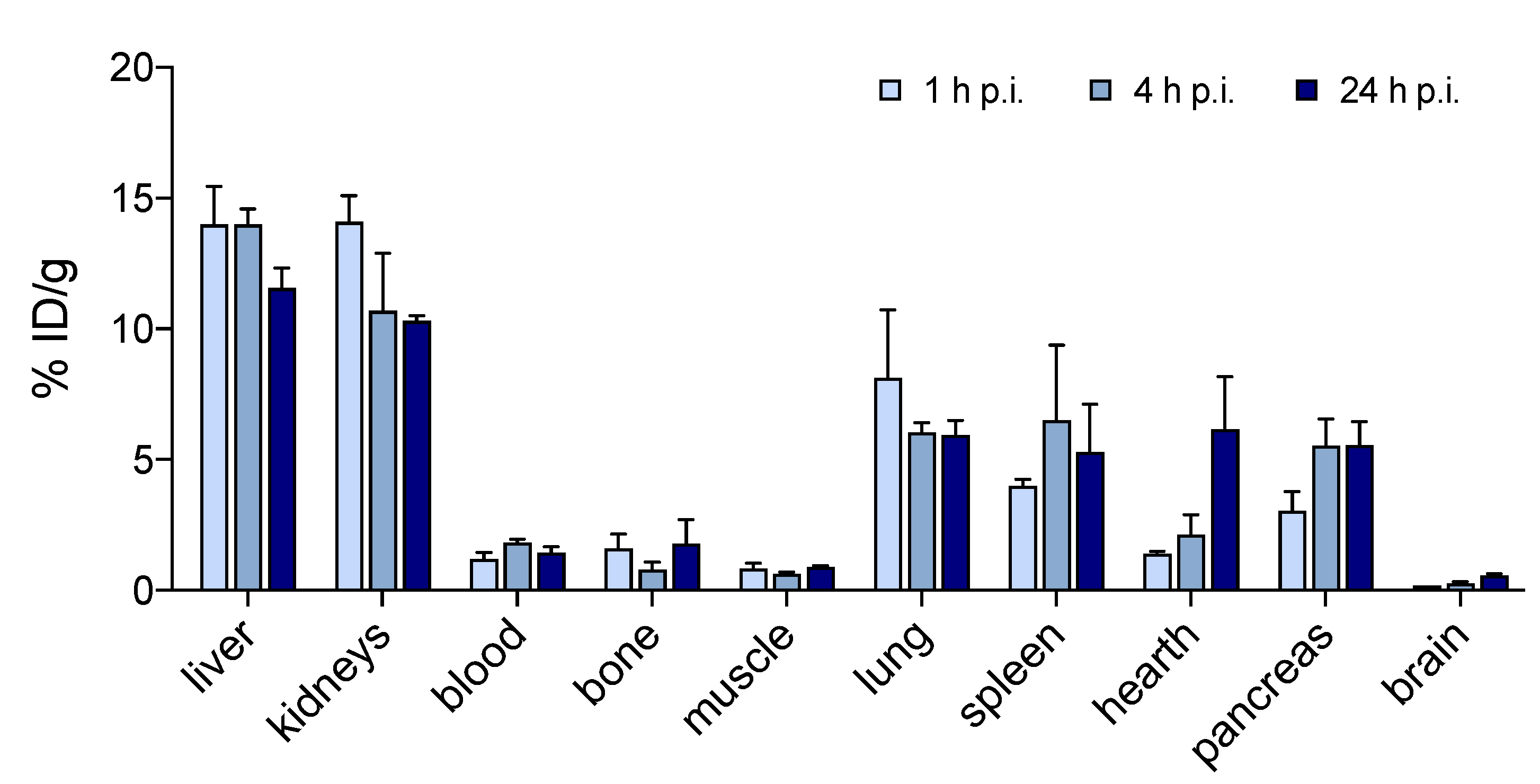
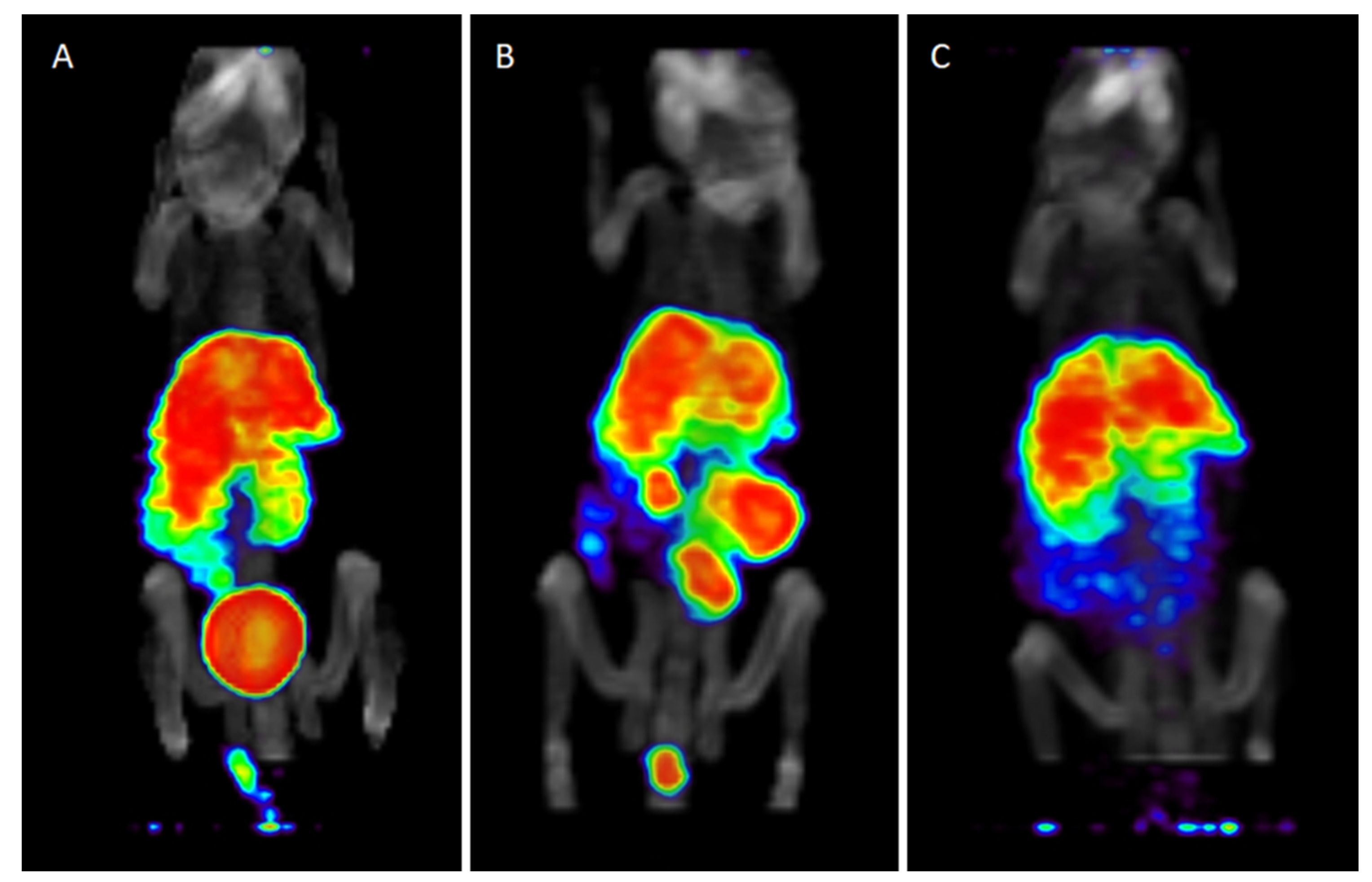
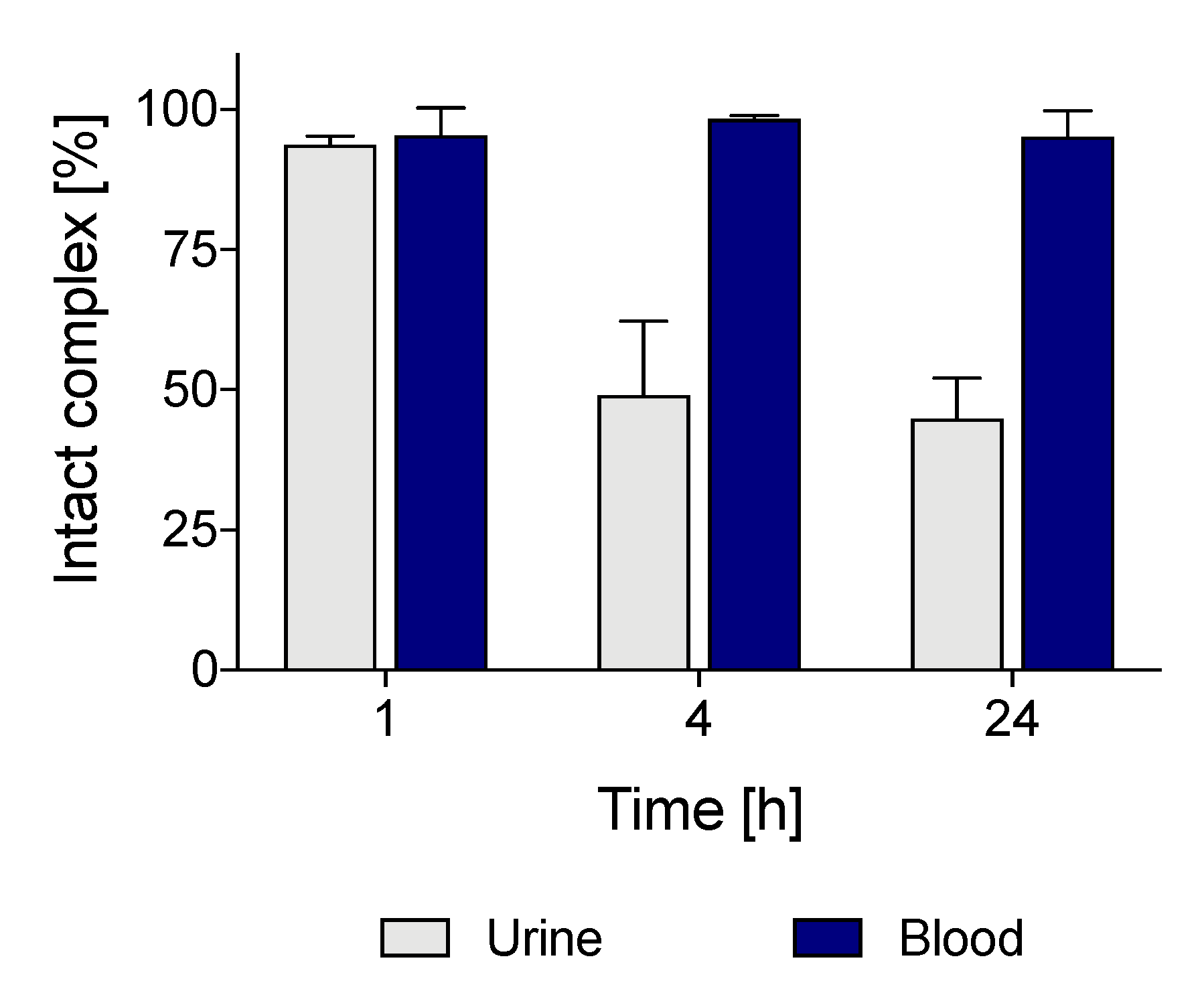
2.6. Small-Animal Imaging, Biodistribution and In Vivo Stability
3. Materials and Methods
3.1. Materials
3.2. Copper-64 Production
3.3. Copper-64 Radiolabelling
3.4. Competition Assays
3.5. Stability Assays with DOTA and Cysteine
3.6. Stability in Phosphate-Buffered Saline
3.7. Human Serum Stability
3.8. Small Animal Imaging, Biodistribution and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Tosato, M.; Dalla Tiezza, M.; May, N.V.; Isse, A.A.; Nardella, S.; Orian, L.; Verona, M.; Vaccarin, C.; Alker, A.; Mäcke, H.; et al. Copper Coordination Chemistry of Sulfur Pendant Cyclen Derivatives: An Attempt to Hinder the Reductive-Induced Demetallation in 64/67Cu Radiopharmaceuticals. Inorg. Chem. 2021, 60, 11530–11547. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.; Orvig, C. Tumour Targeting with Radiometals for Diagnosis and Therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Tosato, M.; Asti, M.; Dalla Tiezza, M.; Orian, L.; Häussinger, D.; Vogel, R.; Köster, U.; Jensen, M.; Andrighetto, A.; Pastore, P.; et al. Highly Stable Silver(I) Complexes with Cyclen-Based Ligands Bearing Sulfide Arms: A Step Toward Silver-111 Labeled Radiopharmaceuticals. Inorg. Chem. 2020, 59, 10907–10919. [Google Scholar] [CrossRef] [PubMed]
- Frindel, M.; Camus, N.; Rauscher, A.; Bourgeois, M.; Alliot, C.; Barré, L.; Gestin, J.F.; Tripier, R.; Faivre-Chauvet, A. Radiolabeling of HTE1PA: A New Monopicolinate Cyclam Derivative for Cu-64 Phenotypic Imaging. In Vitro and in Vivo Stability Studies in Mice. Nucl. Med. Biol. 2014, 41, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.; Voss, S.D.; Dunning, P.; Snay, E.; Fahey, F.; Smith, S.V.; Huston, J.S.; Meares, C.F.; Treves, S.T.; Packard, A.B. Imaging Cancer