Comparison of the Antioxidant Properties of Green Macroalgae from Diverse European Water Habitats by Use of Several Semi-Quantitative Assays
Abstract
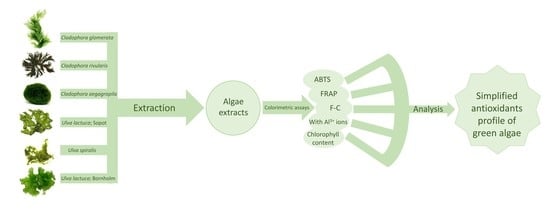
:1. Introduction
2. Results and Discussion
2.1. Optimization of Extraction Process
2.2. Yields of Extractions
2.3. The Content of Chlorophyll
2.4. Antioxidative Potential
2.5. Impact of Environment
3. Materials and Methods
3.1. Algae Studied
3.2. Reagents
3.3. Methodology
3.3.1. Optimization of Extraction Process
3.3.2. Extraction in a Soxhlet Apparatus
3.3.3. Semi-Quantitative Colorimetric Assays
3.3.4. Determination of the Content of Chlorophyll
3.4. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Montero, L.; del Pilar Sánchez-Camargo, A.; Ibáñez, E.; Gilbert-López, B. Phenolic Compounds from Edible Algae: Bioactivity and Health Benefits. Curr. Med. Chem. 2017, 25, 4808–4826. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemodanov, A.; Robin, A.; Golberg, A. Design of Marine Macroalgae Photobioreactor Integrated into Building to Support Seagriculture for Biorefinery and Bioeconomy. Bioresour. Technol. 2017, 241, 1084–1093. [Google Scholar] [CrossRef]
- Hernández, I.; Pérez-Pastor, A.; Mateo, J.J.; Megina, C.; Vergara, J.J. Growth dynamics of Ulva rotundata (Chlorophyta) in a fish farm: Implications for biomitigation at a large scale1. J. Phycol. 2008, 44, 1080–1089. [Google Scholar] [CrossRef]
- Jones, C.S.; Mayfield, S.P. Algae Biofuels: Versatility for the Future of Bioenergy. Curr. Opin. Biotechnol. 2012, 23, 346–351. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Seaweeds Used as Human Food. Available online: https://www.fao.org/3/y4765e/y4765e0b.htm (accessed on 24 February 2022).
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Sun, L.; Zhao, R.; Du, L.; Song, J.; Zhang, L.; He, G.; Zhang, Y.; Zhang, J. Polyphenols: Potential Source of Drugs for the Treatment of Ischaemic Heart Disease. Pharmacol. Ther. 2016, 162, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H. Bin Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Michalak, I.; Messyasz, B. Concise Review of Cladophora spp.: Macroalgae of Commercial Interest. J. Appl. Phycol. 2020, 33, 133–166. [Google Scholar] [CrossRef]
- Hiraoka, M. Massive Ulva Green Tides Caused by Inhibition of Biomass Allocation to Sporulation. Plants 2021, 10, 2482. [Google Scholar] [CrossRef]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; De Clerck, O.; Coates, J.C. The Green Seaweed Ulva: A Model System to Study Morphogenesis. Front. Plant Sci. 2015, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Rybak, A.S. Species of Ulva (Ulvophyceae, Chlorophyta) as Indicators of Salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef] [Green Version]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical Composition and Functional Properties of Ulva lactuca Seaweed Collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Yaich, H.; Amira, A.B.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of Extraction Procedures on Structural, Thermal and Antioxidant Properties of ulvan from Ulva lactuca Collected in Monastir Coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ghareib, H.R. Bioactivity of Ulva lactuca L. Acetone Extract on Germination and Growth of Lettuce and Tomato Plants. Afr. J. Biotechnol. 2009, 8, 3832–3838. [Google Scholar]
- Flodin, C.; Whitfield, F.B. 4-Hydroxybenzoic Acid: A Likely Precursor of 2,4,6-Tribromophenol in Ulva lactuca. Phytochemistry 1999, 51, 249–255. [Google Scholar] [CrossRef]
- Fahprathanchai, P.; Saenphet, K.; Peerapornpisal, Y.; Aritajat, S. Toxicological evaluation of cladophora glomerata kützing and microspora floccosa thuret in albino rats. Southeast Asian J. Trop. Med. Public Health 2006, 206, 206–209. [Google Scholar]
- Marks, J.C.; Power, M.E. Nutrient Induced Changes in the Species Composition of Epiphytes on Cladophora glomerata Kütz. (Chlorophyta). Hydrobiol. 2001, 450, 187–196. [Google Scholar] [CrossRef]
- Lill, J.O.; Salovius-Laurén, S.; Harju, L.; Rajander, J.; Saarela, K.E.; Lindroos, A.; Heselius, S.J. Temporal Changes in Elemental Composition in Decomposing Filamentous Algae (Cladophora glomerata and Pilayella littoralis) Determined with PIXE and PIGE. Sci. Total Environ. 2012, 414, 646–652. [Google Scholar] [CrossRef]
