Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials
Abstract
:1. Introduction
2. Results
2.1. Drug Substance
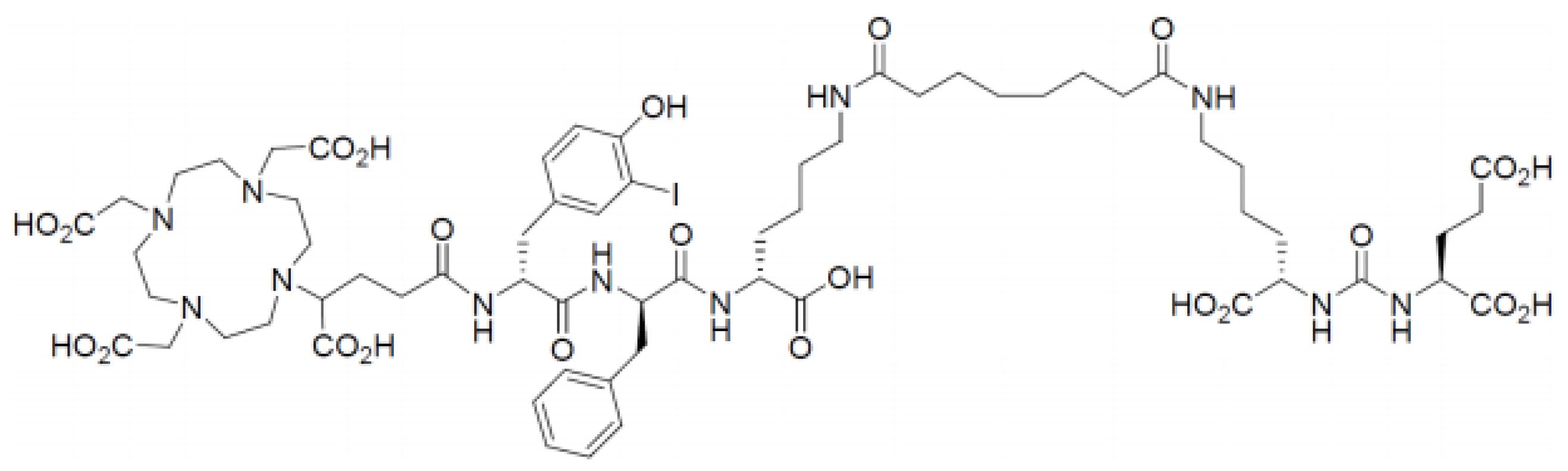
2.1.1. PSMA-I&T
- (R)-DOTAGA-D-Tyr(3-I)-D-Phe-D-Lys[Sub-Lys-CO-Glu]-OH (supplied as acetate salt)
- Synonyms (R)-DOTAGA-(I-y)fk(Sub-KuE) Structure
- Molecular formula: C63H92IN11O23
- Molecular weight: 1498.37 g/mol
2.1.2. Lutetium-177
2.2. Investigational Medicinal Product (IMP) under Test
Description and Composition of the IMP
2.3. Quality Controls
2.3.1. Acceptance Criteria
2.3.2. Validation of the Analytical Procedures
2.3.3. Bioburden
- Total aerobic microbial count (TAMC) < 1 cfu/mL,
- Total yeast and mold count (TYMC) < 1 cfu/mL,
- where <1 cfu/mL means absence of colonies.