Using PET–the Effect of the Bifunctional Chelator on the Biodistribution of a 64Cu-Labeled Antibody. Nucl. Med. Biol. 2011, 38, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litau, S.; Seibold, U.; Vall-Sagarra, A.; Fricker, G.; Wängler, B.; Wängler, C. Comparative Assessment of Complex Stabilities of Radiocopper Chelating Agents by a Combination of Complex Challenge and in Vivo Experiments. ChemMedChem 2015, 10, 1200–1208. [Google Scholar] [CrossRef]
- Anderson, C.J.; Wadas, T.J.; Wong, E.H.; Weisman, G.R. Cross-Bridged Macrocyclic Chelators for Stable Complexation of Copper Radionuclides for PET Imaging. Q. J. Nucl. Med. Mol. Imag. 2008, 52, 185–192. [Google Scholar]
- Lima, L.M.P.; Halime, Z.; Marion, R.; Camus, N.; Delgado, R.; Platas-Iglesias, C.; Tripier, R. Monopicolinate Cross-Bridged Cyclam Combining Very Fast Complexation with Very High Stability and Inertness of Its Copper(II) Complex. Inorg. Chem. 2014, 53, 5269–5279. [Google Scholar] [CrossRef]
- Wadas, T.J.; Anderson, C.J. Radiolabeling of TETA- and CB-TE2A-Conjugated Peptides with Copper. Nat. Protoc. 2006, 1, 3062–3068. [Google Scholar] [CrossRef]
- Yang, H.; Gao, F.; McNeil, B.; Zhang, C.; Yuan, Z.; Zeisler, S.; Kumlin, J.; Zeisler, J.; Bénard, F.; Ramogida, C.; et al. Synthesis of DOTA-Pyridine Chelates for 64Cu Coordination and Radiolabeling of αMSH Peptide. EJNMMI Radiopharm. Chem. 2021, 6, 3. [Google Scholar] [CrossRef]
- Borgna, F.; Ballan, M.; Favaretto, C.; Verona, M.; Tosato, M.; Caeran, M.; Corradetti, S.; Andrighetto, A.; Di Marco, V.; Marzaro, G.; et al. Early Evaluation of Copper Radioisotope Production at ISOLPHARM. Molecules 2018, 23, 2437. [Google Scholar] [CrossRef] [Green Version]
- Rylova, S.N.; Stoykow, C.; Del Pozzo, L.; Abiraj, K.; Tamma, M.L.; Kiefer, Y.; Fani, M.; Mäcke, H.R. The Somatostatin Receptor 2 Antagonist 64Cu-NODAGA-JR11 Outperforms 64Cu-DOTA-TATE in a Mouse Xenograft Model. PLoS ONE 2018, 13, e0195802. [Google Scholar]
