Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies
Abstract
:1. Introduction
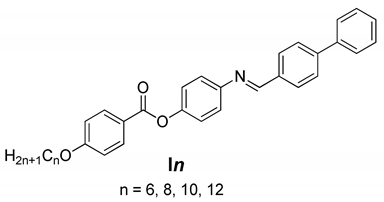
2. Results and Discussion
2.1. Liquid Crystalline Behavior of Series, In
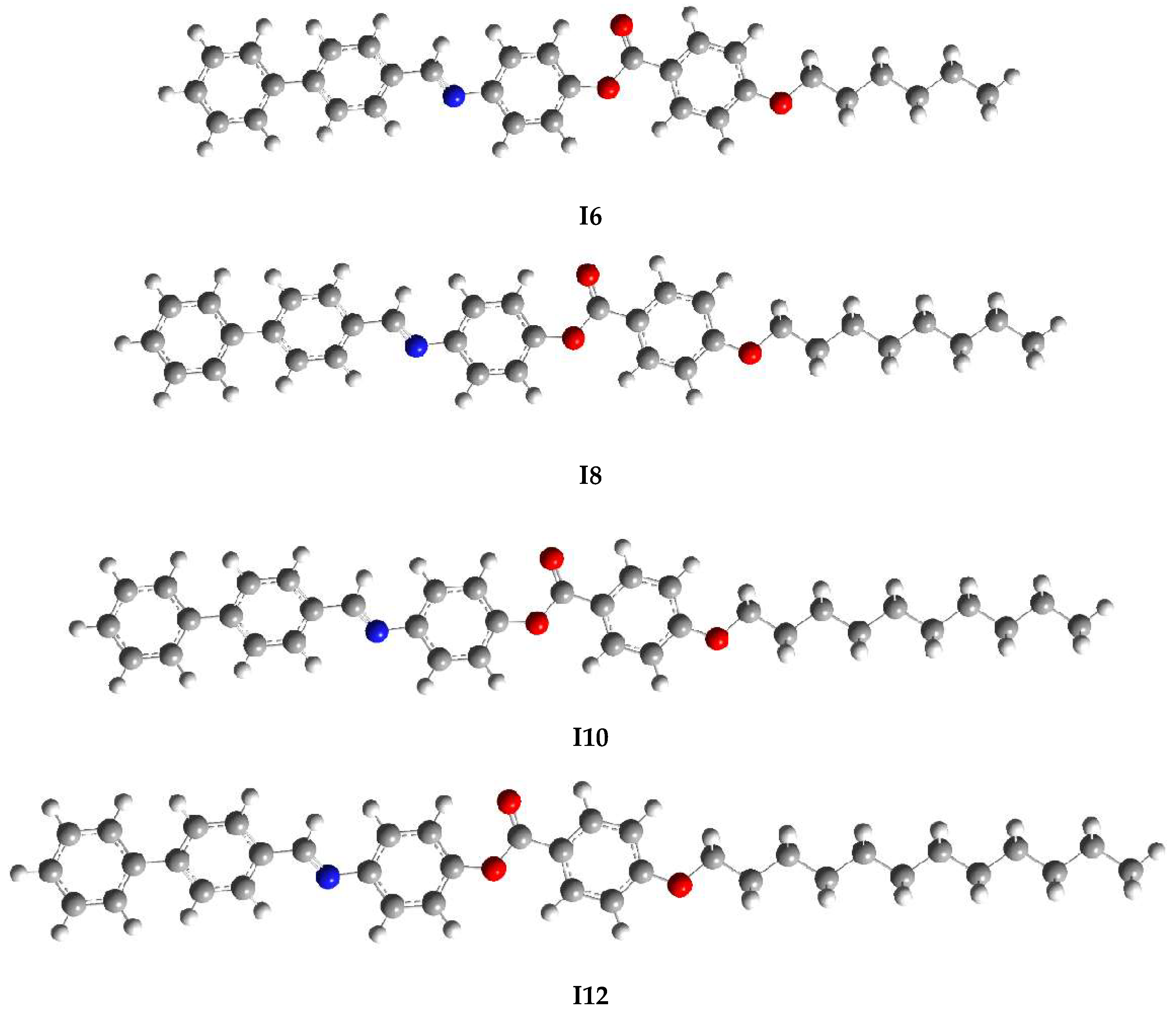
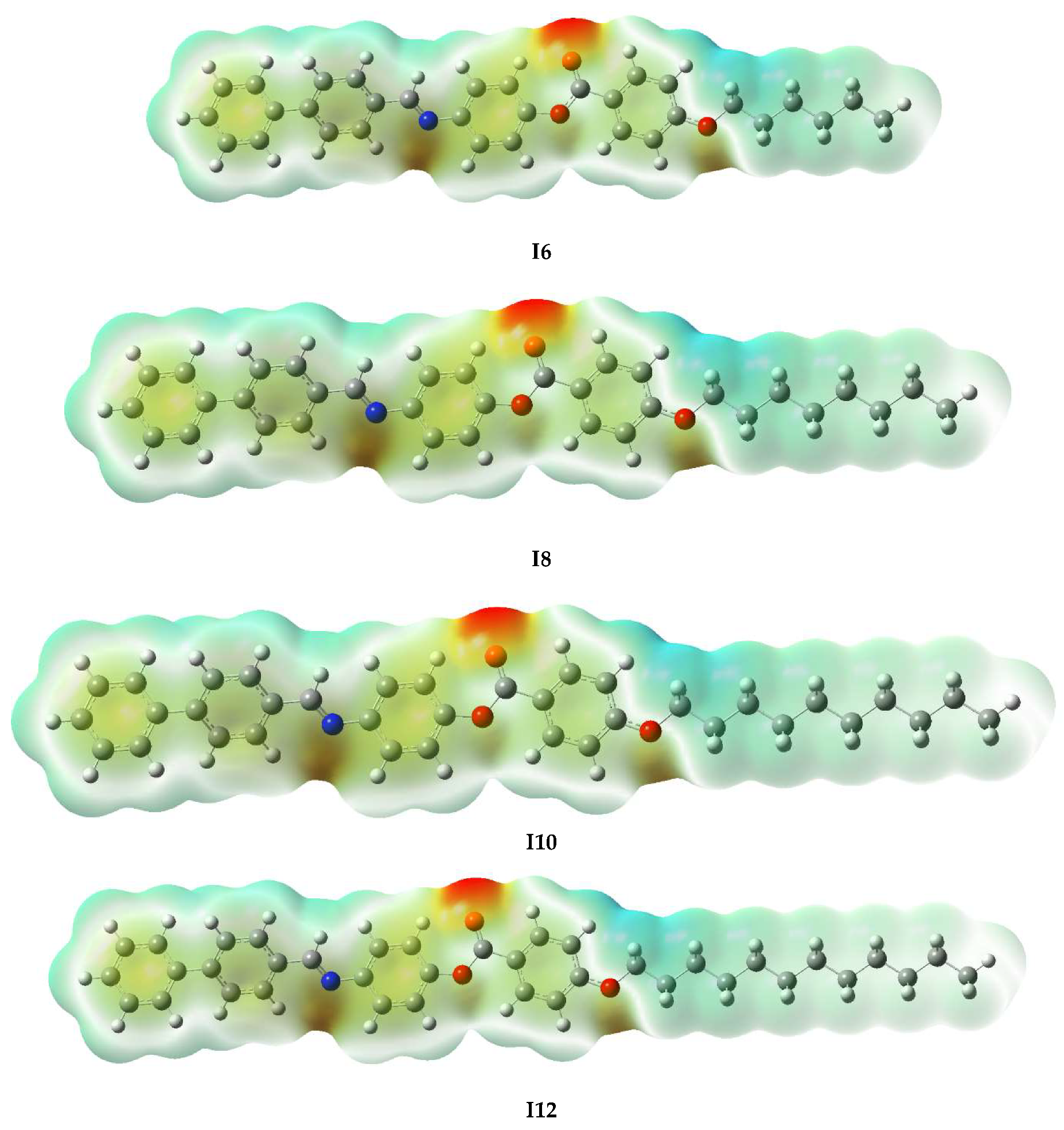
2.2. DFT Studies
3. Experiments
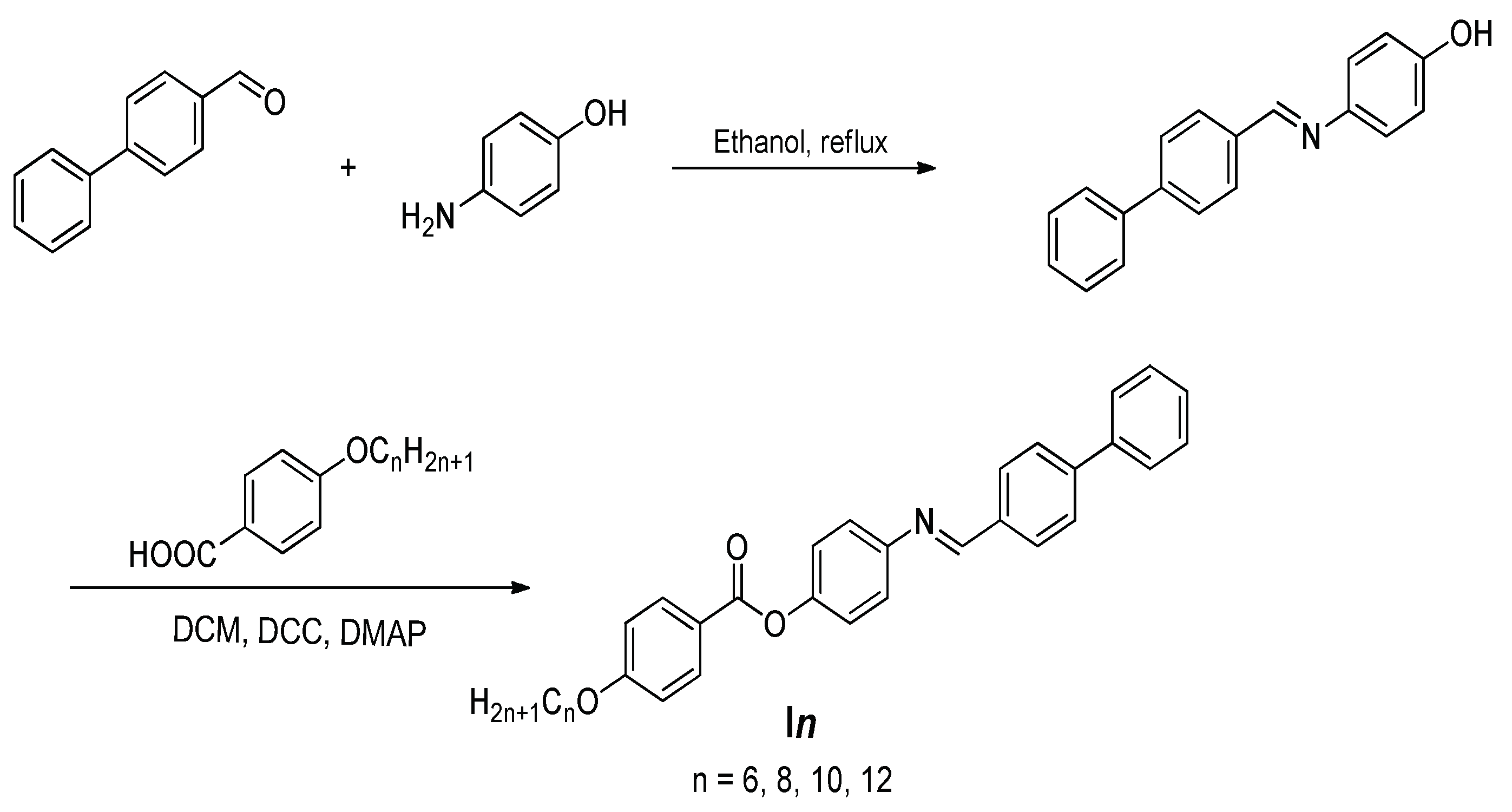
Synthesis
4. Computational Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sudhakar, S.; Narasimhaswamy, T.; Srinivasan, K. Synthesis, characterization and thermal properties of 4, 4′-bis (4-n-alkoxybenzoyloxy) benzylideneanilines and bis (4-benzylidene-4′-n-alkoxyaniline) terephthalates. Liq. Cryst. 2000, 27, 1525–1532. [Google Scholar] [CrossRef]
- Saha, S.K.; Deb, J.; Sarkar, U.; Paul, M.K. Hockey-stick-shaped mesogens based on 1, 3, 4-thiadiazole: Synthesis, mesomorphism, photophysical and DFT studies. Liq. Cryst. 2017, 44, 2203–2221. [Google Scholar] [CrossRef]
- Saha, S.K.; Paul, M.K. Mesomorphic and photophysical behaviour of 1, 3, 4-oxadiazole based hockey stick reactive mesogens. Liq. Cryst. 2019, 46, 386–396. [Google Scholar] [CrossRef]
- Ong, L.-K.; Ha, S.-T.; Yeap, G.-Y.; Lin, H.-C. Heterocyclic pyridine-based liquid crystals: Synthesis and mesomorphic properties. Liq. Cryst. 2018, 45, 1574–1584. [Google Scholar] [CrossRef]
- Quan, Y.-Y.; Wang, D.; He, Q.-Q.; Hu, J.-W.; Tian, M.; Wang, X.-J.; Jia, Y.-G.; Yao, D.-S. V-shaped Schiff’s base liquid crystals based on resorcinol: Synthesis and characterisation. Liq. Cryst. 2020, 47, 737–749. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Osman, F.; Imrie, C.T. Non-symmetric dimers: Effects of varying the mesogenic linking unit and terminal substituent. Liq. Cryst. 2015, 42, 543–554. [Google Scholar] [CrossRef]
- Fritsch, L.; Lavayen, V.; Merlo, A.A. Photochemical behaviour of Schiff base liquid crystals based on isoxazole and isoxazoline ring. A kinetic approach. Liq. Cryst. 2018, 45, 1802–1812. [Google Scholar] [CrossRef]