2.3.4. Batch Analysis and Process Validation
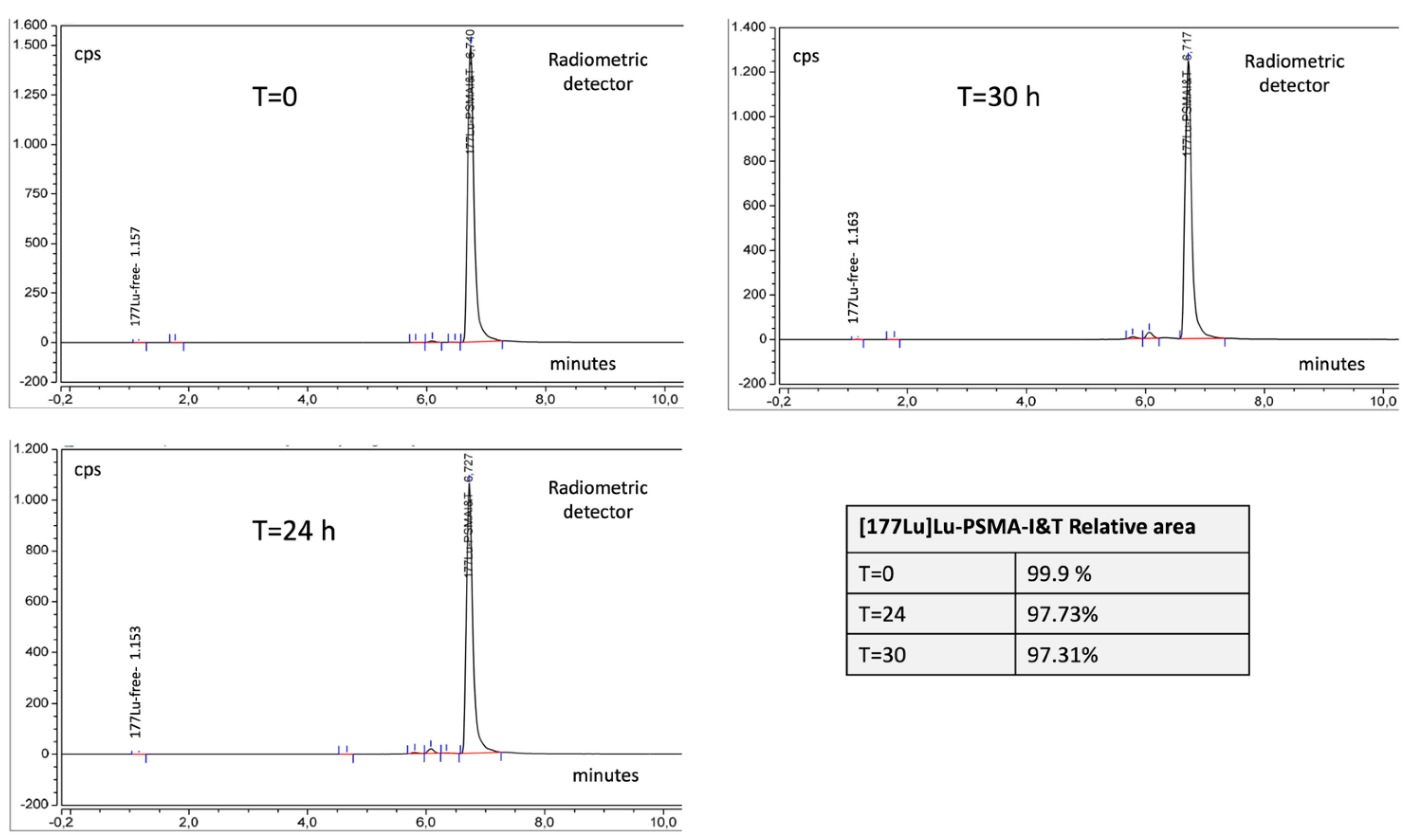
2.3.5. Stability
3. Discussion
4. Materials and Methods
4.1. [177Lu]Lu-PSMA-I&T Manufacturing Process and Process Controls
4.1.1. Reagents
4.1.2. Manufacturing of [177Lu]Lu-PSMA-I&T
4.1.3. In Process Controls (PC)
4.2. Quality Control
4.2.1. Standard Procedures
4.2.2. HPLC Analysis
4.2.3. Thin-Layer Chromatography (TLC)
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroöder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef] [Green Version]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar] [PubMed]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Nan, F.; Conti, P.; Zhang, J.; Ramadan, E.; Bzdega, T.; Wroblewska, B.; Neale, J.H.; Pshenichkin, S.; Wroblewski, J.T. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAA- LADase). J. Med. Chem. 2001, 44, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconj. Chem. 2012, 44, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Benešova, M.; Schäfer, M.; Baüder-Wust, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-labeled PSMA I&T: Optimization of a PSMA-targeted theranostic concept and first proof- of-concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weineisen, M.; Simecek, J.; Schottelius, M.; Schwaiger, M.; Wester, H.J. Synthesis and preclinical evaluation of DOTAGA- conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 2014, 4, 63. Available online: http://www.ejnmmires.com/content/4/1/63 (accessed on 13 April 2022). [CrossRef] [PubMed] [Green Version]
- Norme di Buona Preparazione dei Radiofarmaci per Medicina Nucleare. Ital. Pharm. 2005, 146 (Suppl. S11), 168. Available online: https://www.sifoweb.it/images/pdf/attivita/attivita-scientifica/aree_scientifiche/radiofarmacia/normativa/NBP_Radiofarmaci.pdf (accessed on 2 May 2021).