- Blower, P.J.; Lewis, J.S.; Zweit, J. Copper Radionuclides and Radiopharmaceuticals in Nuclear Medicine. Nucl. Med. Biol. 1996, 23, 957–980. [Google Scholar] [CrossRef]
- Roux, A.; Gillet, R.; Huclier-Markai, S.; Ehret-Sabatier, L.; Charbonnière, L.J.; Nonat, A.M. Bifunctional Bispidine Derivatives for Copper-64 Labelling and Positron Emission Tomography. Org. Biomol. Chem. 2017, 15, 1475–1483. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [Green Version]
- Shokeen, M.; Anderson, C.J. Molecular Imaging of Cancer with Copper-64 Radiopharmaceuticals and Positron Emission Tomography (PET). Acc. Chem. Res. 2009, 42, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Jones-Wilson, T.M.; Deal, K.A.; Anderson, C.J.; McCarthy, D.W.; Kovacs, Z.; Motekaitis, R.J.; Sherry, A.D.; Martell, A.E.; Welch, M.J. The in Vivo Behavior of Copper-64-Labeled Azamacrocyclic Complexes. Nucl. Med. Biol. 1998, 25, 523–530. [Google Scholar] [CrossRef]
- Bass, L.A.; Wang, M.; Welch, M.J.; Anderson, C.J. In Vivo Transchelation of Copper-64 from TETA-Octreotide to Superoxide Dismutase in Rat Liver. Bioconjug. Chem. 2000, 11, 527–532. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Copper Chelation Chemistry and Its Role in Copper Radiopharmaceuticals. Curr. Pharm. Des. 2007, 13, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Anderson, C.J. Chelators for Copper Radionuclides in Positron Emission Tomography Radiopharmaceuticals. J. Label. Comp. Radiopharm. 2014, 57, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boswell, C.A.; Sun, X.; Niu, W.; Weisman, G.R.; Wong, E.H.; Rheingold, A.L.; Anderson, C.J. Comparative in Vivo Stability of Copper-64-Labeled Cross-Bridged and Conventional Tetraazamacrocyclic Complexes. J. Med. Chem. 