2-Phosphonobutane-1,2,4,-Tricarboxylic Acid (PBTC): pH-Dependent Behavior Studied by Means of Multinuclear NMR Spectroscopy
Abstract
:1. Introduction
| pKa | Method | Conditions 1 | Reference | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| PBTC | |||||||
| 1.08 ± 0.42 | 3.99 ± 0.21 | 4.44 ± 0.10 | 4.99 ± 0.14 | 8.59 ± 0.50 | estimation by calculation | water, 20 °C | [13] |
| 1.8 | 4.0 | 4.9 | 6.8 | 10.8 | not specified | not specified | [12] 2 |
| 2.20 ± 0.03 | 4.03 ± 0.01 | 4.97 ± 0.01 | 6.68 ± 0.01 | 10.19 ± 0.01 | potentiometric titration | 0.1 M NaCl, (20 ± 2) °C | [14] |
| 3.865 | 4.145 | 5.17 | 6.83 | 9.038 | potentiometric titration | 0.5 M NaNO3, 25 °C | [8] |
| 0.90 ± 0.02 | 3.92 ± 0.02 | 4.76 ± 0.03 | 6.13 ± 0.03 | 9.79 ± 0.02 | NMR-based titration | 0.5 m NaCl, (25 ± 1) °C | this work |
| (R,S)/(S,R) PPTC 3 | |||||||
| 1.12 ± 0.02 | 3.75 ± 0.01 | 4.78 ± 0.01 | 6.63 ± 0.01 | 9.13 ± 0.01 | NMR-based titration | not specified | [20] |
| (R,R)/(S,S) PPTC 3 | |||||||
| 1.02 ± 0.07 | 3.37 ± 0.03 | 4.87 ± 0.03 | 6.30 ± 0.02 | 9.19 ± 0.02 | NMR-based titration | not specified | [20] |
2. Results
2.1. NMR Signal Assignment
2.2. pKa Determination from NMR pH-Titration Series
2.3. DFT-Calculated Deprotonation Sequence
2.4. pH-Dependent Scalar Spin–Spin Coupling Constants
3. Discussion
4. Materials and Methods
4.1. Starting Material
4.2. Sample Preparation
4.3. NMR Spectroscopy
4.4. DFT Calculation
4.5. Data Processing Software
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Valsami-Jones, E. Phosphorus in Environmental Technology: Principles and Applications; IWA Publishing: London, UK, 2005. [Google Scholar]
- Demadis, K.D.; Lykoudis, P. Chemistry of Organophosphonate Scale Growth lnhibitors: 3. Physicochemical Aspects of 2-Phosphonobutane-1,2,4-Tricarboxylate (PBTC) And Its Effect on CaCO3 Crystal Growth. Bioinorg. Chem. Appl. 2005, 3, 546865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demadis, K.D.; Lykoudis, P.; Raptis, R.G.; Mezei, G. Phosphonopolycarboxylates as Chemical Additives for Calcite Scale Dissolution and Metallic Corrosion Inhibition Based on a Calcium-Phosphonotricarboxylate Organic−Inorganic Hybrid. Cryst. Growth Des. 2006, 6, 1064–1067. [Google Scholar] [CrossRef]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Rott, E.; Happel, O.; Armbruster, D.; Minke, R. Behavior of PBTC, HEDP, and Aminophosphonates in the Process of Wastewater Treatment. Water 2020, 12, 53. [Google Scholar] [CrossRef] [Green Version]
- Clearfield, A.; Demadis, K.D. (Eds.) Metal Phosphonate Chemistry: From Synthesis to Applications; Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar]
- Huang, Y.; Zheng, H.; Li, H.; Zhao, C.; Zhao, R.; Li, S. Highly selective uranium adsorption on 2-phosphonobutane-1,2,4-tricarboxylic acid-decorated chitosan-coated magnetic silica nanoparticles. Chem. Eng. J. 2020, 388, 124349. [Google Scholar] [CrossRef]
- Salvadó, V.; Escoda, M.L.S.; de la Torre, F. A study of the complex formation between trivalent ions (Al3+, Fe3+) and 2-phosphonobutane-1,2,4-tricarboxylic acid and their industrial applications. Polyhedron 1999, 18, 3275–3280. [Google Scholar] [CrossRef]
- Spinthaki, A.; Matheis, J.; Hater, W.; Demadis, K.D. Antiscalant-Driven Inhibition and Stabilization of “Magnesium Silicate” under Geothermal Stresses: The Role of Magnesium–Phosphonate Coordination Chemistry. Energy Fuels 2018, 32, 11749–11760. [Google Scholar] [CrossRef]
- Armbruster, D.; Müller, U.; Happel, O. Characterization of phosphonate-based antiscalants used in drinking water treatment plants by anion-exchange chromatography coupled to electrospray ionization time-of-flight mass spectrometry and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2019, 1601, 189–204. [Google Scholar] [CrossRef]
- Rickert, J.; Thielen, G. Influence of a Long-Term Retarder on the Hydration of Clinker and Cement. In Cement, Concrete, and Aggregates; ASTM International: West Conshohocken, PA, USA, 2004; Volume 26, pp. 1–10. [Google Scholar] [CrossRef] [Green Version]
- Szczygieł, B.; Drela, I. Evaluation of Organic Ligands as Copper Complexing Agents Using the Isotachophoresis Method. Pol. J. Environ. Stud. 2008, 17, 479–483. [Google Scholar]
- European Chemicals Agency (ECHA). 2-Phosphonobutane-1,2,4-Tricarboxylic Acid. Dissociation Constants Estimated by Calculation. European Chemicals Agency (ECHA) Study Report 2009. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/14870/4/22 (accessed on 29 March 2022).
