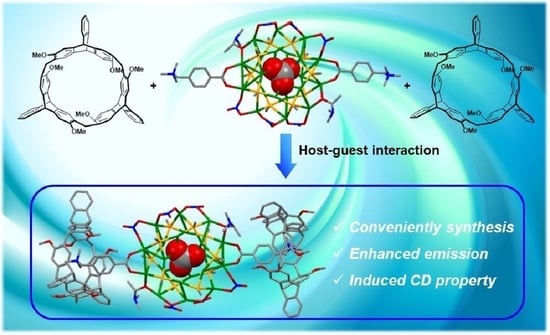
Chiral Nanocluster Complexes Formed by Host−Guest Interaction between Enantiomeric 2,6-Helic[6]arenes and Silver Cluster Ag20: Emission Enhancement and Chirality Transfer
Abstract
:1. Introduction
2. Results
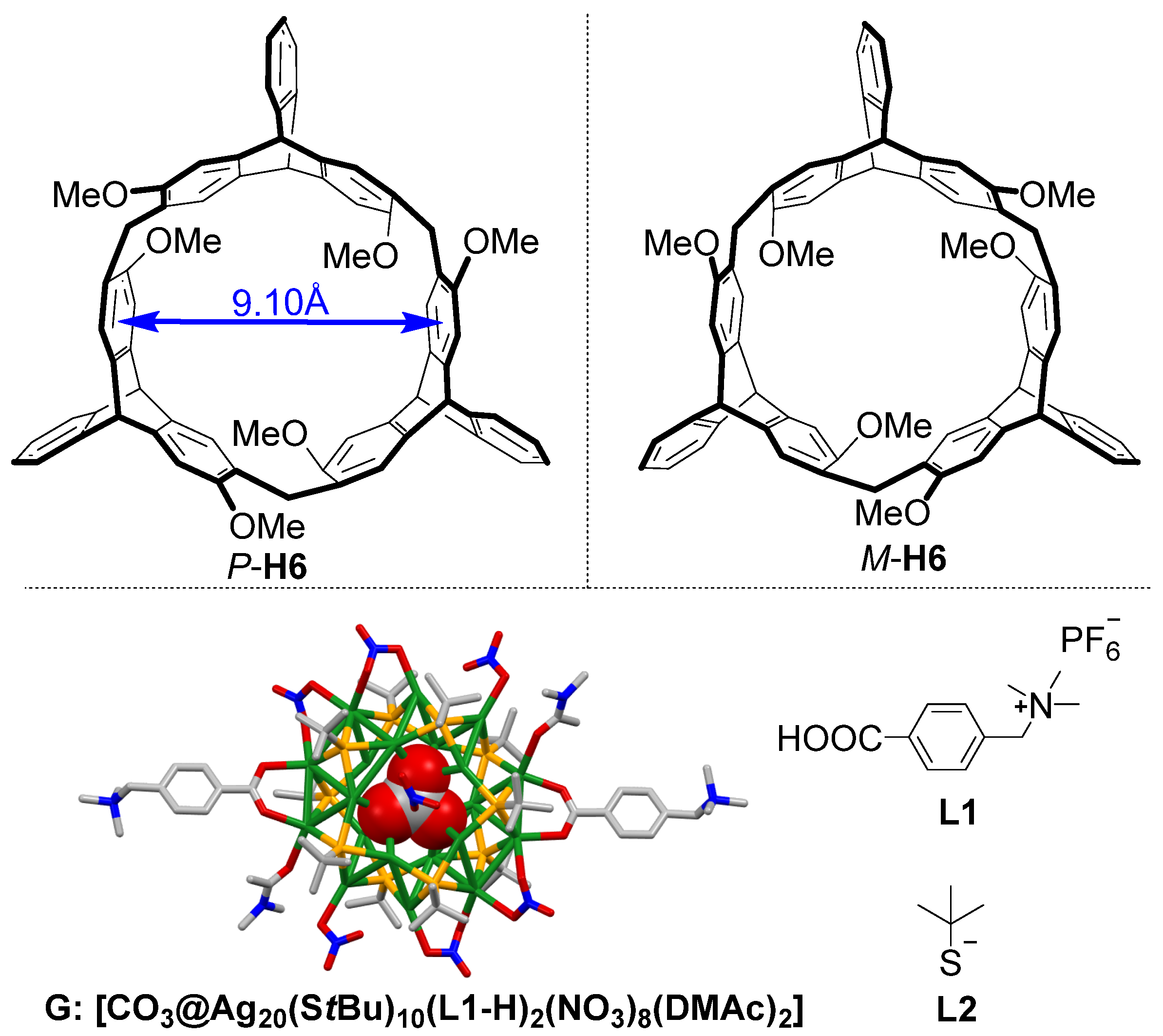
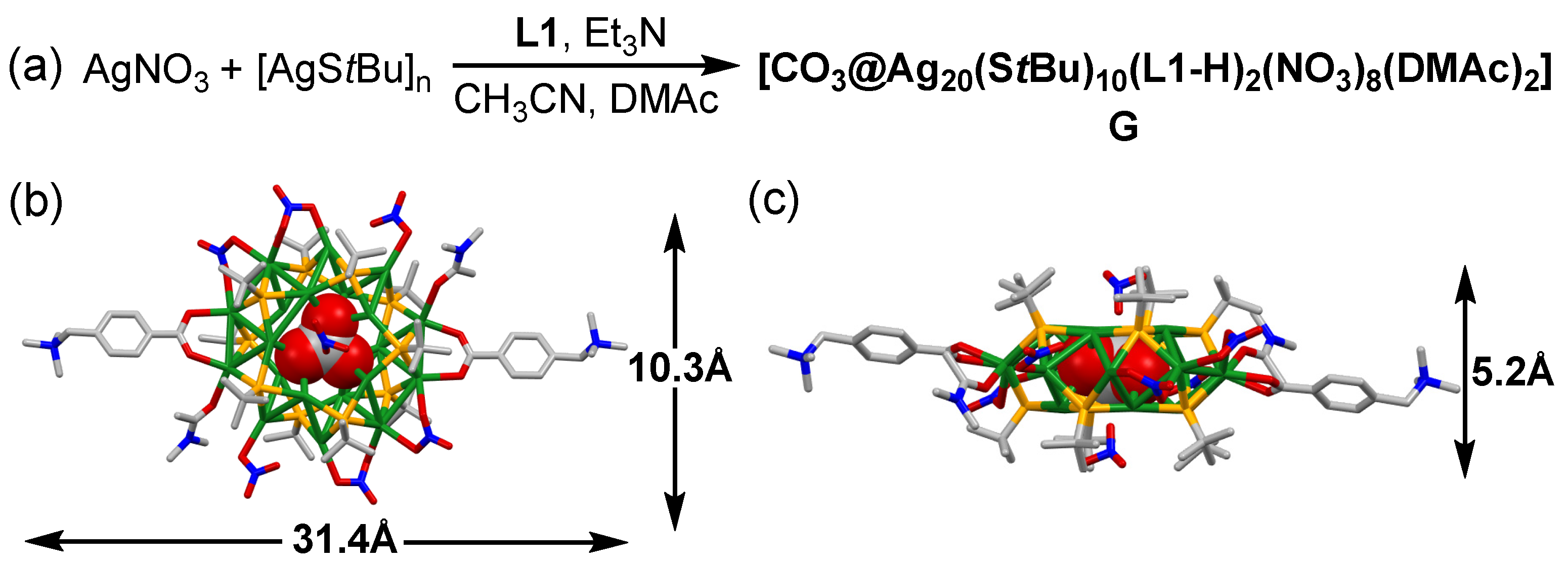
2.1. Synthesis and Structures of the Silver Cluster
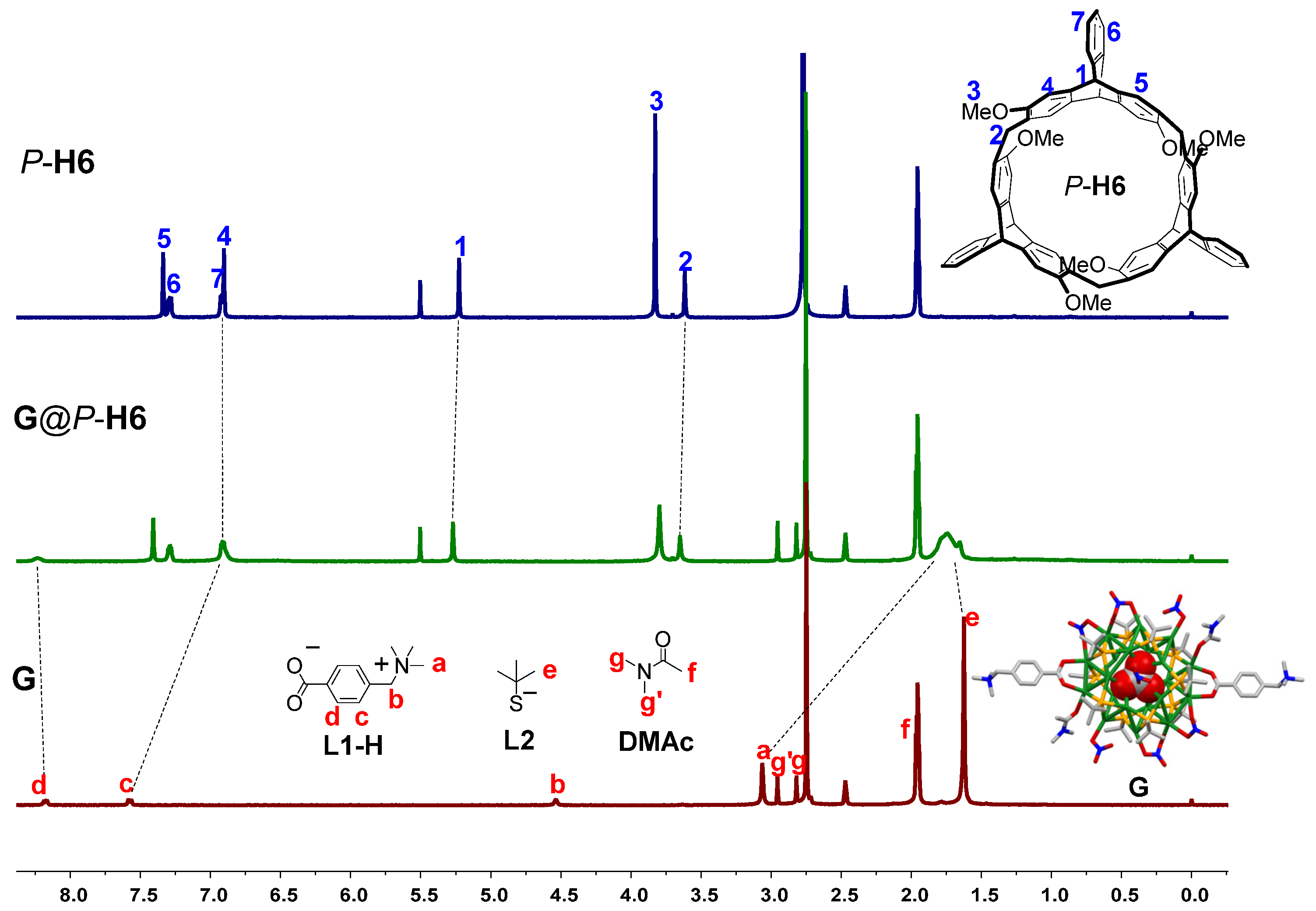
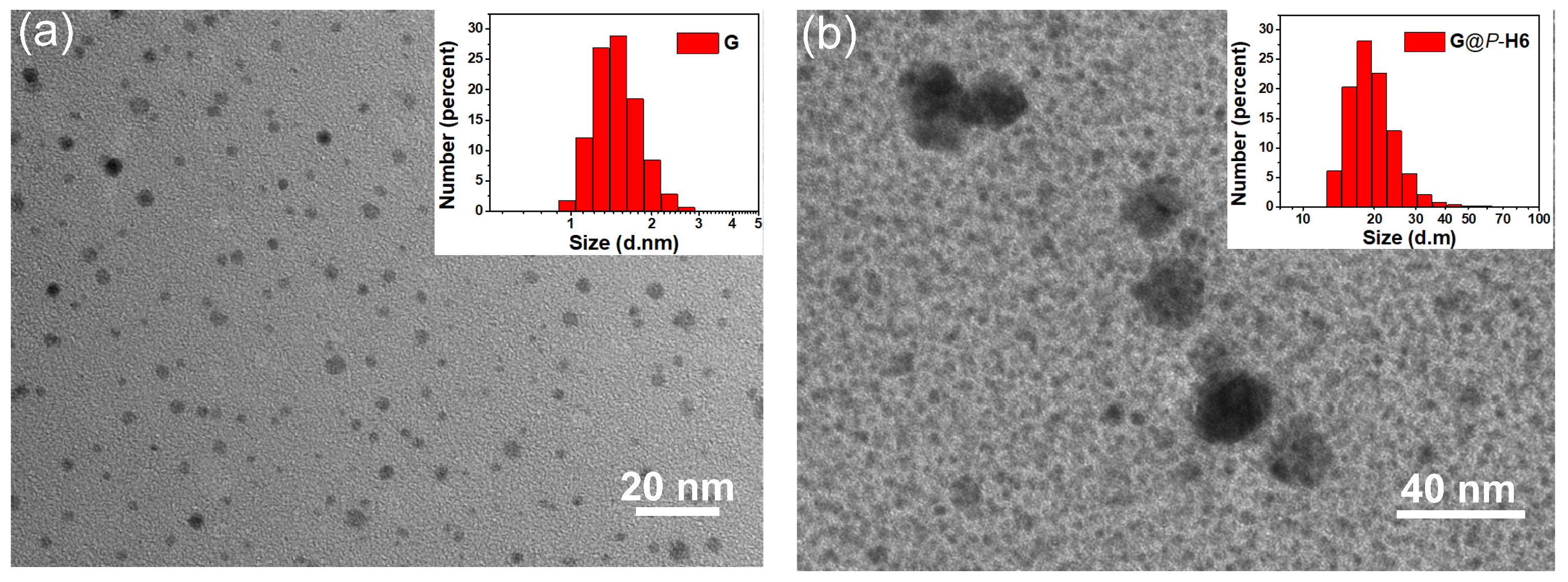
2.2. Study on the Formation of the Nanocluster Complexes
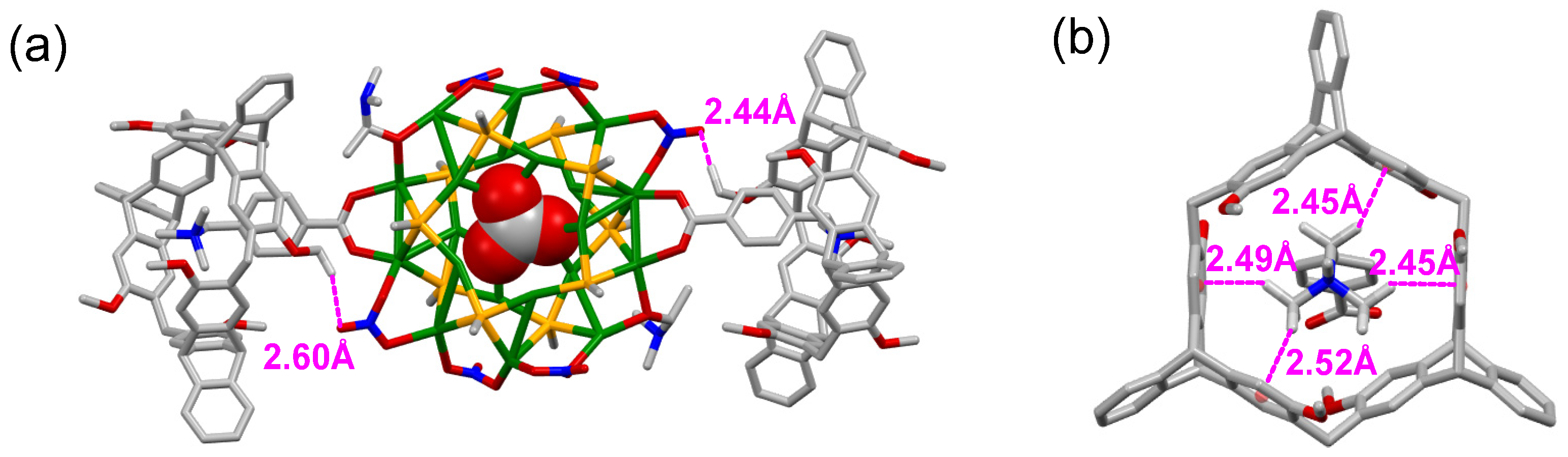
2.3. DFT Calculation of the Nanocluster Complexes
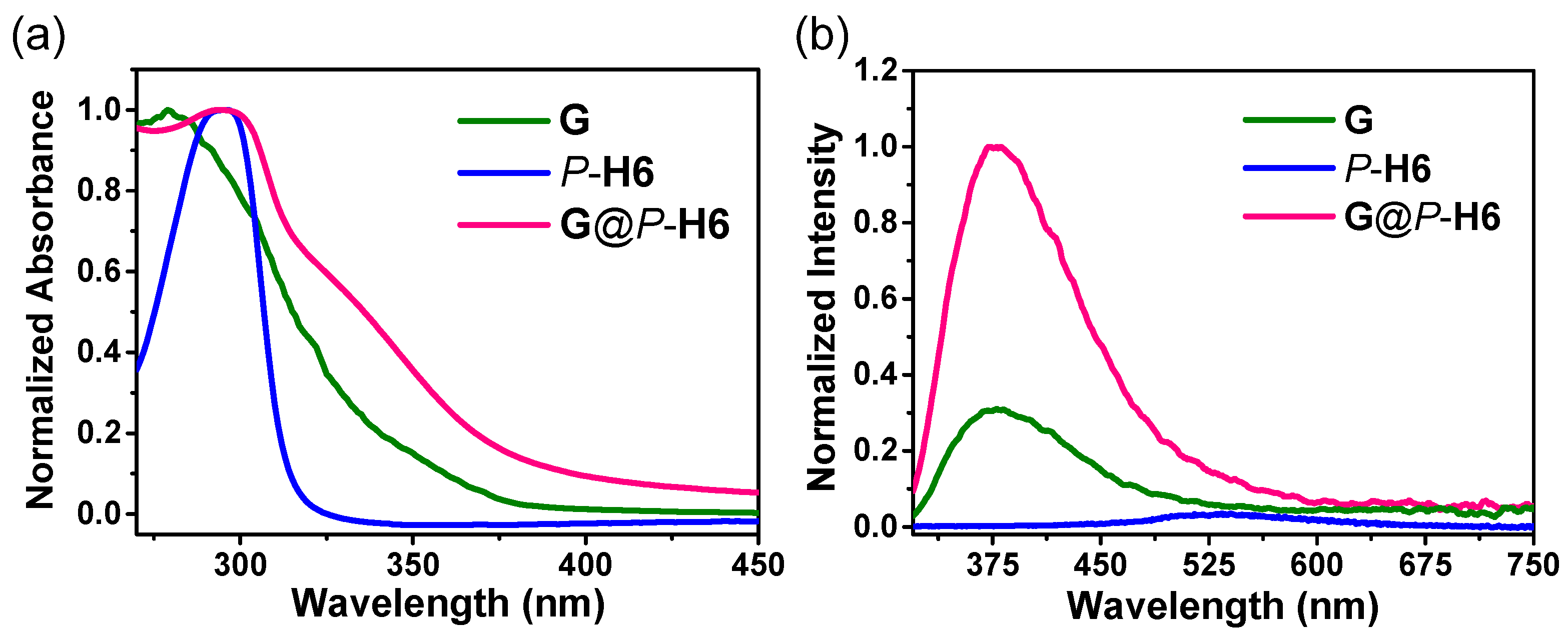
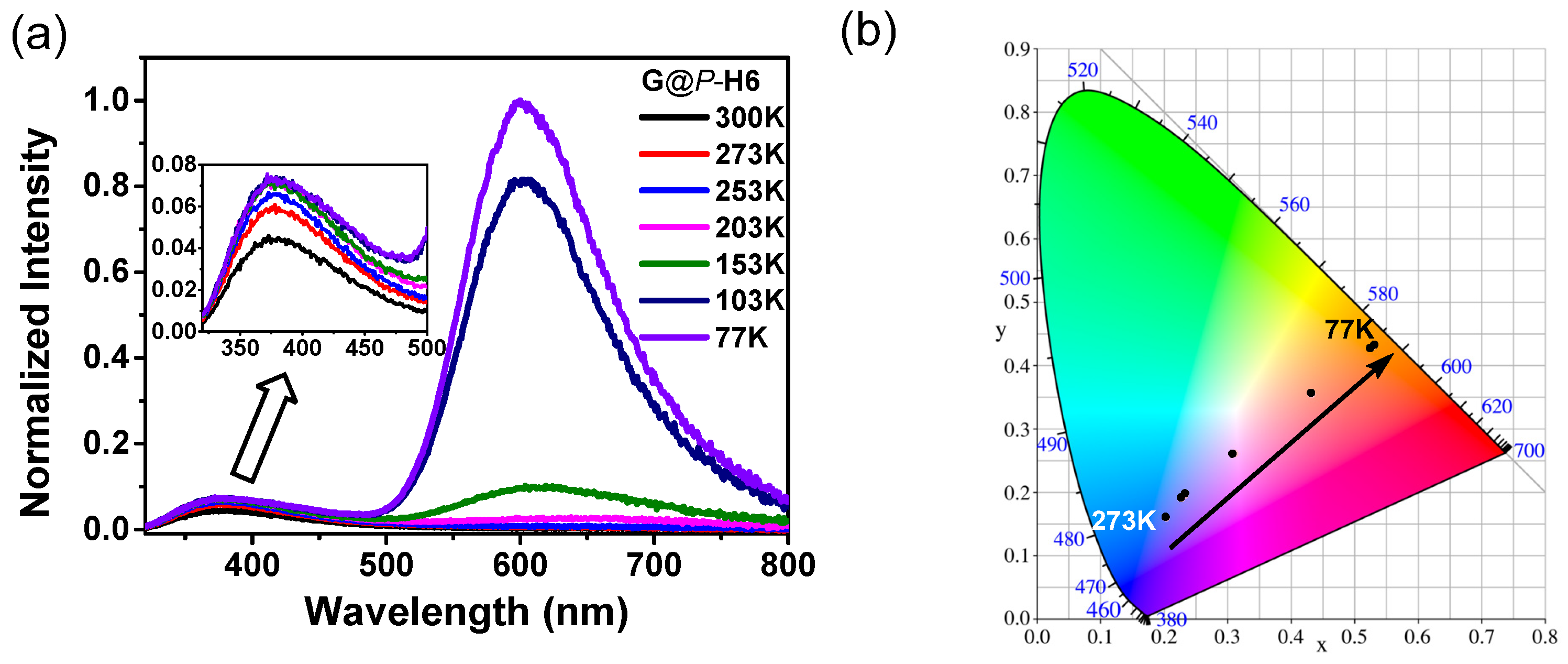
2.4. Photophysical Properties of the Nanocluster Complexes
3. Conclusions
4. Materials and Methods
4.1. General Methods
4.2. Synthetic Procedure of the Silver Cluster and the Nanocluster Complexes
4.3. Preparation of the Samples for CD Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, L.; Moura, A.F.D.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral inorganic nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Gao, H.; Qiu, M.; Jin, W.; Deng, S.; Wong, K.-Y.; Lei, D. Chirality transfer from sub-nanometer biochemical molecules to sub-micrometer plasmonic metastructures: Physiochemical mechanisms, biosensing, and bioimaging opportunities. Adv. Mater. 