2004, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.; Weisman, G.R.; Hill, D.C.; Reed, D.P.; Rogers, M.E.; Condon, J.S.; Fagan, M.A.; Calabrese, J.C.; Lam, K.C.; Guzei, I.A.; et al. Synthesis and Characterization of Cross-Bridged Cyclams and Pendant-Armed Derivatives and Structural Studies of Their Copper(II) Complexes. J. Am. Chem. Soc. 2000, 122, 10561–10572. [Google Scholar] [CrossRef]
- Ait-Mohand, S.; Fournier, P.; Dumulon-Perreault, V.; Kiefer, G.E.; Jurek, P.; Ferreira, C.L.; Bénard, F.; Guérin, B. Evaluation of 64Cu-Labeled Bifunctional Chelate-Bombesin Conjugates. Biocong. Chem. 2011, 22, 1729–1735. [Google Scholar] [CrossRef]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Mäcke, H.R. PET of Somatostatin Receptor-Positive Tumors Using 64Cu- and 68Ga Somatostatin Antagonists: The Chelate Makes the Difference. J. Nucl. Med. 2011, 52, 1110–1118. [Google Scholar] [CrossRef] [Green Version]
- Dumont, R.A.; Deininger, F.; Haubner, R.; Mäcke, H.R.; Weber, W.A.; Fani, M. Novel 64Cu- and 68Ga-Labeled RGD Conjugates Show Improved PET Imaging of Aνβ3 Integrin Expression and Facile Radiosynthesis. J. Nucl. Med. 2011, 52, 1276–1284. [Google Scholar] [CrossRef] [Green Version]
- Woodin, K.S.; Heroux, K.J.; Boswell, C.A.; Wong, E.H.; Weisman, G.R.; Niu, W.; Tomellini, S.A.; Anderson, C.J.; Zakharov, L.N.; Rheingold, A.L. Kinetic Inertness and Electrochemical Behavior of Copper(II) Tetraazamacrocyclic Complexes: Possible Implications for In Vivo Stability. Eur. J. Inorg. Chem. 2005, 2005, 4829–4933. [Google Scholar] [CrossRef]
- Cooper, M.S.; Ma, M.T.; Sunassee, K.; Shaw, K.P.; Williams, J.D.; Paul, R.L.; Donnelly, P.S.; Blower, P.J. Comparison of 64Cu-Complexing Bifunctional Chelators for Radioimmunoconjugation: Labeling Efficiency, Specific Activity, and in Vitro/in Vivo Stability. Bioconjug. Chem. 2012, 23, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Boros, E.; Holland, J.P. Chemical Aspects of Metal Ion Chelation in the Synthesis and Application Antibody-based Radiotracers. J. Label. Comp. Radiopharm. 2018, 61, 652–671. [Google Scholar] [CrossRef]
