1. Introduction
The steady depletion of fossil fuels and their hazardous byproducts has led to the search for alternative renewable energy sources [
1,
2]. The availability of renewable energy sources varies depending on weather and location, which has drawn the attention of many researchers and has expedited the development of efficient energy storage and energy transport technologies. With its byproduct, water, hydrogen is one of the most promising energy carriers for automotive applications. [
3,
4,
5]. However, hydrogen storage technology is very challenging for many researchers due to its low density with the known possibilities provided by compressed and liquid hydrogen [
6] for several applications. However, when it comes to the use of hydrogen in our daily lives, several issues come up. To address the key issues such as low density and very low temperature of liquid hydrogen, i.e., 20.2 K [
7,
8], two possible methods are high-pressure tanks and cryogenics, but they are difficult to implement for daily life applications [
9,
10].
Light element hydrides (LiH, MgH
2, LiNH
2, NaAlH
4, etc.) are suitable candidates for hydrogen storage in solid form due to their increased gravimetric and volumetric hydrogen densities at normal conditions [
11,
12,
13,
14,
15]. Several studies of hydride and their combinations for hydrogen storage have been reported [
16,
17]. Additionally, the combination of solid hydrides (LiH and LiNH
2), known as an amide-imide (M-N-H) hydrogen system, is also extensively studied [
18,
19]. Chen et al. published the first report on the LiNH
2 system, well-known for storing hydrogen up to 10.4 wt% via the following conversion reactions [
20]:
Kojima et al. found the desorption enthalpy change as 65.6 kJ mol
−1 [
21] for the same reaction, while Ichikawa et al. reported the reaction through the evolution of ammonia from LiNH
2, i.e.,
Hydrogen can be desorbed as per these reactions below the temperature of 300 °C. Several catalysts and additives have been explored to improve the reaction kinetics. In this direction, potassium and sodium hydride has attained significant attention due to their extraordinary performance. The KH addition reduced the hydrogen desorption temperature of the Mg(NH
2)-LiH system from 186 °C to 107 °C, according to Wang and coworkers. The diffusion of potassium into amide and imide, which, when paired with nitrogen, weakens the N-H and Li-N bonds and so promotes dehydrogenation, has been proposed as the basis for this exceptional performance [
22]. Similarly, Teng et al. reported the improved hydrogen desorption kinetics of the LiH-NH
3 system by adding a small amount of KH. The improved reactivity of KH with NH
3 (emitted from Mg(NH
2)
2), resulting in KNH
2 and H
2, was suggested as a probable reason for the improvement. By solid-solid interaction, this in situ produced KNH
2 reacts with LiH and forms LiNH
2 and KH as a reaction product [
23].
The LiNH
2-KH composite system was explored in our recent work, and the ammonolysis rate was greatly improved. The reaction product was discovered to be a double-cation amide phase LiK(NH
2)
2 [
24,
25]. Because of the eutectic melting phenomena, the reaction temperature was determined to be lower than the individual melting points of LiNH
2 and KH. Basically, eutectic melting is the lowering of the melting temperature of a mixture of two materials as compared to their respective melting temperatures without any change in their phases. This destabilization of the associated species allows low-temperature hydrogen release and has sparked much interest in hydrogen storage materials, particularly complicated hydrides, melting at lower temperatures. Low-melting-point hydrogen storage materials act like ionic liquids and enable quick vehicle refilling, like present fossil-fuel technologies. Several studies on the eutectic melting of metal borohydrides have been published [
26,
27]. After the discovery of the eutectic phenomenon for the LiNH
2-KH system, its detailed investigation has become imperative. In the present study, along with the addition to KH, NaH additive was also considered to visualize and understand the eutectic phenomenon in amide-hydride systems. The thermal studies were performed using the differential scanning calorimetry (DSC). XRD was used to validate the presence of individual phases and their intactness, while the SEM technique was used to investigate the unique characteristic of eutectic melting through morphological characterization. Using DSC thermograms, a complete pseudo-binary phase diagram was produced for a number of compositions with varied atomic ratios.
2. Results and Discussion
DSC and mass spectroscopy were used on these samples to determine the thermal characteristics of LiNH
2-MH complexes. As shown in
Figure 1a, DSC measurements were carried out in a 0.1 MPa Ar environment up to 400 °C at a scan rate of 5 °C/min. In previous research, we looked into the thermal decomposition of the LiNH
2 -NaH (1:1 ratio) system [
28]. This system does not show any sharp/significant peak up to 200 °C, but only a broad exothermic hump was observed, which was suggested due to the ionic mobility of LiNH
2 and NaH, resulting in the formation of Li
3Na (NH
2)
4 [
29]. The above report also clearly stated further disintegration.