- Altowyan, A.S.; Ahmed, H.A.; Gomha, S.M.; Mostafa, A.M. Optical and thermal investigations of new schiff base/ester systems in pure and mixed states. Polymers 2021, 13, 1687. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsu, C.-C.; Chen, L.-W.; Cheng, Y.-L. The effect of position of (S)-2-octyloxy tail on the formation of frustrated blue phase and antiferroelectric phase in Schiff base liquid crystals. Soft Matter 2014, 10, 9343–9351. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Naoum, M.M.; Al-Zahrani, S.A.; Ahmed, H.A. New nitro-laterally substituted azomethine derivatives; Synthesis, mesomorphic and computational characterizations. Molecules 2021, 26, 1927. [Google Scholar] [CrossRef]
- Abberley, J.P.; Killah, R.; Walker, R.; Storey, J.; Imrie, C.T.; Salamończyk, M.; Zhu, C.; Gorecka, E.; Pociecha, D. Heliconical smectic phases formed by achiral molecules. Nat. Commun. 2018, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Salamończyk, M.; Vaupotič, N.; Pociecha, D.; Walker, R.; Storey, J.; Imrie, C.T.; Wang, C.; Zhu, C.; Gorecka, E. Multi-level chirality in liquid crystals formed by achiral molecules. Nat. Commun. 2019, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Imrie, C.T. Non-symmetric liquid crystal dimers: How to make molecules intercalate. Liq. Cryst. 2006, 33, 1449–1485. [Google Scholar] [CrossRef]
- Ooi, Y.-H.; Yeap, G.-Y.; Han, C.-C.; Lin, H.-C.; Kubo, K.; Ito, M.M. Synthesis and smectogenic properties of novel phloroglucinol-based star-shaped liquid crystals containing three peripheral alkyloxylated Schiff base arms. Liq. Cryst. 2013, 40, 516–527. [Google Scholar] [CrossRef]