- Sharma, A.K.; Schultz, J.W.; Prior, J.T.; Rath, N.P.; Mirica, L.M. Coordination Chemistry of Bifunctional Chemical Agents Designed for Applications in 64Cu PET Imaging for Alzheimer’s Disease. Inorg. Chem. 2017, 56, 13801–13814. [Google Scholar] [CrossRef] [Green Version]
- Le Fur, M.; Beyler, M.; Le Poul, N.; Lima, L.M.P.; Le Mest, Y.; Delgado, R.; Iglesias, C.P.; Patineca, V.; Tripier, R. Improving the Stability and Inertness of Cu(II) and Cu(I) Complexes with Methylthiazolyl Ligands by Tuning the Macrocyclic Structure. Dalton Trans. 2016, 45, 7406–7420. [Google Scholar] [CrossRef]
- Tosato, M.; Verona, M.; Doro, R.; Dalla Tiezza, M.; Orian, L.; Andrighetto, A.; Pastore, P.; Marzaro, G.; Di Marco, V. Toward Novel Sulphur-Containing Derivatives of Tetraazacyclododecane: Synthesis, Acid–Base Properties, Spectroscopic Characterization, DFT Calculations, and Cadmium(II) Complex Formation in Aqueous Solution. New J. Chem. 2020, 44, 8337–8350. [Google Scholar] [CrossRef]
- Tosato, M.; Pelosato, M.; Franchi, S.; Isse, A.A.; May, N.V.; Zanoni, G.; Mancin, F.; Pastore, P.; Badocco, D.; Asti, M.; et al. When Ring Makes the Difference: Coordination Properties of Cu2+/Cu+ Complexes with Sulfur-Pendant Polyazamacrocycles for Radiopharmaceutical Applications. New J. Chem. 2022, 46, 10012–10025. [Google Scholar] [CrossRef]
- van der Meulen, N.P.; Hasler, R.; Blanc, A.; Farkas, R.; Benešová, M.; Talip, Z.; Müller, C.; Schibli, R. Implementation of a New Separation Method to Produce Qualitatively Improved 64Cu. J. Label. Comp. Radiopharm. 2019, 62, 460–470. [Google Scholar] [CrossRef]