- Guideline on the Requirements for the Chemical and Pharmaceutical Quality Documentation Concerning Investigational Medicinal Products in Clinical Trials—EMA/CHMP/QWP/545525/2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-requirements-chemical-pharmaceutical-quality-documentation-concerning-investigational_en.pdf (accessed on 13 April 2022).
- ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology, CPMP/ICH/381/95. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 13 April 2022).
- Sosabowski, J.K.; Mather, S.J. Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat. Protoc. 2006, 1, 972–976. [Google Scholar] [CrossRef] [PubMed]
- The European Pharmacopoeia: Radiopharmaceuticals Preparation; General Monograph 07/2016:0125; European Directorate for the Quality of the Medicines (EDQM): Strasbourg, France, 2020.

| Test | Method | Specification | Unit |
|---|---|---|---|
| [177Lu]LuCl3 in HCl 0.04M pH 1–2 Activity per Vial value decay corrected to ART | n.a. | 90–110 of the activity stated in the label | % |
| Volume delivered | n.a. | 0.4–0.8 mL According to the radioactivity ordered | mL |
| Appearance | Visual test | Clear and colorless solution | n.a. |
| Identity Lu-177 | Gamma Spectrometry | 113 KeV gamma line 208 KeV gamma line | n.a. |
| Identity Chloride | Eu. Phar. | White precipitate visible | n.a. |
| Specific activity value decay corrected to ART | ICP-MS | ≥3000 | GBq/mg |
| Radionuclidic purity Radiochemical purity | Gamma Spectrometry TLC | Yb-175 ≤ 0.01 Sum of impurities ≤ 0.01 ≥99.0 as 177LuCl3 | % % % |
| Chemical purity | ICP-MS | Fe ≤ 0.25 Cu ≤ 0.5 Zn ≤ 0.5 Pb ≤ 0.5 Yb-176 ≤ 0.1 Sum of impurities ≤ 0.5 | µg/GBq |
| Radiolabeling yield | TLC | ≥99.0 | % |
| Sterility | Eu. Phar. | Sterile | n.a. |
| Bacterial endotoxins | Eu. Phar. | ≤20 | EU/mL |
| Components | Function | Amount/Activity |
|---|---|---|
| [177Lu]LuCl3 | Active Pharmaceutical Ingredient (API) | 18,350–31,040 MBq Activity Reference Time (ART) |
| PSMA-I&T | Precursor | 500–800 µg (334–534 nmol) |
| Water for injection | For reconstitution of PSMA-I&T | 0.5–1 mL |
| Gentisic/ascorbic buffer composition: | ||
| Gentisic acid | Radical scavenger | 16.8 mg (109 µmol) |