- Hinojosa, A.R.C.; de Souza, S.P.; Alves, T.V.; dos Santos, I.T.O.; Silva, E.O.; Gonçalves, I.L.; Merlo, A.A.; Junkes, C.F.; Bechtold, I.H.; Vieira, A.A. Shining rings: The effect of the rigid core and benzazole heterocycles on the properties of luminescent calamitic liquid crystals. J. Mol. Liq. 2021, 338, 116614. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Khushaim, M.S.; Gomh, S.M.; Ahmed, H.A.; Naoum, M.M. Mesophase behavior of four ring ester/azomethine/ester liquid crystals in pure and mixed states. Liq. Cryst. 2022, 49, 1–8. [Google Scholar] [CrossRef]
- Paterson, D.A.; Crawford, C.A.; Pociecha, D.; Walker, R.; Storey, J.M.; Gorecka, E.; Imrie, C.T. The role of a terminal chain in promoting the twist-bend nematic phase: The synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkyloxyanilinebenzylidene-4′-oxy) hexanes. Liq. Cryst. 2018, 45, 2341–2351. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Strachan, G.J.; Storey, J.M.; Gorecka, E.; Imrie, C.T. Molecular curvature, specific intermolecular interactions and the twist-bend nematic phase: The synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkylanilinebenzylidene-4′-oxy) hexanes (CB6O. m). Soft Matter 2019, 15, 3188–3197. [Google Scholar] [CrossRef]
- Walker, R.; Majewska, M.; Pociecha, D.; Makal, A.; Storey, J.M.; Gorecka, E.; Imrie, C.T. Twist-bend nematic glasses: The synthesis and characterisation of pyrene-based nonsymmetric dimers. ChemPhysChem 2021, 22, 461–470. [Google Scholar] [CrossRef]
- Forsyth, E.; Paterson, D.A.; Cruickshank, E.; Strachan, G.J.; Gorecka, E.; Walker, R.; Storey, J.M.; Imrie, C.T. Liquid crystal dimers and the twist-bend nematic phase: On the role of spacers and terminal alkyl chains. J. Mol. Liq. 2020, 320, 114391. [Google Scholar] [CrossRef]
- Alamro, F.S.; Al-Kadhi, N.S.; Gomha, S.M.; Popoola, S.A.; Khushaim, M.S.; Alhaddad, O.A.; Ahmed, H.A. Experimental and Theoretical Investigations of Three-Ring Ester/Azomethine Materials. Materials 2022, 15, 2312. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; El-Atawy, M.A. Synthesis, optical and DFT characterizations of laterally fluorinated phenyl cinnamate liquid crystal non-symmetric system. Symmetry 2021, 13, 1145. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Bedowr, N.S.; Khushaim, M.S.; El-Atawy, M.A. New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer. Polymers 2022, 14, 1256. [Google Scholar] [CrossRef]