- Kornev, V.I.; Kropacheva, T.N.; Sorokina, U.V. Coordination compounds of oxovanadium(IV) with organophosphonic complexones in aqueous solutions. Russ. J. Inorg. Chem. 2015, 60, 403–408. [Google Scholar] [CrossRef]
- Popov, K.; Rönkkömäki, H.; Lajunen, L.H.J. Guidelines for NMR measurements for determination of high and low pKa values (IUPAC Technical Report). Pure Appl. Chem. 2006, 78, 663–675. [Google Scholar] [CrossRef]
- Szakács, Z.; Hägele, G. Accurate determination of low pK values by 1H NMR titration. Talanta 2004, 62, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Szakács, Z.; Hägele, G.; Tyka, R. 1H/31P NMR pH indicator series to eliminate the glass electrode in NMR spectroscopic pKa determinations. Anal. Chim. Acta 2004, 522, 247–258. [Google Scholar] [CrossRef]
- Szakács, Z.; Kraszni, M.; Noszál, B. Determination of microscopic acid–base parameters from NMR–pH titrations. Anal. Bioanal. Chem. 2004, 378, 1428–1448. [Google Scholar] [CrossRef] [PubMed]
- Hägele, G. Protolysis and Complex Formation of Organophosphorus Compounds—Characterization by NMR-Controlled Titrations. Molecules 2019, 24, 3238. [Google Scholar] [CrossRef] [Green Version]
- Hägele, G. NMR controlled titrations characterizing organophosphorus compounds. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 361–363. [Google Scholar] [CrossRef]
- Hägele, G.; Arendt, C.; Kropp, H.W.; Majer, H.J.; Ollig, J. Protonation and Metal Complex Formation of Phosphorus Containing Acids and Bases. Phosphorus Sulfur Silicon Relat. Elem. 1996, 109, 205–208. [Google Scholar] [CrossRef]