| Sodium acetate | Buffer solution | 32.4 mg (395 µmol) |
| Sodium hydroxide | pH balance buffer | 9.6 mg (240 µmol) |
| Ascorbic acid | Radical scavenger | 31.2 mg (177 µmol) |
| Ascorbic acid solution in NaCl 0.9% 20 mg/mL | Diluent and radical scavenger | 17–25 ml |
| Parameter | Method | Acceptance Criteria |
|---|---|---|
| [177Lu]Lu-PSMA-I&T activity | Dose calibrator | 17,980–29,790 MBq |
| Radioactive concentration | Dose calibrator | 1057–1192 MBq/mL |
| Volume | 17–25 mL | |
| Appearance | Visual test | Clear and colorless solution |
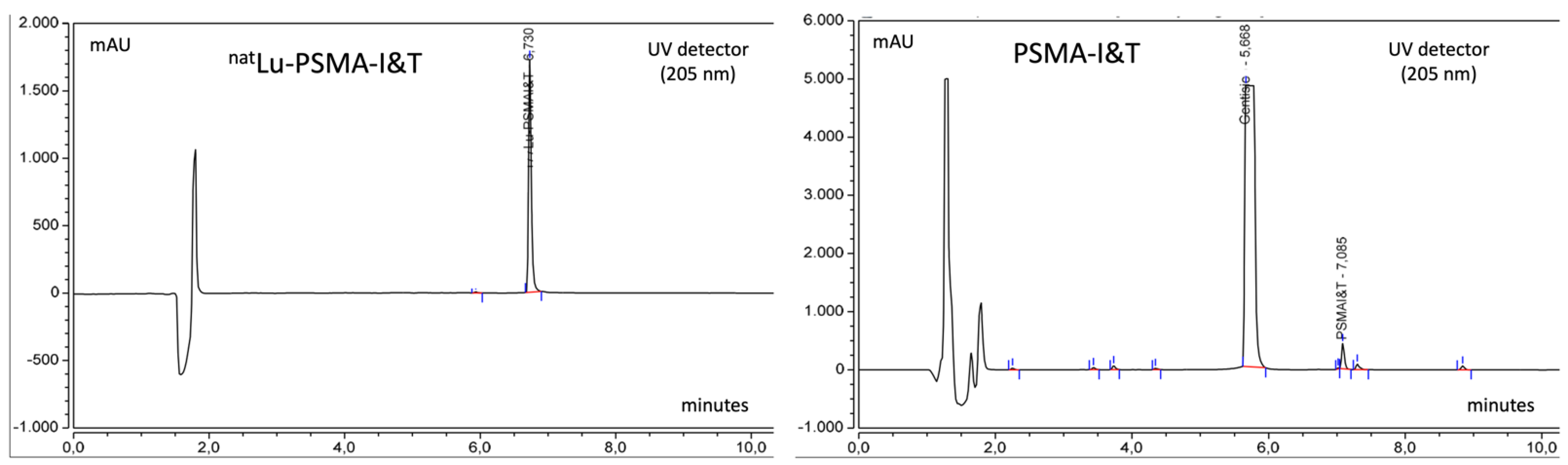
| Identification | HPLC | Rt [177Lu]Lu-PSMA-I&T ± 0.2 min vs. Rt natLu-PSMA-I&T reference standard |
| Radionuclidic identity | Gamma Spectrometry | 113 KeV gamma line 208 KeV gamma line |
| Yb-175 content | Gamma Spectrometry | Yb-175 ≤ 0.01% |
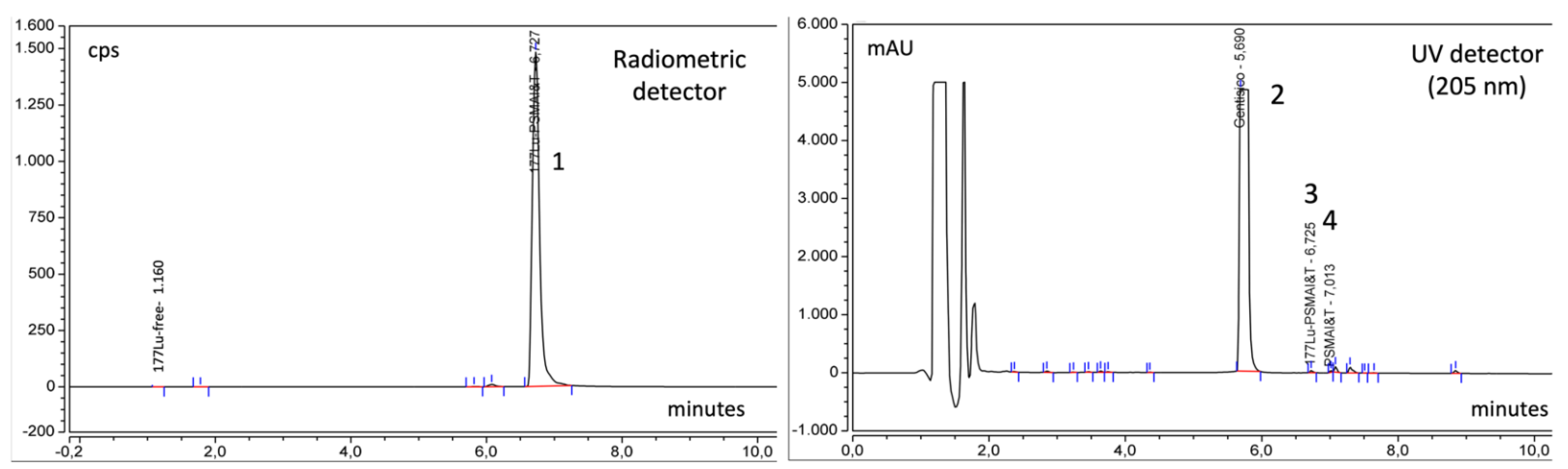
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% |
| pH | pH strips | 4.5–5.5 |
| Filter integrity | Bubble Point Test | ≥50 psi |
| Sterility | Sterility Test (Eur. Ph.) | Sterile |
| Bacterial endotoxins | Eur. Ph. | ≤175 EU/V |
| Chemical Purity UV Detector | ||
|---|---|---|
| Parameters | Acceptance Criteria | Results |
| Specificity | Rs natLuPSMA I&T and PSMA-I&T Rs ≥ 1.5 | Comply |
| Precision | CV% PSMA-I&T ≤ 5% CV% natLuPSMA-I&T ≤ 5% | <4% <3% |
| Linearity | R2 PSMA-I&T ≥ 0.99 R2 natLuPSMA-I&T ≥ 0.99 | ≥0.999 >0.999 |
| LOQ (µg/mL) | Experimental | PSMA-I&T = 6.8 natLuPSMA-I&T = 13.2 |
| LOD (µg/mL) | Experimental | PSMA-I&T = 2.2 natLuPSMA-I&T = 4.3 |
| Range | 80–120% | Comply |
| Accuracy | Average bias < 5% | Comply |
| Radiochemical Purity Radiodetector | ||
| Parameters | Acceptance Criteria | Results |
| Specificity | Difference tR ± 5% RT compared with RT natLuPSMA-I&T | ±4% |
| Precision | CV% ≤ 5% | ≤3.2% |
| Linearity | R2 ≥ 0.99 | ≥0.999 |
| LOQ | n.a. | n.a. |
| LOD | n.a. | n.a. |
| Range | n.a. | n.a. |
| Parameter | Method | Acceptance Criteria | Batch 08/04/2021 | Batch 15/04/2021 | Batch 07/05/2021 |
|---|---|---|---|---|---|
| [177Lu]Lu-PSMA-I&T Activity | Dose Calibrator | 17,980–29,790 MBq | 17,980 MBq | 21,720 MBq | 29,790 MBq |