2020, 32, 1907151. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Shi, L.; Zhu, Y.; Hou, K.; Zheng, Y.; Tang, Z. Tunable chiral metal organic frameworks toward visible light-driven asymmetric catalysis. Sci. Adv. 2017, 3, e1701162. [Google Scholar] [CrossRef] [Green Version]
- Gross, E.; Liu, J.H.; Alayoglu, S.; Marcus, M.A.; Fakra, S.C.; Toste, F.D.; Somorjai, G.A. Asymmetric catalysis at the mesoscale: Gold nanoclusters embedded in chiral self-assembled monolayer as heterogeneous catalyst for asymmetric reactions. J. Am. Chem. Soc. 2013, 135, 3881–3886. [Google Scholar] [CrossRef]
- Pendry, J.B. A chiral route to negative refraction. Science 2004, 306, 1353–1355. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Du, W.; Qin, C.; Liu, D.; Tang, L.; Liu, Y.; Wang, S.; Zhu, M. Assembly of the thiolated [Au1Ag22(S-Adm)12]3+ superatom complex into a framework material through direct linkage by SbF6–@anions. Angew. Chem. Int. Ed. 2020, 59, 7542–7547. [Google Scholar]
- Han, Z.; Dong, X.-Y.; Luo, P.; Li, S.; Wang, Z.-Y.; Zang, S.-Q.; Mak, T.C.W. Ultrastable atomically precise chiral silver clusters with more than 95% quantum efficiency. Sci. Adv. 2020, 6, eaay0107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.-M.; Dong, X.-Y.; Wang, Z.-Y.; Li, H.-Y.; Li, S.-J.; Zhao, X.; Zang, S.-Q. AIE triggers the circularly polarized luminescence of atomically precise enantiomeric copper(I) alkynyl clusters. Angew. Chem. Int. Ed. 2020, 59, 10052–10058. [Google Scholar]