- Kubíček, V.; Böhmová, Z.; Ševčíková, R.; Vaněk, J.; Lubal, P.; Poláková, Z.; Michalicová, R.; Kotek, J.; Hermann, P. NOTA Complexes with Copper(II) and Divalent Metal Ions: Kinetic and Thermodynamic Studies. Inorg. Chem. 2018, 57, 3061–3072. [Google Scholar] [CrossRef]
- Gillet, R.; Roux, A.; Brandel, J.; Huclier-Markai, S.; Camerel, F.; Jeannin, O.; Nonat, A.M.; Charbonniere, L.J. A Bispidol Chelator with a Phosphonate Pendant Arm: Synthesis, Cu(II) Complexation, and 64Cu Labeling. Inorg. Chem. 2017, 56, 11738–11752. [Google Scholar] [CrossRef]
- Silva, R.A.D.; Jain, S.; Lears, K.A.; Chong, H.S.; Kang, C.S.; Sun, X.; Rogers, B.E. Copper-64 Radiolabeling and Biological Evaluation of Bifunctional Chelators for Radiopharmaceutical Development. Nucl. Med. Biol. 2012, 39, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Shuvaev, S.; Suturina, E.A.; Rotile, N.J.; Astashkin, C.A.; Ziegler, C.J.; Ross, A.W.; Walker, T.L.; Caravan, P.; Taschner, I.S. Revisiting Dithiadiaza Macrocyclic Chelators for Copper-64 PET Imaging. Dalton Trans. 2020, 49, 14088–14098. [Google Scholar] [CrossRef]
- Wu, N.; Kang, C.S.; Sin, I.; Ren, S.; Liu, D.; Ruthengael, V.C.; Lewis, M.R.; Chong, H.S. Promising Bifunctional Chelators for Copper 64-PET Imaging: Practical 64Cu Radiolabeling and High in Vitro and in Vivo Complex Stability. J. Biol. Inorg. Chem. 2016, 21, 177–184. [Google Scholar] [CrossRef]
- Sun, X.; Wuest, M.; Kovacs, Z.; Sherry, D.A.; Motekaitis, R.; Wang, Z.; Martell, A.E.; Welch, M.J.; Anderson, C.J. In Vivo Behavior of Copper-64-Labeled Methanephosphonate Tetraaza Macrocyclic Ligands. J. Biol. Inorg. Chem. 2003, 8, 217–225. [Google Scholar] [CrossRef]
- Zarschler, K.; Kubeil, M.; Stephan, H. Establishment of Two Complementary in Vitro Assays for Radiocopper Complexes Achieving Reliable and Comparable Evaluation of in Vivo Stability. RSC Adv. 2014, 4, 10157–10164. [Google Scholar] [CrossRef] [Green Version]