| Radioactive concentration | Dose Calibrator | 1057–1192 MBq/mL | 1058 MBq/mL | 1086 MBq/mL | 1192MBq/mL |
| Volume | - | 17–25 ml | 17 mL | 20 mL | 25 mL |
| Appearance | Visual test | Clear and Colorless Solution | Complies | Complies | Complies |
| Identification | HPLC | Rt [177Lu]Lu-PSMA-I&T ± 0.2 min vs. Rt natLu-PSMA-I&T reference standard | +0.012 | +0.03 | +0.01 |
| Radionuclidic identity | Gamma Spectrometry | 113 KeV gamma line 208 KeV gamma line | Comply | Comply | Comply |
| Yb-175 content | Gamma Spectrometry | Yb-175 ≤ 0.01% | Comply | Comply | Comply |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0 100% | 0 100% | 0 100% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.02% 99.3% | 0.02% 99.4% | 0.01% 99.4% |
| Chemical purity | HPLC | PSMA-I&T ≤ 0.1 mg/Vmax Sum of impurities ≤ 0.5 mg/Vmax | Complies | Complies | Complies |
| pH | pH Strips | 4.5–5.5 | 5 | 5 | 5 |
| Filter integrity | Bubble Point Test | ≥50 psi | ≥50 psi | ≥50 psi | ≥50 psi |
| Sterility | Sterility Test (Eur. Ph.) | Sterile | Sterile | Sterile | Sterile |
| Bacterial endotoxins | Eur. Ph. | ≤175 EU/V | ≤10 EU/V | ≤10 EU/V | ≤10 EU/V |
| T0 Stability Test | |||||
|---|---|---|---|---|---|
| Parameter | Method | Acceptance Criteria | Batch 08/04/2021 | Batch 15/04/2021 | Batch 07/05/2021 |
| Appearance | Visual test | Clear and Colorless Solution | Complies | Complies | Complies |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0 100% | 0 100% | 0 100% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.02% 99.3% | 0.02% 99.4% | 0.01% 99.4% |
| pH | pH Strips | 4.5–5.5 | 5 | 5 | 5 |
| 24 h stability test | |||||
| Parameter | Method | Acceptance Criteria | Batch 08/04/2021 | Batch 15/04/2021 | Batch 07/05/2021 |
| Appearance | Visual test | Clear and Colorless Solution | Complies | Complies | Complies |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.1% 99.9% | 0.1% 99.9% | 0.1% 99.9% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.01% 98% | 0.02% 98% | 0.04% 97.7% |
| pH | pH Strips | 4.5–5.5 | 5 | 5 | 5 |
| 30 h stability test | |||||
| Parameter | Method | Acceptance Criteria | Batch 08/04/2021 | Batch 15/04/2021 | Batch 07/05/2021 |