- Kong, Y.-J.; Yan, Z.-P.; Li, S.; Su, H.-F.; Li, K.; Zheng, Y.-X.; Zang, S.-Q. Photoresponsive propeller-like chiral AIE copper(I) clusters. Angew. Chem. Int. Ed. 2020, 59, 5336–5340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, J.; Qiu, X.; Zhao, S.; Tang, Z. Optical activity of chiral metal nanoclusters. Acc. Mater. Res. 2021, 2, 21–35. [Google Scholar] [CrossRef]
- Li, Y.; Higaki, T.; Du, X.; Jin, R. Chirality and surface bonding correlation in atomically precise metal nanoclusters. Adv. Mater. 2020, 32, 1905488. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, C.; Dong, X.-Y.; Zang, S.-Q.; Mak, T.C.W. Shell engineering to achieve modification and assembly of atomically-precise silver clusters. Chem. Soc. Rev. 2021, 50, 2297–2319. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L. Macrocycle-encircled polynuclear metal clusters: Controllable synthesis, reactivity studies, and applications. Acc. Chem. Res. 2018, 51, 2535–2545. [Google Scholar] [CrossRef]
- Guy, K.; Ehni, P.; Paofai, S.; Forschner, R.; Roiland, C.; Amela-Cortes, M.; Cordier, S.; Laschat, S.; Molard, Y. Lord of the crowns: A new precious in the kingdom of clustomesogens. Angew. Chem. Int. Ed. 2018, 57, 11692–11696. [Google Scholar] [CrossRef]