- Khushaim, M.S.; Alalawy, H.H.; Naoum, M.M.; Ahmed, H.A. Experimental and computational simulations of nematogenic liquid crystals based on cinnamic acid in pure and mixed state. Liq. Cryst. 2021, 48, 1493–1504. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; El-Atawy, M.A.; Al-Zahrani, S.A.; Omar, A.Z. Induced Nematic Phase of New Synthesized Laterally Fluorinated Azo/Ester Derivatives. Molecules 2021, 26, 4546. [Google Scholar] [CrossRef] [PubMed]
- Mandle, R.J.; Sebastián, N.; Martinez-Perdiguero, J.; Mertelj, A. On the molecular origins of the ferroelectric splay nematic phase. Nat. Commun. 2021, 12, 4962. [Google Scholar] [CrossRef] [PubMed]
- Mandle, R.J.; Cowling, S.J.; Goodby, J.W. Rational Design of Rod-Like Liquid Crystals Exhibiting Two Nematic Phases. Chem. A Eur. J. 2017, 23, 14554–14562. [Google Scholar] [CrossRef]
- Manabe, A.; Bremer, M.; Kraska, M. Ferroelectric nematic phase at and below room temperature. Liq. Cryst. 2021, 48, 1079–1086. [Google Scholar] [CrossRef]
- Brown, S.; Cruickshank, E.; Storey, J.M.; Imrie, C.T.; Pociecha, D.; Majewska, M.; Makal, A.; Gorecka, E. Multiple Polar and Non-polar Nematic Phases. ChemPhysChem 2021, 22, 2506–2510. [Google Scholar] [CrossRef]
- Dunmur, D.; Toriyama, K. Handbook of Liquid Crystals; Demus, D., Goodby, J., Gray, G.W., Spiess, H.W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; p. 341. [Google Scholar]
- Kelker, H.; Scheurle, B. Eine flüssig-kristalline (nematische) Phase mit besonders niedrigem Erstarrungspunkt. Angew. Chem. 1969, 81, 903–904. [Google Scholar] [CrossRef]
- Goodby, J.W. Nano-objects-sculpting and shape in molecular material design (The Pierre Gilles de Gennes ILCS prize lecture). Liq. Cryst. 2019, 46, 1901–1924. [Google Scholar] [CrossRef]