In a similar way, the thermal decomposition of the LiNH
2-KH (1:1 ratio) system has been analyzed in this study. Two endothermic peaks appeared at 193 °C and 347 °C, along with a minor exothermic shoulder peak at 149 °C. It is noteworthy here that only H
2 evolution occurred for the entire temperature range, as evident from the corresponding MS signals (
Figure 1b; solid lines). Only a small amount of NH
3 (
Figure 1b; Dashed lines) was detected at higher temperatures (>350 °C). This exothermic peak at 149 °C can be speculated as to the formation of a double-cation amide phase, comparable to the Li
3Na(NH
2)
4 in the other system. It is noteworthy here that no melting was observed during the thermal heating of both systems under the Ar atmosphere, as evident from the absence of any sharp endothermic peaks in the studied range. It must be due to the transformation of initial species to some complex amides such as Li
3Na(NH
2)
4 and Li
3K(NH
2)
4 during milling/heating at very low temperatures.
XRD investigations were performed after this temperature, and the results are shown in
Figure 2a. The XRD analysis indicates the presence of Li
3K(NH
2)
4 in addition to the initial phases, i.e., LiNH
2 and KH for the sample heated up to 150 °C. The endothermic peaks at 193 °C and 347 °C are very similar to the reaction of LiNH
2 and NaH. Heating up to 250 °C transformed the mixture of Li
3K(NH
2)
4, LiNH
2, and KH into K
2Li(NH
2)
3 and KH, which is evident from the XRD profile (
Figure 2a). Further heating to higher temperatures leads to the decomposition of these amides and the evolution of H
2. It also shows the final reaction product to be the potassium metal only (top panel of
Figure 2a).
FTIR experiments were conducted at various stages of heating to better explain this phenomenon and establish the presence and transition of amide-imide.
Figure 2b shows a summary of the findings. The presence of LiNH
2 is confirmed by two distinctive peaks in the FTIR spectra of a milled sample at 3312 and 3259 cm
−1. Furthermore, a distortion in the peak at 3259 cm
−1 suggests a minor interaction between LiNH
2 and KH, resulting in the formation of a small percentage of double-cation amide, which was too small to be seen in the XRD profile. Heating the sample up to 150 °C transformed it into double-cation amide along with the starting materials. This is supported by the FTIR spectra, which show an additional set of peaks. Since the new peaks are shifted toward the lower-frequency side (red shifted as compared to the peak of LiNH
2), these can be considered as K-substituted LiNH
2 structure, i.e., Li
3K(NH
2)
4. A clear peak at 3298 cm
−1 and overlapped peak at 3253 cm
−1 (
Figure 2b) are in suitable agreement with the observations of Dong et al. [
29], where they reported Li
3K(NH
2)
4 as an important intermediate compound.
Further heating to a higher temperature, i.e., 250 °C, increases the reactivity between KH, LiNH2, and previously formed Li3K(NH2)4, which turns them into another double-cation phase K2Li(NH2)3 with higher K content. This can again be seen in the FTIR spectra, where a red shift in the peaks is observed as compared to that of the Li3K(NH2)4, and the new peaks are developed at 3286 and 3227 cm−1. Additionally, a broad characteristic peak around 3160 cm−1 corresponds to the existence of Li2NH. However, the presence of Li2NH could not be confirmed through XRD, indicating the presence of amorphous characteristics. Further heating up to 350 °C causes the disappearance of the peaks in the FTIR spectra, suggesting the decomposition of the amide-imide phase into a metallic state, also evidenced in the XRD profile.
The reaction atmosphere has a significant impact on the thermal behavior of hydrogen storage materials [
30,
31]. The existence of gas species in the reaction field can have a considerable impact on the reaction mechanism of such a complex system. It is due to the fact that the presence of Ar works as a vacuum condition where the thermodynamics can not be changed; however, the presence of H
2 in the reaction field creates a back pressure of hydrogen toward the reaction and affect the thermodynamic significantly due to which the reaction pathways can be altered drastically. Generally, it is seen that the presence of hydrogen in the reaction field shifts the decomposition temperature to the higher side [
31]. In contrast to the decomposition under the Ar atmosphere reported in this work, it was observed in our previous studies that the LiNH
2-KH system did not undergo decomposition even under a small H
2 pressure of 0.5 MPa, and this system revealed the possibility of eutectic melting. To understand the mechanism and its relevance to other systems, detailed investigations were performed on LiNH
2-MH (M = Na, K) systems under 0.5 MPa H
2.
Figure 3 shows the DSC thermograms of both samples during heating and cooling cycles.