- Lalioti, V.; Muruais, G.; Tsuchiya, Y.; Pulido, D.; Sandoval, I.V. Molecular Mechanisms of Copper Homeostasis. Front. Biosci. 2009, 14, 4878–4903. [Google Scholar] [CrossRef] [Green Version]
- Cutler, C.S.; Wuest, M.; Anderson, C.J.; Reichert, D.E.; Sun, Y.; Martell, A.E.; Welch, M.J. Labeling and in Vivo Evaluation of Novel Copper(II) Dioxotetraazamacrocyclic Complexes. Nucl. Med. Biol. 2000, 27, 375–380. [Google Scholar] [CrossRef]
- Sun, X.; Wuest, M.; Weisman, G.R.; Wong, E.H.; Reed, D.P.; Boswell, C.A.; Motekaitis, R.; Martell, A.E.; Welch, M.J.; Anderson, C.J. Radiolabeling and In Vivo Behavior of Copper-64-Labeled Cross-Bridged Cyclam Ligands. J. Med. Chem. 2002, 45, 468–477. [Google Scholar] [CrossRef]
- Gotzmann, C.; Braun, F.; Bartholomä, M.D. Synthesis, 64Cu-Labeling and PET Imaging of 1,4,7-Triazacyclononane Derived Chelators with Pendant Azaheterocyclic Arms. RSC Adv. 2016, 6, 119–131. [Google Scholar] [CrossRef]
- Kun, E. The Metabolism of Sulfur-Containing Compounds. In Metabolic Pathways, 2nd ed.; Greenberg, D.M., Ed.; Academic Press: New York, NY, USA, 1961; pp. 237–261. [Google Scholar]
- Nizou, G.; Favaretto, C.; Borgna, F.; Grundler, P.V.; Saffon-Merceron, N.; Platas-Iglesias, C.; Fougère, O.; Rousseaux, O.; van der Meulen, N.P.; Müller, C.; et al. Expanding the Scope of Pyclen-Picolinate Lanthanide Chelates to Potential Theranostic Applications. Inorg. Chem. 2020, 59, 11736–11748. [Google Scholar] [CrossRef]
- Morselli, L.; Lunardon, M.; Stevanato, L.; Andrighetto, A. Characterization of a Cost-Effective γ-Counter Based on a Lanthanum Bromo-Chloride Scintillator; INFN-LNL Report 266; INFN-LNL: Legnaro, Italy, 2022; ISSN 1828-8561. [Google Scholar]


| Zn2+ | Ni2+ | |
|---|---|---|
| [64Cu][Cu(DO4S)]2+ | 100 ± 1 | 100 ± 2 |