- Jiang, T.; Qu, G.; Wang, J.; Ma, X.; Tian, H. Cucurbiturils brighten Au nanoclusters in water. Chem. Sci. 2020, 11, 3531–3537. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhuang, S.; Liao, L.; Wang, C.; Xia, N.; Gan, Z.; Gu, W.; Li, J.; Deng, H.; Wu, Z. A dual purpose strategy to endow gold nanoclusters with both catalysis activity and water solubility. J. Am. Chem. Soc. 2020, 142, 973–977. [Google Scholar] [CrossRef]
- Muhammed, M.A.H.; Cruz, L.K.; Emwas, A.-H.; El-Zohry, A.M.; Moosa, B.; Mohammed, O.F.; Khashab, N.M. Pillar[5]arene-stabilized silver nanoclusters: Extraordinary stability and luminescence enhancement induced by host-guest interactions. Angew. Chem. Int. Ed. 2019, 58, 15665–15670. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Han, Z.; Yan, B.-J.; Li, H.-Y.; Dong, X.-Y.; Zang, S.-Q. Cations controlling the chiral assembly of luminescent atomically precise copper(I) clusters. Angew. Chem. Int. Ed. 2019, 58, 12143–12148. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wang, H.; Wan, K.W.; Guo, J.; He, C.T.; Yu, Y.; Zhao, L.; Zhang, Y.; Lv, J.; Shi, L.; et al. Enantioseparation of Au20(PP3)4Cl4 clusters with intrinsically chiral cores. Angew. Chem. Int. Ed. 2018, 57, 9059–9063. [Google Scholar] [CrossRef] [PubMed]