- Mandle, R.J.; Stock, N.; Cowling, S.J.; Parker, R.R.; Hart, S.; Whitwood, A.C.; Goodby, J.W. Condensation of free volume in structures of nematic and hexatic liquid crystals. Liq. Cryst. 2019, 46, 114–123. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Alhaddad, O.A.; Ahmed, H.A. Experimental and geometrical structure characterizations of new synthesized laterally fluorinated nematogenic system. Liq. Cryst. 2021, 48, 2106–2116. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-Atawy, M.A. Synthesis, mesomorphic and geometrical approaches of new non-symmetrical system based on central naphthalene moiety. Liq. Cryst. 2021, 48, 1940–1952. [Google Scholar] [CrossRef]
- Capar, M.I.; Cebe, E. Molecular dynamic study of the odd-even effect in some 4-n-alkyl-40-cyanobiphenyls. Phys. Rev. E 2006, 73, 061711. [Google Scholar] [CrossRef]
- Capar, M.I.; Cebe, E. Odd–even effects in the homologous series of alkyl-cyanobiphenyl liquid crystals: A molecular dynamic study. J. Comput. Chem. 2007, 28, 2140–2146. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid crystal dimers and higher oligomers: Between monomers and polymers. Chem. Soc. Rev. 2007, 36, 2096–2124. [Google Scholar] [CrossRef]
- Saha, R.; Babakhanova, G.; Parsouzi, Z.; Rajabi, M.; Gyawali, P.; Welch, C.; Jákli, A. Oligomeric odd-even effect in liquid crystals. Mater. Horiz. 2019, 6, 1905–1912. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G.R. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino) phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Mostafa, A.M.; Naoum, M.M. Thermal and Mesomorphic Investigations of 1:1 Supramolecular Assemblies of 4-[(4-(n-Alkoxy) phenylimino) methyl] benzoic Acids Having Symmetrical and Un-Symmetrical Terminal Chain Lengths. Symmetry 2021, 13, 1785. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Popoola, S.A.; Aboelnaga, A. Synthesis, Phase Behavior and Computational Simulations of a Pyridyl-Based Liquid Crystal System. Molecules 2021, 26, 6416. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.W. Molecular Structure and The Properties of Liquid Crystals; Academic Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Imrie, C.; Taylor, L. The preparation and properties of low molar mass liquid crystals possessing lateral alkyl chains. Liq. Cryst. 1989, 6, 1–10. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Storey, J.M.; Gorecka, E.; Imrie, C.T. Remarkable smectic phase behaviour in odd-membered liquid crystal dimers: The CT6O. m series. J. Mater. Chem. C 2021, 9, 5167–5173. [Google Scholar] [CrossRef]
- Date, R.; Imrie, C.; Luckhurst, G.; Seddon, J. Smectogenic dimeric liquid crystals. The preparation and properties of the α, ω-bis (4-n-alkylanilinebenzylidine-4′-oxy) alkanes. Liq. Cryst. 1992, 12, 203–238. [Google Scholar] [CrossRef]
- Paterson, D.A.; Abberley, J.P.; Harrison, W.T.; Storey, J.M.; Imrie, C.T. Cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 44, 127–146. [Google Scholar] [CrossRef]
- Hickey, A.L.; Rowley, C.N. Benchmarking quantum chemical methods for the calculation of molecular dipole moments and polarizabilities. J. Phys. Chem. A 2014, 118, 3678–3687. [Google Scholar] [CrossRef]
- Emam, S.M.; Tolan, D.A.; El-Nahas, A.M. Synthesis, structural, spectroscopic, and thermal studies of some transition-metal complexes of a ligand containing the amino mercapto triazole moiety. Appl. Organomet. Chem. 2020, 34, e5591. [Google Scholar] [CrossRef]
- Tolan, D.A.; Kashar, T.I.; Yoshizawa, K.; El-Nahas, A.M. Synthesis, spectral characterization, density functional theory studies, and biological screening of some transition metal complexes of a novel hydrazide–hydrazone ligand of isonicotinic acid. Appl. Organomet. Chem. 2021, 35, e6205. [Google Scholar] [CrossRef]