For the LiNH2-KH sample, a reversible peak is obtained at around 240 °C, whereas the melting temperatures of individual LiNH2 and KH are 390 °C and 400 °C. Furthermore, it is important to note that the temperatures of these melting and solidification peaks did not change with varying hydrogen pressures (not shown here). Due to the existence of relatively identical peak positions, any disintegration during heating could be ruled out (the origin of the cooling peak coincides with the origin of the heating peak). The LiNH2-NaH sample showed a similar set of peaks but at a higher temperature, 321 °C, which is lower than the individual melting temperatures of LiNH2 and NaH. These DSC results point to eutectic melting as a possibility. Furthermore, the physical appearance of both samples after the above-mentioned DSC experiments indicated the sign of melting.
XRD was performed on both the samples after melting to obtain scientific evidence of this low-temperature eutectic melting. The XRD and FTIR profiles of both the samples before and after melting (
Figure 4a,b) can be used to establish the eutectic melting phenomenon as they reflect the intact nature of participating components. They suggest the improvement in crystallinity after melting without changing the existing phases or introducing the new phases. Additionally, the FTIR spectra of both the samples (
Figure 4c,d) also do not show any new peak after melting. In fact, the observed distortion in the low-frequency peak for both as prepared samples disappeared after melting. This suggests the dissolution of a small fraction of double-cation amide under the hydrogen atmosphere during melting. These findings support the hypothesis of eutectic melting in these systems.
According to the basic definition of eutectic melting, atom diffusion into the other phase element or preferential segregation of the two-phase elements can result in the production of unique microstructures. The samples were subjected to scanning electron microscopy to validate this.
Figure 5 shows the SEM results, which demonstrate the presence of distinctive lamellar microstructures in both samples. This indicates that the growth is coupled, implying that the phases of LiNH
2, NaH, and KH form a liquid at the same interface, whether it is isothermal or planar. Rutter and coworkers reported this phenomenon occurring at the interface of all the phases [
32]. The large-sized grains observed in these samples can be attributed to the perfect lamellar structure [
30,
31,
32]. Efforts have been made by researchers to find out the different microstructures of eutectics. According to Chadwick et al., the growth of lamellar eutectics alloys is caused by the simultaneous edgewise growth of phases of both materials in the presence of constant heat flow [
33]. The confirmation of eutectic melting through all of the foregoing evidence prompted the investigation of the exact eutectic composition of the LiNH
2-NaH and LiNH