- Shibu, E.S.; Pradeep, T. Quantum clusters in cavities: Trapped Au15 in cyclodextrins. Chem. Mater. 2011, 23, 989–999. [Google Scholar] [CrossRef]
- Das, T.; Poria, D.K.; Purkayastha, P. NIR-emitting chiral gold nanoclusters coated with γ-cyclodextrin are pH sensitive: Application as biomarker. Nanomedicine. 2016, 12, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-W.; Li, P.-F.; Meng, Z.; Wang, H.-X.; Han, Y.; Chen, C.-F. Triptycene-based chiral macrocyclic hosts for highly enantioselective recognition of chiral guests containing a trimethylamino group. Angew. Chem. Int. Ed. 2016, 55, 5304–5308. [Google Scholar]
- Chen, C.-F.; Han, Y. Triptycene-derived macrocyclic arenes: From calixarenes to helicarenes. Acc. Chem. Res. 2018, 51, 2093–2106. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Han, Y.; Shi, Q.; Chen, C.-F. Directional transportation of a helic[6]arene along a nonsymmetric molecular axle. J. Org. Chem. 2019, 84, 5872–5876. [Google Scholar] [CrossRef]
- Guo, Y.; Han, Y.; Chen, C.-F. Construction of chiral nanoassemblies based on host-guest complexes and their responsive CD and CPL properties: Chirality transfer from 2,6-helic[6]arenes to a stilbazolium derivative. Front. Chem. 2019, 7, 543. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Han, Y.; Chen, C.-F. Synthesis of chiral helic[1]triptycene[3]arenes and their enantioselective recognition towards chiral guests containing aminoindan groups. Molecules 2021, 26, 536. [Google Scholar] [CrossRef]