- Lindner, A. 1-Phosphonopropan-1,2,3-Tricarbonsäure—NMR-und Konformationsanalytische Untersuchungen; Heinreich-Heine-Universität Düsseldorf: Düsseldorf, Germany, 2000. [Google Scholar]
- Hagen, R.; Roberts, J.D. Nuclear magnetic resonance spectroscopy. Carbon-13 spectra of aliphatic carboxylic acids and carboxylate anions. J. Am. Chem. Soc. 1969, 91, 4504–4506. [Google Scholar] [CrossRef]
- Kretzschmar, J.; Brendler, E.; Wagler, J.; Schmidt, A.-C. Kinetics and activation parameters of the reaction of organoarsenic(V) compounds with glutathione. J. Hazard. Mater. 2014, 280, 734–740. [Google Scholar] [CrossRef]
- Kretzschmar, J.; Tsushima, S.; Drobot, B.; Steudtner, R.; Schmeide, K.; Stumpf, T. Trimeric uranyl(VI)–citrate forms Na+, Ca2+, and La3+ sandwich complexes in aqueous solution. Chem. Commun. 2020, 56, 13133–13136. [Google Scholar] [CrossRef]
- Demadis, K.D.; Raptis, R.G.; Baran, P. Chemistry of Organophosphonate Scale Growth Inhibitors: 2. Structural Aspects of 2-Phosphonobutane-1,2,4-Tricarboxylic Acid Monohydrate (PBTC.H2O). Bioinorg. Chem. Appl. 2005, 3, 606490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, S.J. Understanding Hydrogen Bonds: Theoretical and Experimental Views; The Royal Society of Chemistry: London, UK, 2021; pp. 368–400. [Google Scholar]
- Lapper, R.D.; Smith, I.C.P. Carbon-13 and proton nuclear magnetic resonance study of the conformations of 2′,3′-cyclic nucleotides. J. Am. Chem. Soc. 1973, 95, 2880–2884. [Google Scholar] [CrossRef]