| [64Cu][Cu(DO3S)]2+ | 95 ± 2 | 91 ± 2 |
| [64Cu][Cu(DO3SAm)]2+ | 90 ± 2 | 99 ± 2 |
| [64Cu][Cu(DO2A2S)] | 64 ± 5 | 73 ± 3 |
| [64Cu][Cu(TRI4S)]2+ | 100 ± 1 | 100 ± 1 |
| Complex | DOTA | Cysteine | ||||
|---|---|---|---|---|---|---|
| 0 h | 7 h | 24 h | 0 h | 3 h | 24 h | |
| [64Cu][Cu(DO4S)]2+ | 100 | 92 | 88 | 100 | 100 | 83 |
| [64Cu][Cu(DO3S)]2+ | 100 | 100 | 100 | 100 | 100 | 100 |
| [64Cu][Cu(DO3SAm)]2+ | 100 | 80 | 38 | 100 | 83 | 72 |
| [64Cu][Cu(DO2A2S)] | 91 | 90 | 87 | 100 | 85 | 74 |
| [64Cu][Cu(TRI4S)]2+ | − | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosato, M.; Verona, M.; Favaretto, C.; Pometti, M.; Zanoni, G.; Scopelliti, F.; Cammarata, F.P.; Morselli, L.; Talip, Z.; van der Meulen, N.P.; et al. Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation. Molecules 2022, 27, 4158. https://doi.org/10.3390/molecules27134158
Tosato M, Verona M, Favaretto C, Pometti M, Zanoni G, Scopelliti F, Cammarata FP, Morselli L, Talip Z, van der Meulen NP, et al. Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation. Molecules. 2022; 27(13):4158. https://doi.org/10.3390/molecules27134158
Chicago/Turabian StyleTosato, Marianna, Marco Verona, Chiara Favaretto, Marco Pometti, Giordano Zanoni, Fabrizio Scopelliti, Francesco Paolo Cammarata, Luca Morselli, Zeynep Talip, Nicholas P. van der Meulen, and et al. 2022. "Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation" Molecules 27, no. 13: 4158. https://doi.org/10.3390/molecules27134158
APA StyleTosato, M., Verona, M., Favaretto, C., Pometti, M., Zanoni, G., Scopelliti, F., Cammarata, F. P., Morselli, L., Talip, Z., van der Meulen, N. P., Di Marco, V., & Asti, M. (2022). Chelation of Theranostic Copper Radioisotopes with S-Rich Macrocycles: From Radiolabelling of Copper-64 to In Vivo Investigation. Molecules, 27(13), 4158. https://doi.org/10.3390/molecules27134158