- Unpaprom, Y.; Whangchai, N.; Prasongpol, P. Antibacterial, Antifungal Properties and Chemical Composition of Freshwater Macroalage, Cladophora glomerata. J. Biol. Med. Open Access Res. 2020, 1, 107. [Google Scholar]
- Akköz, C.; Arslan, D.; Ünver, A.; Özcan, M.M.; Yilmaz, B. Chemical Composition, total phenolic and mineral contents of Enteromorpha intestinalis (L.) kütz. and Cladophora glomerata (L.) kütz. seaweeds. J. Food Biochem. 2011, 35, 513–523. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Stefanov, K.; Dimitrova-Konaklieva, S.; Najdenski, H.; Tsvetkova, I.; Popov, S. Chemical Composition and Biological Activity of the Brackish-Water Green Alga Cladophora rivularis (L.) Hoek. Bot. Mar. 2004, 47, 215–221. [Google Scholar] [CrossRef]
- Boedeker, C.; Leliaert, F.; Timoshkin, O.A.; Vishnyakov, V.S.; Díaz-Martínez, S.; Zuccarello, G.C. The Endemic Cladophorales (Ulvophyceae) of Ancient Lake Baikal Represent a Monophyletic Group of Very Closely Related but Morphologically Diverse Species. J. Phycol. 2018, 54, 616–629. [Google Scholar] [CrossRef]
- Yoshii, Y.; Hanyuda, T.; Wakana, I.; Miyaji, K.; Arai, S.; Ueda, K.; Inouye, I. Carotenoid compositions of cladophora balls (Aegagropila linnaei) and some members of the cladophorales (ulvophyceae, chlorophyta): Their taxonomic and evolutionary implication1. J. Phycol. 2004, 40, 1170–1177. [Google Scholar] [CrossRef]
- Hanyuda, T.; Wakana, I.; Arai, S.; Miyaji, K.; Watano, Y.; Ueda, K. Phylogenetic relationships within cladophorales (ulvophyceae, chlorophyta) inferred from 18S rrna gene sequences, with special reference to Aegagropila linnaei. J. Phycol. 2002, 38, 564–571. [Google Scholar] [CrossRef]
- Shutova, V.V.; Tyutyaev, E.V.; Veselova, T.V.; Choob, V.V.; Maksimov, G.V. Dark Adaptation and Conformations of Carotenoids in the Cells of Cladophora aegagropila (L). Rabenh. Cell Biophys. 2017, 62, 728–733. [Google Scholar] [CrossRef]
- Messyasz, B.; Leska, B.; Fabrowska, J.; Pikosz, M.; Cieslak, A.; Schroeder, G. Effects of Organic Compounds on the Macroalgae Culture of Aegagropila linnaei. Open Chem. 2015, 13, 1040–1044. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancement of Microwave-Assisted Extraction of Bioactive Compounds from Cabbage Outer Leaves via the Application of Ultrasonic Pretreatment. Sep. Purif. Technol. 2015, 144, 37–45. [Google Scholar] [CrossRef]
- Bellis, V.J. Unialgal cultures of Cladophora glomerata (L.) kütz.12. J. Phycol. 1968, 4, 19–23. [Google Scholar] [CrossRef]
- Piotrowicz, Z.; Tabisz, Ł.; Łȩska, B. Material Balance for Stepwise Extraction of Freshwater Algae Biomass with Heavy Metal Sequestration. Ecol. Chem. Eng. 2019, 26, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Malkin, S.Y.; Sorichetti, R.J.; Wiklund, J.A.; Hecky, R.E. Seasonal Abundance, Community Composition, and Silica Content of Diatoms Epiphytic on Cladophora glomerata. J. Great Lakes Res. 2009, 35, 199–205. [Google Scholar] [CrossRef]
- Piotrowicz, Z.; Tabisz, Ł.; Waligórska, M.; Pankiewicz, R.; Łęska, B. Phenol-Rich Alternatives for Rosa x Damascena Mill. Efficient Phytochemical Profiling Using Different Extraction Methods and Colorimetric Assays. Sci. Rep. 2021, 11, 23883. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowicz, Z.; Tabisz, Ł.; Łęska, B.; Messyasz, B.; Pankiewicz, R. Comparison of the Antioxidant Properties of Green Macroalgae from Diverse European Water Habitats by Use of Several Semi-Quantitative Assays. Molecules 2022, 27, 3812. https://doi.org/10.3390/molecules27123812
Piotrowicz Z, Tabisz Ł, Łęska B, Messyasz B, Pankiewicz R. Comparison of the Antioxidant Properties of Green Macroalgae from Diverse European Water Habitats by Use of Several Semi-Quantitative Assays. Molecules. 2022; 27(12):3812. https://doi.org/10.3390/molecules27123812
Chicago/Turabian StylePiotrowicz, Zuzanna, Łukasz Tabisz, Bogusława Łęska, Beata Messyasz, and Radosław Pankiewicz. 2022. "Comparison of the Antioxidant Properties of Green Macroalgae from Diverse European Water Habitats by Use of Several Semi-Quantitative Assays" Molecules 27, no. 12: 3812. https://doi.org/10.3390/molecules27123812
APA StylePiotrowicz, Z., Tabisz, Ł., Łęska, B., Messyasz, B., & Pankiewicz, R. (2022). Comparison of the Antioxidant Properties of Green Macroalgae from Diverse European Water Habitats by Use of Several Semi-Quantitative Assays. Molecules, 27(12), 3812. https://doi.org/10.3390/molecules27123812