- Haemers, M.; Ottinger, R.; Reisse, J.; Zimmermann, D. Direct NMR evidence of the axial preference of the substituent on phosphorus in 2-chloro- and 2-methoxy-1,2,3-dioxaphosphorinanes. Tetrahedron Lett. 1971, 12, 461–464. [Google Scholar] [CrossRef]
- Borisenko, A.A.; Sergeyev, N.M.; Nifant’ev, E.Y.; Ustynyuk, Y.A. Carbon-13 nuclear magnetic resonance of 1,3,2-dioxaphosphorinan-2-ones. J. Chem. Soc. Chem. Commun. 1972, 7, 406–407. [Google Scholar] [CrossRef]
- Wetzel, R.B.; Kenyon, G.L. 1-phosphabicyclo[2.2.1]heptane 1-oxide and 1-phosphabicyclo[2.2.2]octane 1-oxide. Syntheses and some properties relative to their monocyclic and acyclic analogs. J. Am. Chem. Soc. 1974, 96, 5189–5198. [Google Scholar] [CrossRef]
- Gray, G.A.; Cremer, S.E. Carbon-13 nuclear magnetic resonance of organophosphorus compounds. III. Phosphorus heterocycles. J. Org. Chem. 1972, 37, 3458–3469. [Google Scholar] [CrossRef]
- McFarlane, W.; Whiffen, D.H. A 13C and 31P magnetic double resonance study of organo-phosphorus compounds. Proc. R. Soc. Ser. A 1968, 306, 185–199. [Google Scholar] [CrossRef]
- Mavel, G.; Green, M.J. Carbon–phosphorus coupling in representative organophosphorus compounds. Chem. Commun. 1968, 13, 742–743. [Google Scholar] [CrossRef]
- Benezra, C. NMR of phosphonates. VI. Variation of vicinal phosphorus-31-carbon-carbon-proton couplings with dihedral angle in phosphonates. J. Am. Chem. Soc. 1973, 95, 6890–6894. [Google Scholar] [CrossRef]