| Appearance | Visual Test | Clear and Colorless Solution | Complies | Complies | Complies |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.1% 99.9% | 0.2% 99.8% | 0.1% 99.9% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.04% 97.5% | 0.04% 97.4% | 0.07% 97.3% |
| pH | pH Strips | 4.5–5.5 | 5 | 5 | 5 |
| T0 Stability Test | ||||
|---|---|---|---|---|
| Parameter | Method | Acceptance Criteria | [177Lu]Lu-PSMA-I&T | [177Lu]Lu-PSMA-617 |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.3% 99.7% | 0 100% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.2% 99.1% | 0.02% 99.8% |
| 24 h stability test | ||||
| Parameter | Method | Acceptance Criteria | [177Lu]Lu-PSMA-I&T | [177Lu]Lu-PSMA-617 |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 3.3% 96.7% | 0.1% 99.9% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.2% 94.6% | 0.04% 97.4% |
| 30 h stability test | ||||
| Parameter | Method | Acceptance Criteria | [177Lu]Lu-PSMA-I&T | [177Lu]Lu-PSMA-617 |
| Radiochemical purity | TLC | [177Lu]Lu colloids ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 3.5% 96.5% | 0.2% 99.8% |
| Radiochemical purity | HPLC | [177Lu]Lu ≤ 3% [177Lu]Lu-PSMA-I&T ≥ 97% | 0.2% 93.2% | 0.06% 97.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Iorio, V.; Boschi, S.; Cuni, C.; Monti, M.; Severi, S.; Paganelli, G.; Masini, C. Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials. Molecules 2022, 27, 4143. https://doi.org/10.3390/molecules27134143
Di Iorio V, Boschi S, Cuni C, Monti M, Severi S, Paganelli G, Masini C. Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials. Molecules. 2022; 27(13):4143. https://doi.org/10.3390/molecules27134143
Chicago/Turabian StyleDi Iorio, Valentina, Stefano Boschi, Cristina Cuni, Manuela Monti, Stefano Severi, Giovanni Paganelli, and Carla Masini. 2022. "Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials" Molecules 27, no. 13: 4143. https://doi.org/10.3390/molecules27134143
APA StyleDi Iorio, V., Boschi, S., Cuni, C., Monti, M., Severi, S., Paganelli, G., & Masini, C. (2022). Production and Quality Control of [177Lu]Lu-PSMA-I&T: Development of an Investigational Medicinal Product Dossier for Clinical Trials. Molecules, 27(13), 4143. https://doi.org/10.3390/molecules27134143