2-KH systems, which have the lowest eutectic temperature that might be used in future research.
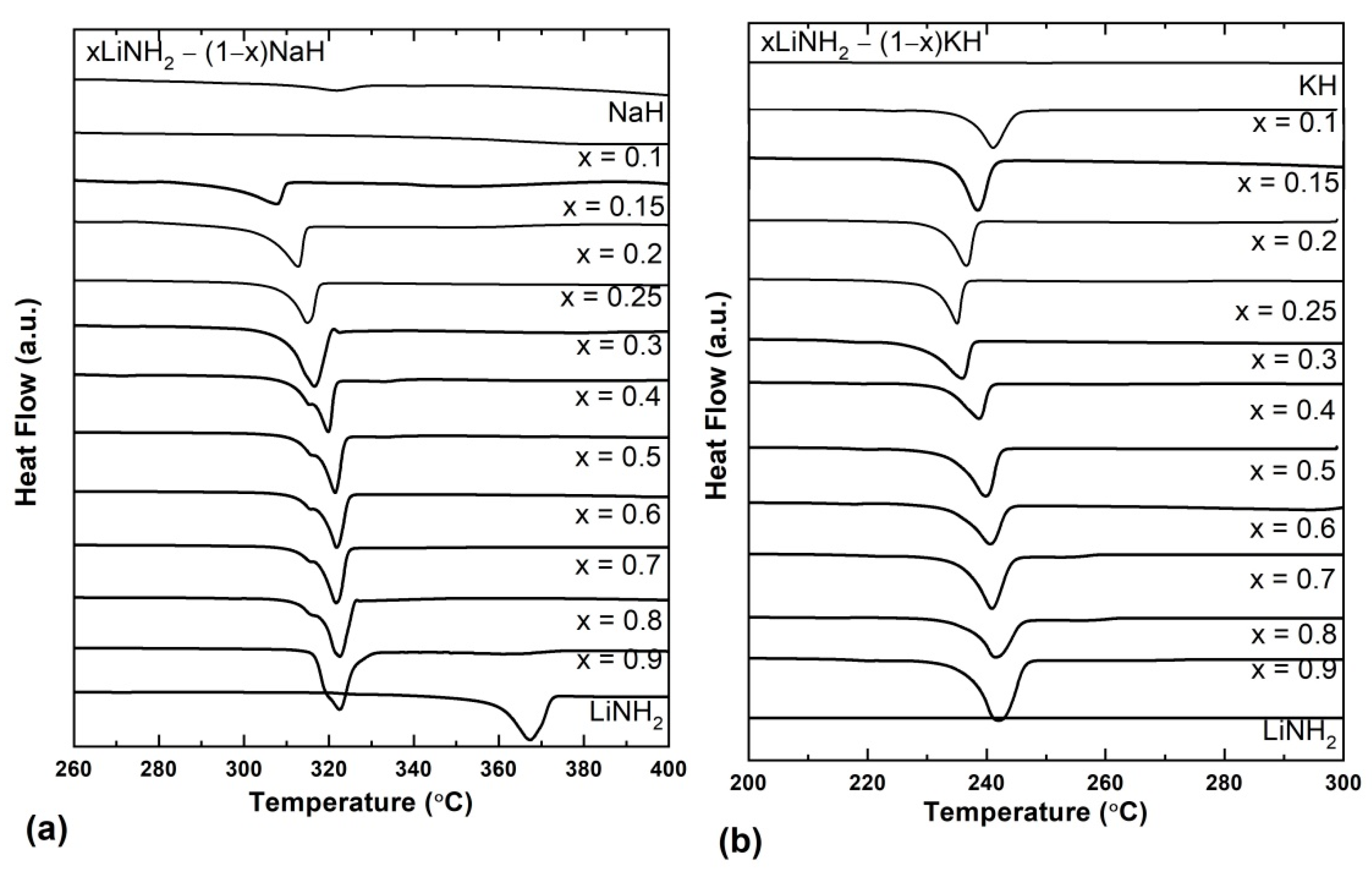
Detailed investigation of the composition and temperature of eutectic melting was carried out using DSC thermograms taking different compositions of xLiNH
2-(1−x)NaH and xLiNH
2-(1−x)KH systems where x = 0, 0.1, 0.15, 0.2, 0.25, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1 for both systems. These DSC curves under 0.5 MPa H
2 are shown in
Figure 6. Let’s first discuss the xLiNH
2-(1−x) NaH compositions shown in
Figure 6a.
The melting temperature of individual LiNH
2 and NaH is 368 °C and >800 °C, respectively. The DSC thermogram shows the absence of any melting peak for NaH in the temperature range of 200–300 °C, which remained the same for the composition 0.1LiNH
2-0.9NaH. When the LiNH
2 content was further increased, i.e., 0.15LiNH
2-0.85NaH, an endothermic peak corresponding to the melting appeared at 307 °C and increased continuously with the increase in the content of LiNH
2. Similar DSC experiments were performed for the xLiNH
2-(1−x) KH compositions, and the results are depicted in
Figure 6b.
The individual melting point of LiNH
2 and KH is 368 °C and 400 °C, respectively. It is observed that the bare samples of LiNH
2 and KH show no melting peaks in the given temperature range. For the composition 0.1LiNH
2 –0.9 KH, an endothermic melting peak was observed at 240 °C, and on further increasing the LiNH
2 content, the melting temperature is decreased. The lowest melting temperature was observed at 235 °C for the 0.25LiNH
2 –0.75 KH composition, which is much lower than that of the individual melting points of the LiNH
2 and KH. XRD of all the studied xLiNH
2-(1−x)NaH and xLiNH
2-(1−x)KH samples suggest no change in the initial phases except for the formation of better crystallinity after melting (
Figures S1 and S2). A similar observation was received from FTIR spectra. The morphological investigation of the series of these samples (
Figures S3 and S4) clearly visualizes the melting phenomena, thus ruling out any possibility of desorption corresponding to DSC endothermic peaks (
Figure 6).
The melting temperatures of each individual composition were determined using the above DSC data, and a phase diagram of these systems was prepared. The pseudo-binary phase diagram for xLiNH
2-(1−x) NaH is shown in
Figure 7a, which clearly shows a reduction in the melting temperature of NaH from more than 800 °C to 320 °C when a small amount of LiNH
2 is mixed in NaH. The melting temperature of NaH is 800 °C, with the addition of LiNH
2, the temperature is reduced systematically while in the composition 0.15LiNH
2 –0.85NaH the eutectic temperature was found to be 307 °C. The confirmation of eutectic composition can be done on the basis of the similar crystal structure and observed morphology. Similarly, for the composition xLiNH
2-(1−x) KH,
Figure 7b suggested the eutectic composition is 0.25LiNH
2 –0.75KH with the eutectic temperature of 242 °C.