- Jakobsen, H.J.; Bundgaard, T.; Hansen, R.S. Assignments and signs of 13C-31P coupling constants from off-resonance proton decoupled 13C spectra. Mol. Phys. 1972, 23, 197–201. [Google Scholar] [CrossRef]
- Buchanan, G.W.; Benezra, C. Stereochemical dependence of 13C shieldings and 13C–31P couplings in phosphonates of known geometry. Is there a Karplus-type relationship for P–C–C–C coupling? Canad. J. Chem. 1976, 54, 231–237. [Google Scholar] [CrossRef]
- Ghiasi, M.; Oskouie, A.A.; Saeidian, H. Dynamic stereochemistry of Topiramate (anticonvulsant drug) in solution: Theoretical approaches and experimental validation. Carbohydr. Res. 2012, 348, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretzschmar, J.; Tsushima, S.; Lucks, C.; Jäckel, E.; Meyer, R.; Steudtner, R.; Müller, K.; Rossberg, A.; Schmeide, K.; Brendler, V. Dimeric and Trimeric Uranyl(VI)–Citrate Complexes in Aqueous Solution. Inorg. Chem. 2021, 60, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Altmaier, M.; Metz, V.; Neck, V.; Müller, R.; Fanghänel, T. Solid-liquid equilibria of Mg(OH)2(cr) and Mg2(OH)3Cl·4H2O(cr) in the system Mg-Na-H-OH-Cl-H2O at 25 °C. Geochim. Cosmochim. Acta 2003, 67, 3595–3601. [Google Scholar] [CrossRef]
- Glasoe, P.K.; Long, F.A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 1960, 64, 188–190. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Willcott, M.R. MestRe Nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]