- Pegu, D.; Deb, J.; Van Alsenoy, C.; Sarkar, U. Theoretical investigation of electronic, vibrational, and nonlinear optical properties of 4-fluoro-4-hydroxybenzophenone. Spectrosc. Lett. 2017, 50, 232–243. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical hardness and density functional theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C. A new equation for calculation of chemical hardness of groups and molecules. Mol. Phys. 2015, 113, 1311–1319. [Google Scholar] [CrossRef]
- Zaki, A.A.; Hagar, M.; Alnoman, R.B.; Jaremko, M.; Emwas, A.-H.; Ahmed, H.A. Mesomorphic, Optical and DFT Aspects of Near to Room-Temperature Calamitic Liquid Crystal. Crystals 2020, 10, 1044. [Google Scholar] [CrossRef]
- Parr, R.G.W. Yang Density Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1989; Volume 1, p. 1989. [Google Scholar]
- Ziegler, T. Approximate density functional theory as a practical tool in molecular energetics and dynamics. Chem. Rev. 1991, 91, 651–667. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-functional theory of the electronic structure of molecules. Annu. Rev. Phys. Chem. 1995, 46, 701–728. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Roy, D.R. Update 1 of: Electrophilicity index. Chem. Rev. 2007, 107, PR46–PR74. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
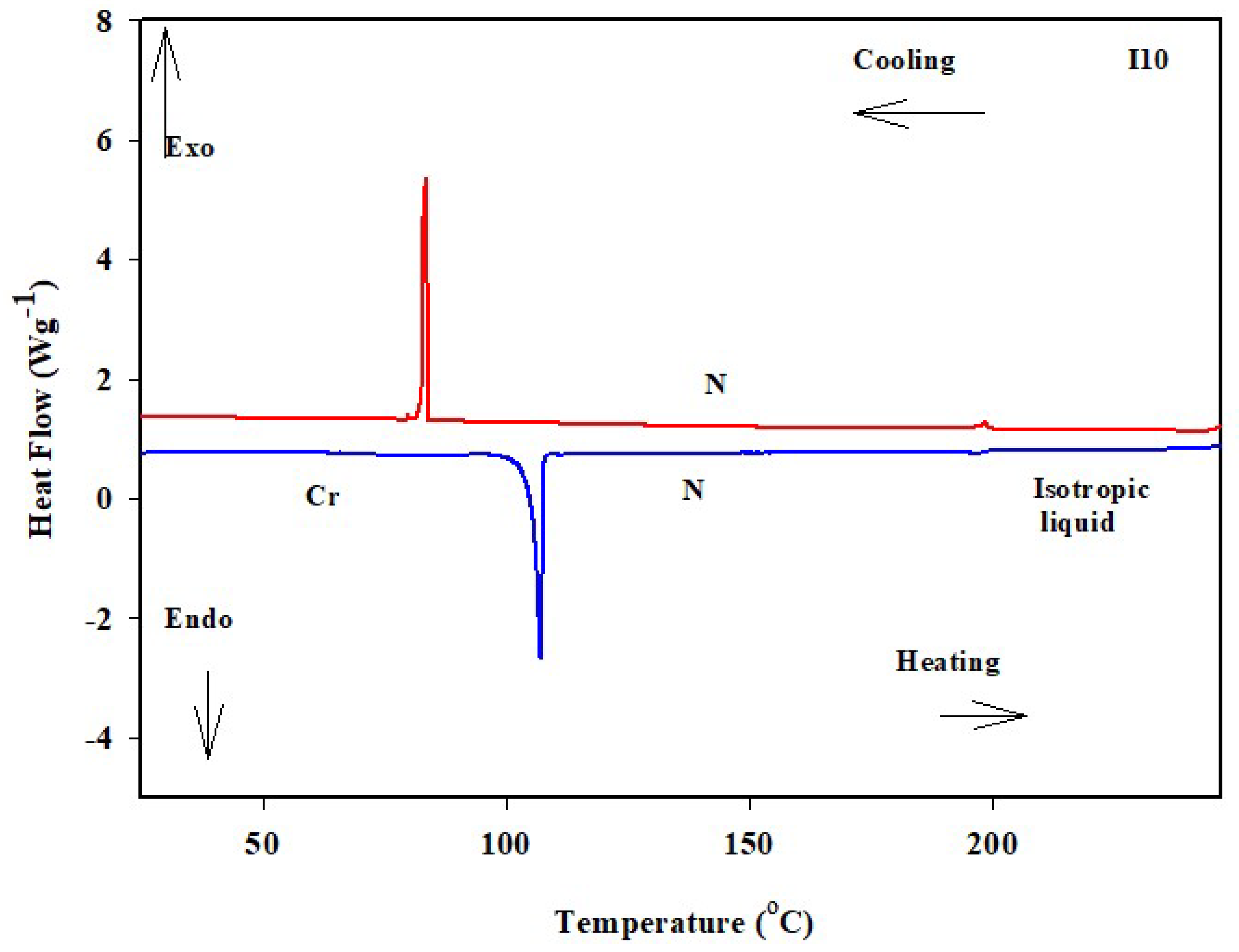

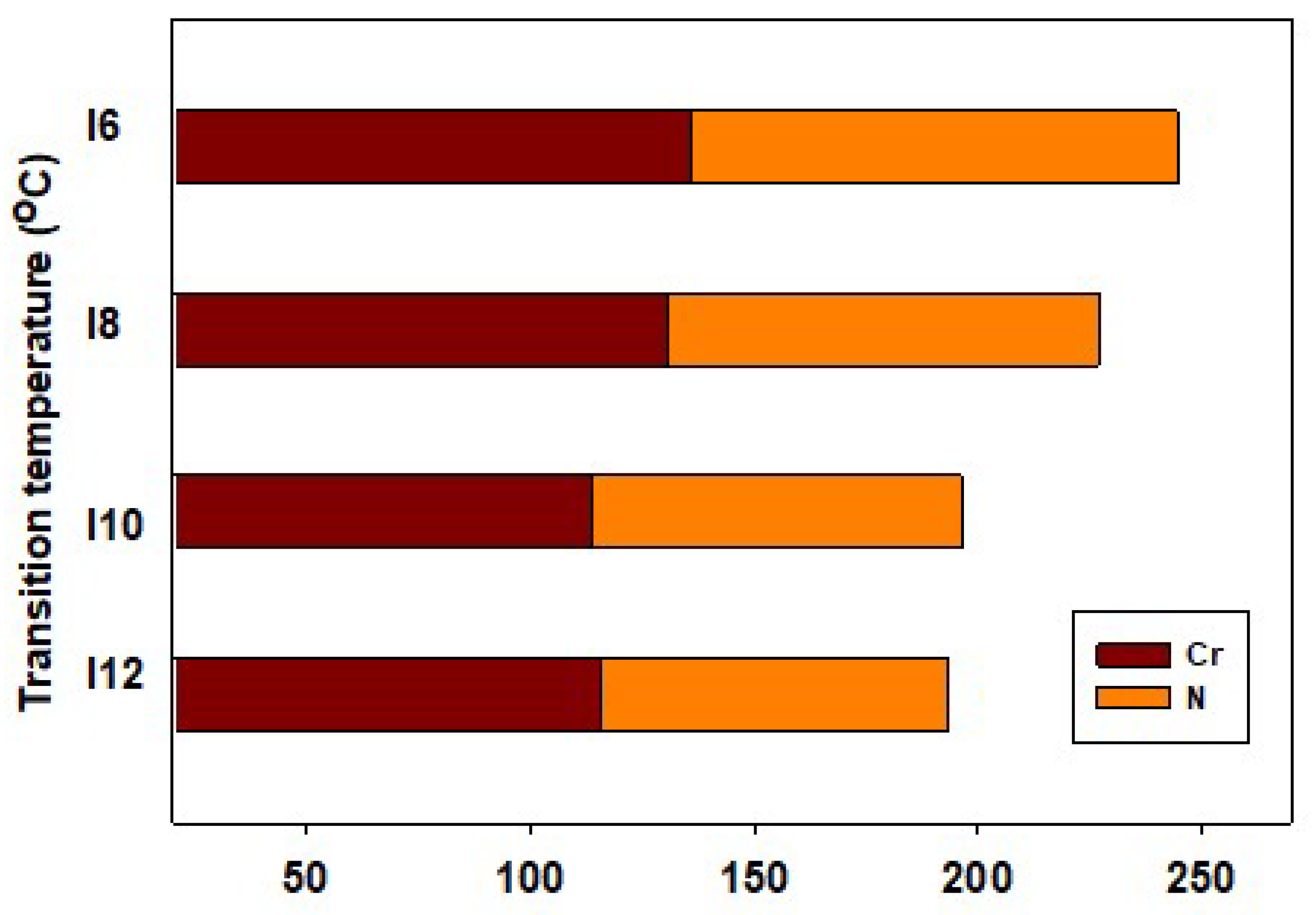
| Comp. | TCr-N | ΔHCr-N | TN-I | ΔHN-I | ΔTN | ΔSN-I/R |
|---|---|---|---|---|---|---|
| I6 | 135.8 | 43.72 | 244.3 | 1.99 | 108.50 | 0.46 |
| I8 | 130.5 | 45.08 | 226.8 | 1.87 | 96.30 | 0.45 |
| I10 | 113.5 | 39.88 | 196.2 | 1.95 | 82.70 | 0.50 |
| I12 | 115.8 | 41.79 | 193.3 | 1.91 | 77.50 | 0.49 |
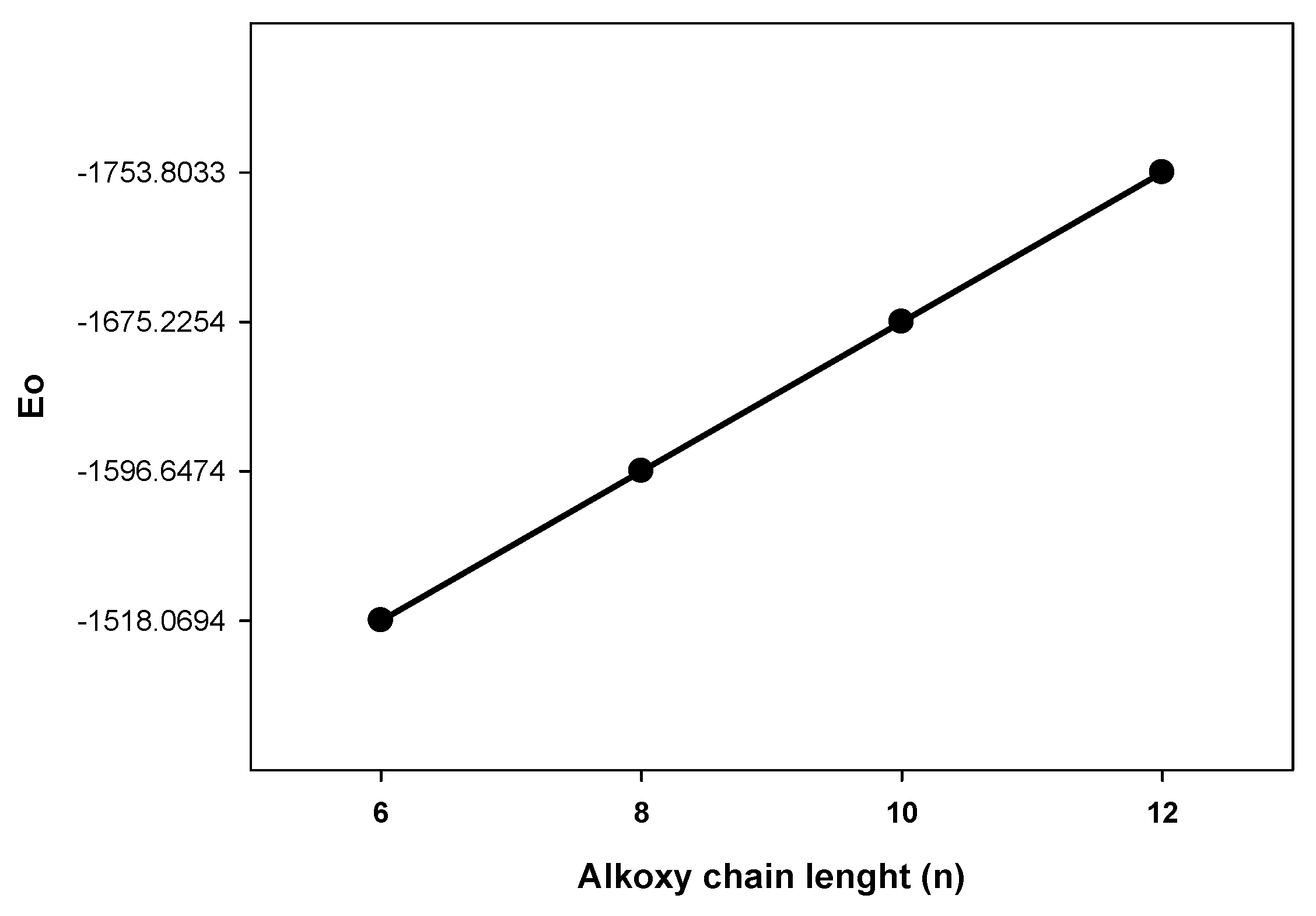
| E0 | E298 | H298 | G298 | |
|---|---|---|---|---|
| I6 | −1518.069423 | −1518.036426 | −1518.035482 | −1518.141578 |
| I8 | −1596.647410 | −1596.611662 | −1596.610718 | −1596.723890 |