- Du, X.-S.; Han, Y.; Chen, C.-F. Helic[6]arene-based chiral pseudo[1]rotaxanes and [1]rotaxanes. Chem. Eur. J. 2022, 28, e202104024. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.-L.; Qin, C.; Wang, H.-N.; Yang, G.-S.; Jiao, Y.-Q.; Huang, P.; Shao, K.-Z.; Su, Z.-M. Serendipitous anion-templated self-assembly of a sandwich-like Ag20S10 macrocycle-based high-nuclearity luminescent nanocluster. Dalton Trans. 2013, 42, 1352–1355. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Lu, H.-F.; Feng, S.-Y.; Zhang, Z.-W.; Sun, G.-X.; Sun, D.-F. Two birds with one stone: Anion templated ball-shaped Ag56 and disc-like Ag20 clusters. Dalton Trans. 2013, 42, 6281–6284. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, X.-S.; Li, B.; Wang, J.-Y.; Li, G.-P.; Gao, G.-G.; Zang, S.-Q. Atom-precise modification of silver(I) thiolate cluster by shell ligand substitution: A new approach to generation of cluster functionality and chirality. J. Am. Chem. Soc. 2018, 140, 594–597. [Google Scholar] [CrossRef]
- Li, S.; Yan, Z.-P.; Li, X.-L.; Kong, Y.-J.; Li, H.-Y.; Gao, G.-G.; Zheng, Y.-X.; Zang, S.-Q. Stepwise achievement of circularly polarized luminescence on atomically precise silver clusters. Adv. Sci. 2020, 7, 2000738. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Deng, Y.-K.; Wang, X.-P.; Sun, D. A temperature-sensitive luminescent Ag20 nanocluster templated by carbonate in situ generated from atmospheric CO2 fixation. New J. Chem. 2013, 37, 2973–2977. [Google Scholar] [CrossRef]
- Zhang, G.-W.; Li, P.-F.; Wang, H.-X.; Han, Y.; Chen, C.-F. Complexation of racemic 2,6-helic[6]arene and its hexamethyl-substituted derivative with quaternary ammonium salts, N-heterocyclic salts, and tetracyanoquinodimethane. Chem. Eur. J. 2017, 23, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, S.; Xiong, L.; Liu, C.; Yu, H.; Wang, S.; Rosi, N.L.; Pei, Y.; Zhu, M. Total structure determination of Au16(S-Adm)12 and Cd1Au14(StBu)12 and implications for the structure of Au15(SR)13. J. Am. Chem. Soc. 2018, 140, 10988–10994. [Google Scholar] [CrossRef]
- Delley, B. An allelectron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Zhou, K.; Qin, C.; Wang, X.-L.; Shao, K.-Z.; Yan, L.-K.; Su, Z.-M. Unexpected 1D self-assembly of carbonate-templated sandwich-like macrocycle-based Ag20S10 luminescent nanoclusters. CrystEngComm 2014, 16, 7860–7864. [Google Scholar] [CrossRef]