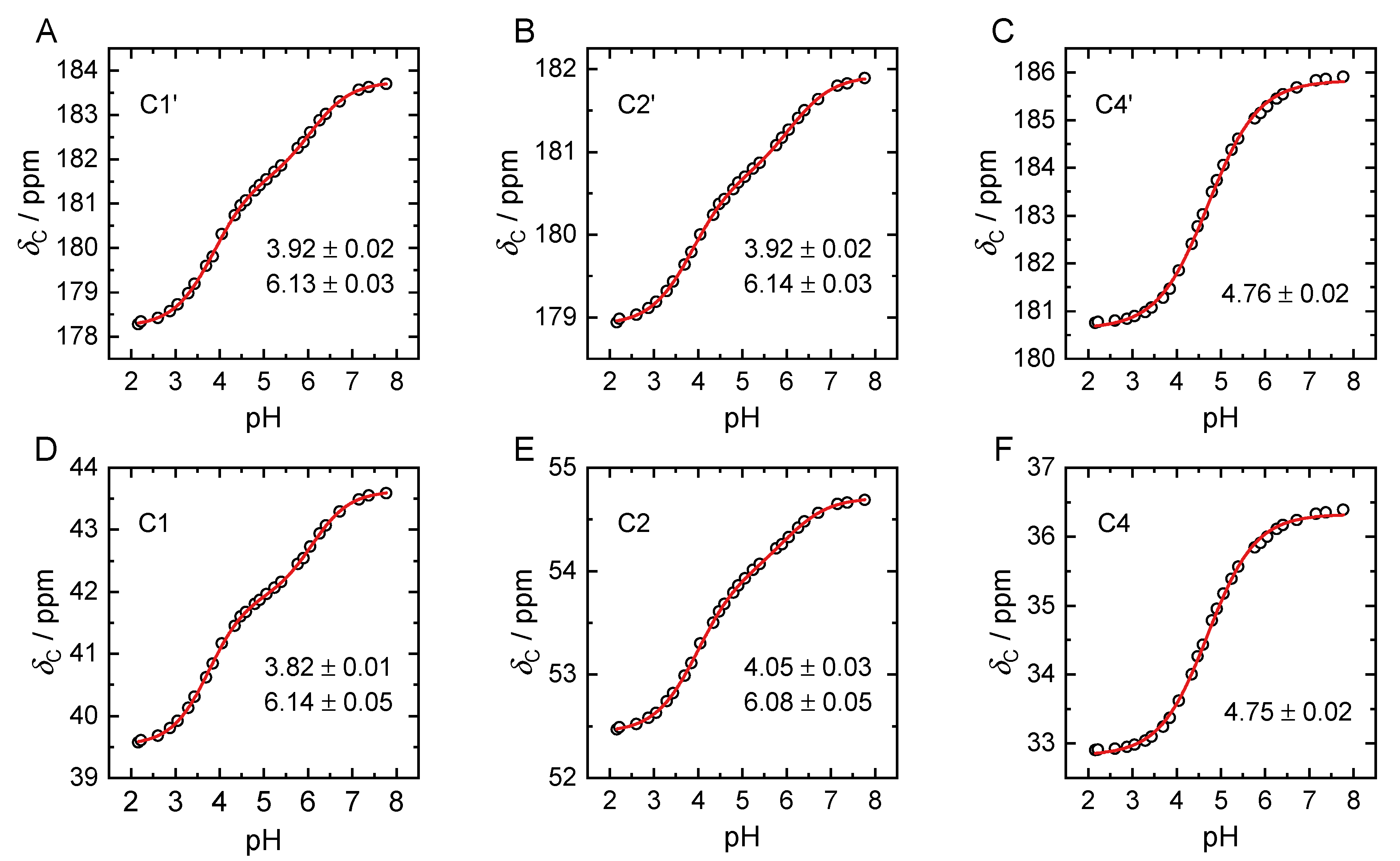
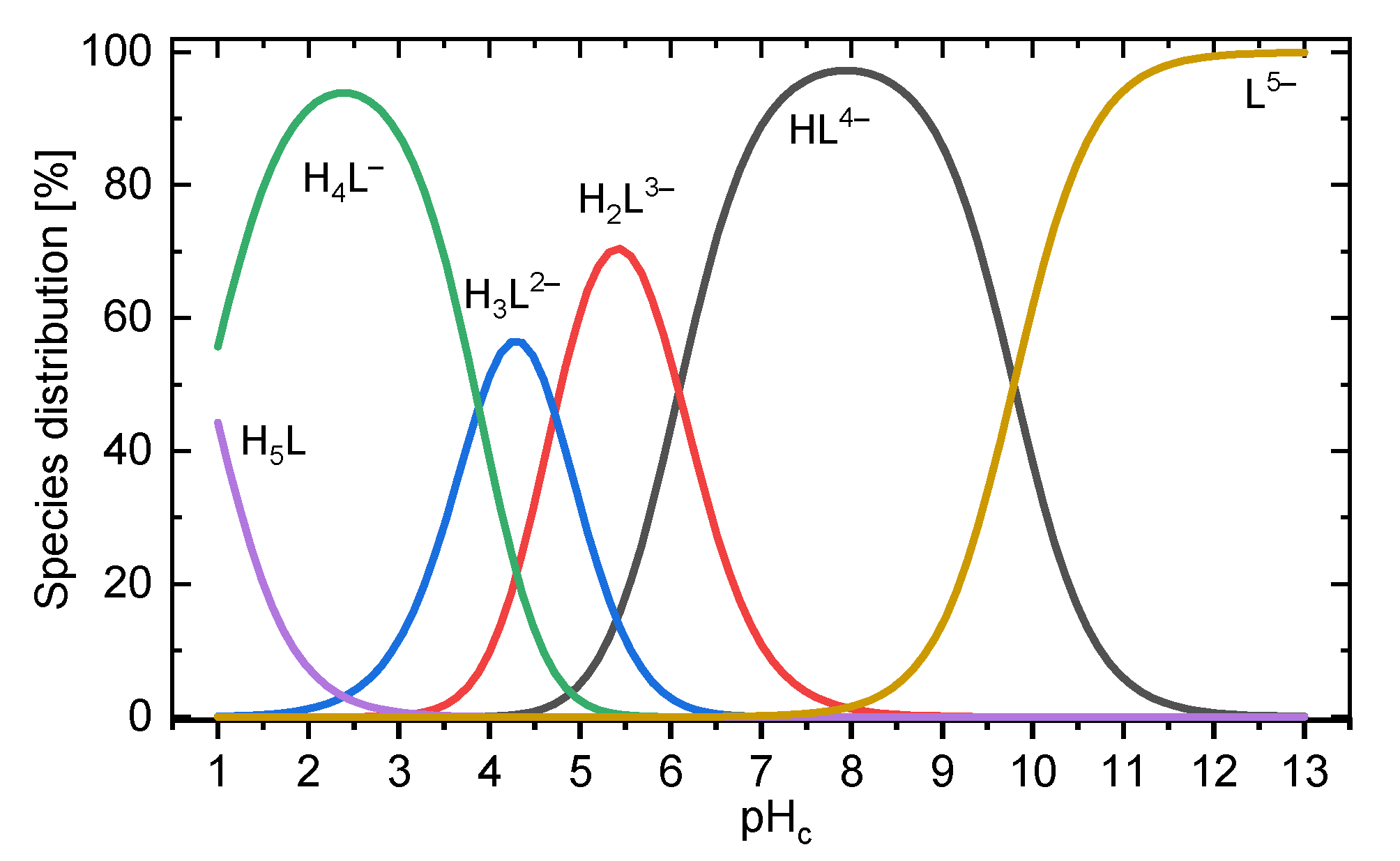

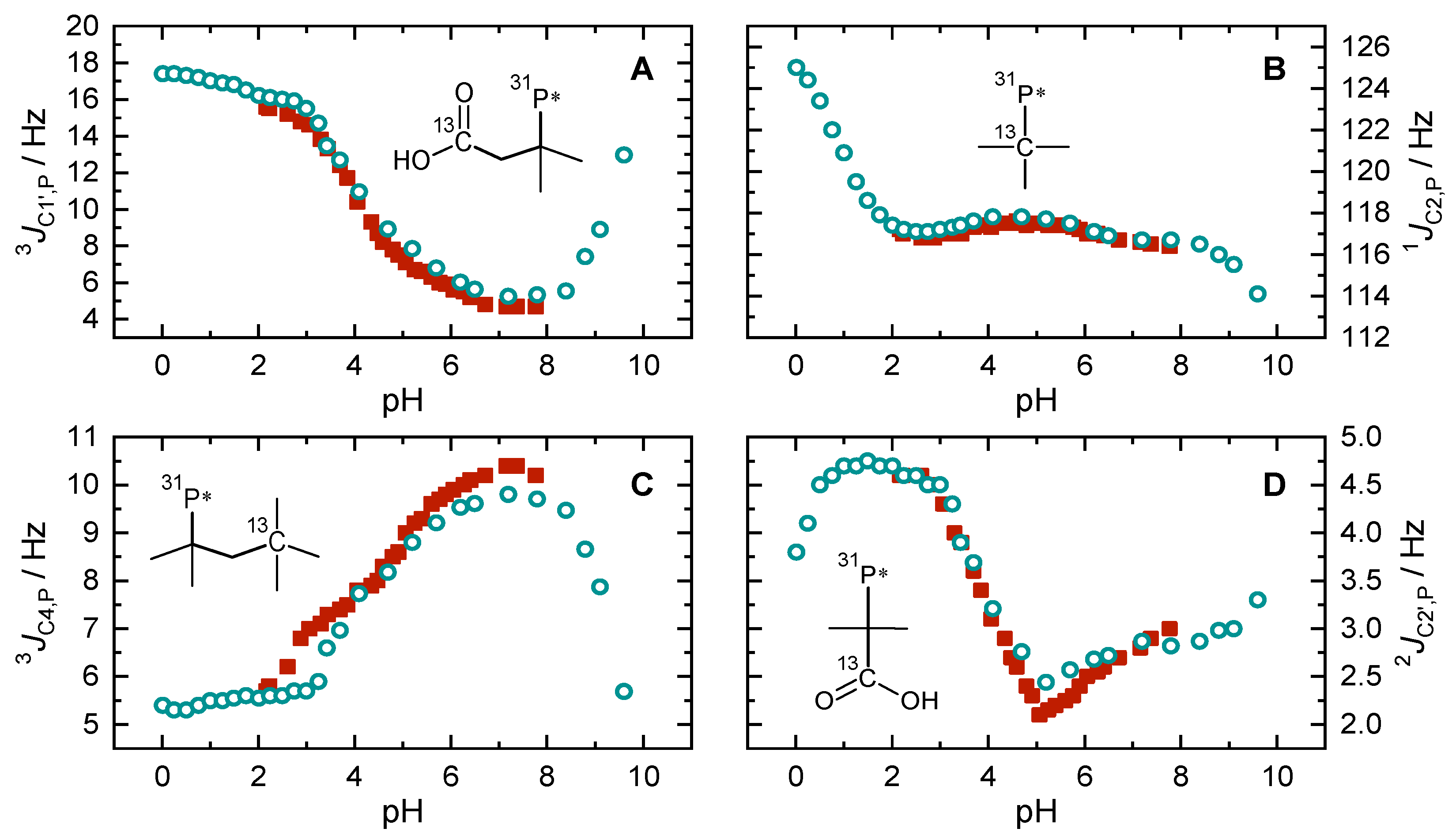

| Species | Carboxylic Groups | |||
|---|---|---|---|---|
| P* 1 | 1′ | 2′ | 4′ | |
| H4L− | ±0.0 | +26.8 | +9.0 | +25.3 |
| H3L2− | - | ±0.0 | +7.6 | +14.8 |
| H2L3− | - | - | +7.7 | ±0.0 |
| HL4− | +8.6 | - | ±0.0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kretzschmar, J.; Wollenberg, A.; Tsushima, S.; Schmeide, K.; Acker, M. 2-Phosphonobutane-1,2,4,-Tricarboxylic Acid (PBTC): pH-Dependent Behavior Studied by Means of Multinuclear NMR Spectroscopy. Molecules 2022, 27, 4067. https://doi.org/10.3390/molecules27134067
Kretzschmar J, Wollenberg A, Tsushima S, Schmeide K, Acker M. 2-Phosphonobutane-1,2,4,-Tricarboxylic Acid (PBTC): pH-Dependent Behavior Studied by Means of Multinuclear NMR Spectroscopy. Molecules. 2022; 27(13):4067. https://doi.org/10.3390/molecules27134067
Chicago/Turabian StyleKretzschmar, Jerome, Anne Wollenberg, Satoru Tsushima, Katja Schmeide, and Margret Acker. 2022. "2-Phosphonobutane-1,2,4,-Tricarboxylic Acid (PBTC): pH-Dependent Behavior Studied by Means of Multinuclear NMR Spectroscopy" Molecules 27, no. 13: 4067. https://doi.org/10.3390/molecules27134067
APA StyleKretzschmar, J., Wollenberg, A., Tsushima, S., Schmeide, K., & Acker, M. (2022). 2-Phosphonobutane-1,2,4,-Tricarboxylic Acid (PBTC): pH-Dependent Behavior Studied by Means of Multinuclear NMR Spectroscopy. Molecules, 27(13), 4067. https://doi.org/10.3390/molecules27134067