| I10 | −1675.225375 | −1675.186884 | −1675.185940 | −1675.306220 |
| I12 | −1753.803278 | −1753.762061 | −1753.761117 | −1753.888488 |
| E (a.u) | I6 | I8 | I10 | I12 |
|---|---|---|---|---|
| Dipole moment (D) | 2.359 | 2.402 | 2.426 | 2.442 |
| Polarizability (α) | 472.08 | 497.42 | 522.50 | 547.42 |
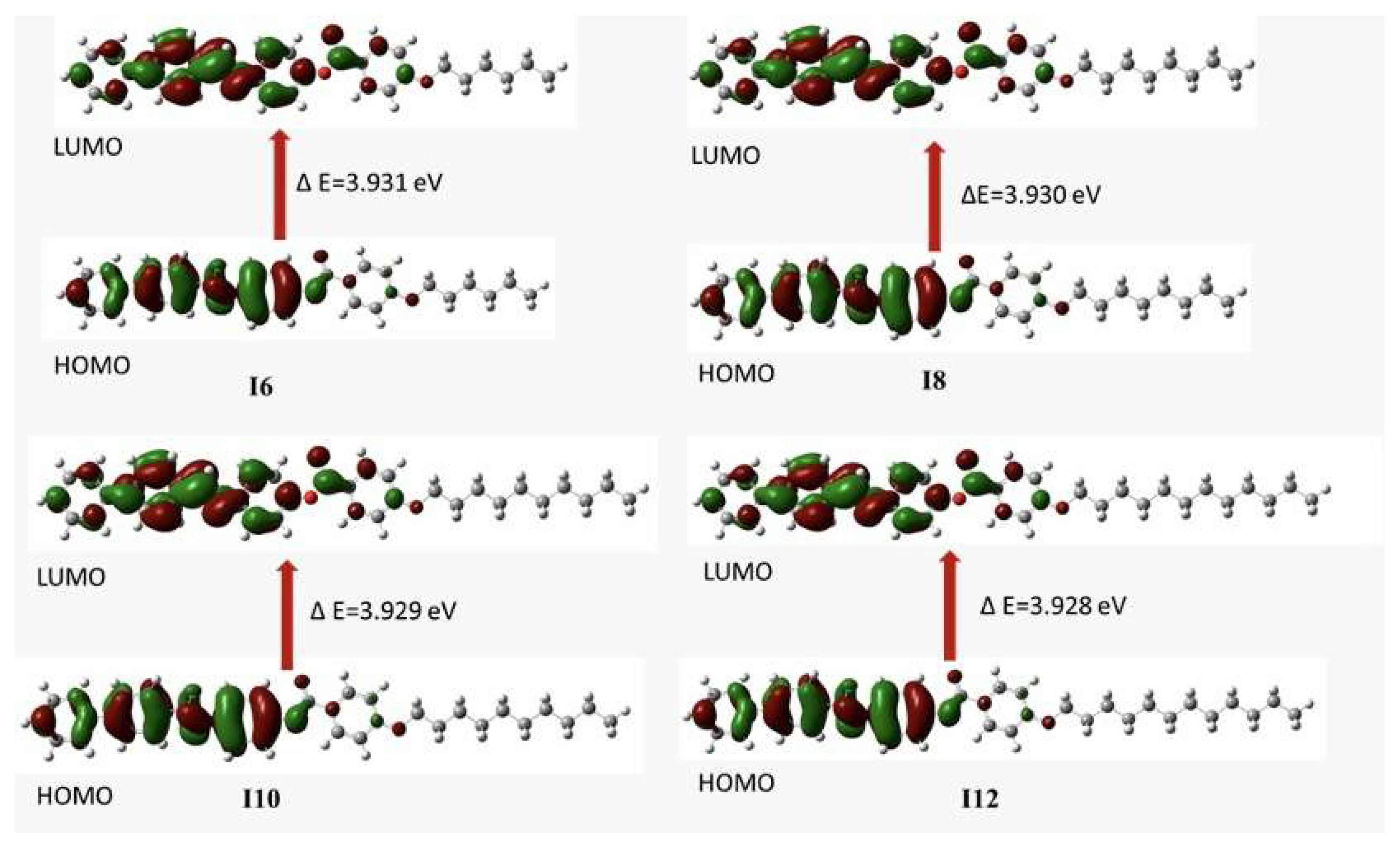
| EHOMO (eV) | −5.950 | −5.948 | −5.948 | −5.948 |
| ELUMO (eV) | −2.019 | −2.018 | −2.019 | −2.019 |
| ΔE (eV) | 3.931 | 3.930 | 3.929 | 3.929 |
| χ (eV) | 3.984 | 3.983 | 3.983 | 3.983 |
| η (eV) | 1.965 | 1.965 | 1.964 | 1.964 |
| σ (eV−1) | 0.508 | 0.508 | 0.509 | 0.509 |
| μ (eV) | −3.984 | −3.983 | −3.983 | −3.983 |
| S (eV−1) | 0.254 | 0.254 | 0.254 | 0.254 |
| ω (eV) | 4.038 | 4.036 | 4.038 | 4.038 |
| ΔNmax | 2.027 | 2.027 | 2.028 | 2.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, F.S.; Tolan, D.A.; El-Nahas, A.M.; Ahmed, H.A.; El-Atawy, M.A.; Al-Kadhi, N.S.; Aziz, S.G.; Shibl, M.F. Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies. Molecules 2022, 27, 4150. https://doi.org/10.3390/molecules27134150
Alamro FS, Tolan DA, El-Nahas AM, Ahmed HA, El-Atawy MA, Al-Kadhi NS, Aziz SG, Shibl MF. Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies. Molecules. 2022; 27(13):4150. https://doi.org/10.3390/molecules27134150
Chicago/Turabian StyleAlamro, Fowzia S., Dina A. Tolan, Ahmed M. El-Nahas, Hoda A. Ahmed, Mohamed A. El-Atawy, Nada S. Al-Kadhi, Saadullah G. Aziz, and Mohamed F. Shibl. 2022. "Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies" Molecules 27, no. 13: 4150. https://doi.org/10.3390/molecules27134150
APA StyleAlamro, F. S., Tolan, D. A., El-Nahas, A. M., Ahmed, H. A., El-Atawy, M. A., Al-Kadhi, N. S., Aziz, S. G., & Shibl, M. F. (2022). Wide Nematogenic Azomethine/Ester Liquid Crystals Based on New Biphenyl Derivatives: Mesomorphic and Computational Studies. Molecules, 27(13), 4150. https://doi.org/10.3390/molecules27134150