- Forment-Aliaga, A.; Coronado, E.; Feliz, M.; Gaita-Ariño, A.; Llusar, R.; Romero, F.M. Cationic Mn12 single-molecule magnets and their polyoxometalate hybrid salts. Inorg. Chem. 2003, 42, 8019–8027. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lei, Z.; Wang, Q.-M. Luminescent molecular Ag-S nanocluster [Ag62S13(SBut)32](BF4)4. J. Am. Chem. Soc. 2010, 132, 17678–17679. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Han, Y.; Chen, C. Chiral Nanocluster Complexes Formed by Host−Guest Interaction between Enantiomeric 2,6-Helic[6]arenes and Silver Cluster Ag20: Emission Enhancement and Chirality Transfer. Molecules 2022, 27, 3932. https://doi.org/10.3390/molecules27123932
Guo Y, Han Y, Chen C. Chiral Nanocluster Complexes Formed by Host−Guest Interaction between Enantiomeric 2,6-Helic[6]arenes and Silver Cluster Ag20: Emission Enhancement and Chirality Transfer. Molecules. 2022; 27(12):3932. https://doi.org/10.3390/molecules27123932
Chicago/Turabian StyleGuo, Yan, Ying Han, and Chuanfeng Chen. 2022. "Chiral Nanocluster Complexes Formed by Host−Guest Interaction between Enantiomeric 2,6-Helic[6]arenes and Silver Cluster Ag20: Emission Enhancement and Chirality Transfer" Molecules 27, no. 12: 3932. https://doi.org/10.3390/molecules27123932
APA StyleGuo, Y., Han, Y., & Chen, C. (2022). Chiral Nanocluster Complexes Formed by Host−Guest Interaction between Enantiomeric 2,6-Helic[6]arenes and Silver Cluster Ag20: Emission Enhancement and Chirality Transfer. Molecules, 27(12), 3932. https://doi.org/10.3390/molecules27123932