Induction of DR5-Dependent Apoptosis by PGA2 through ATF4-CHOP Pathway
Abstract
:1. Introduction
2. Results
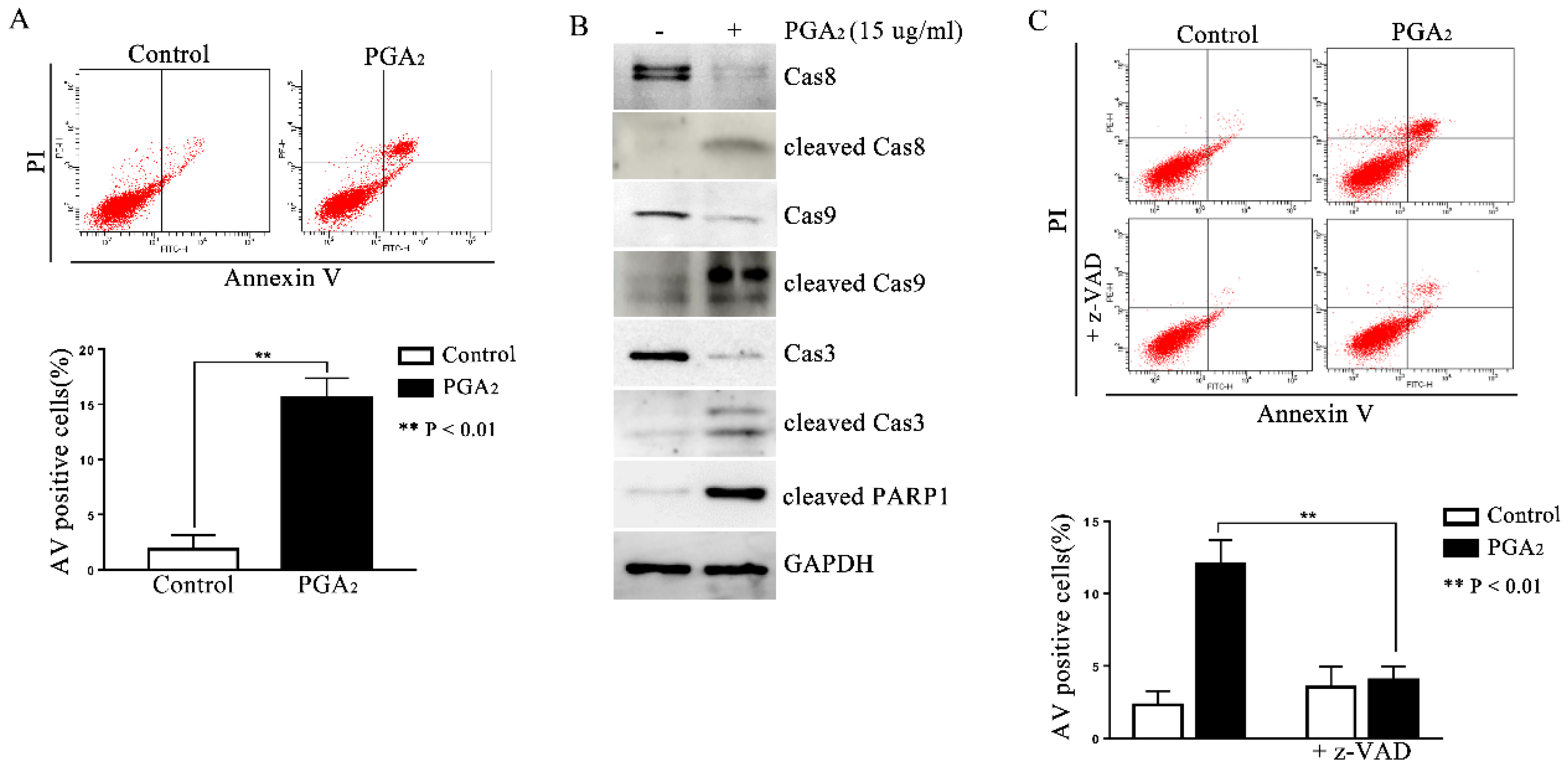
2.1. Induction of Caspase-Dependent Apoptosis in HCT116 p53 −/− Cells by PGA2
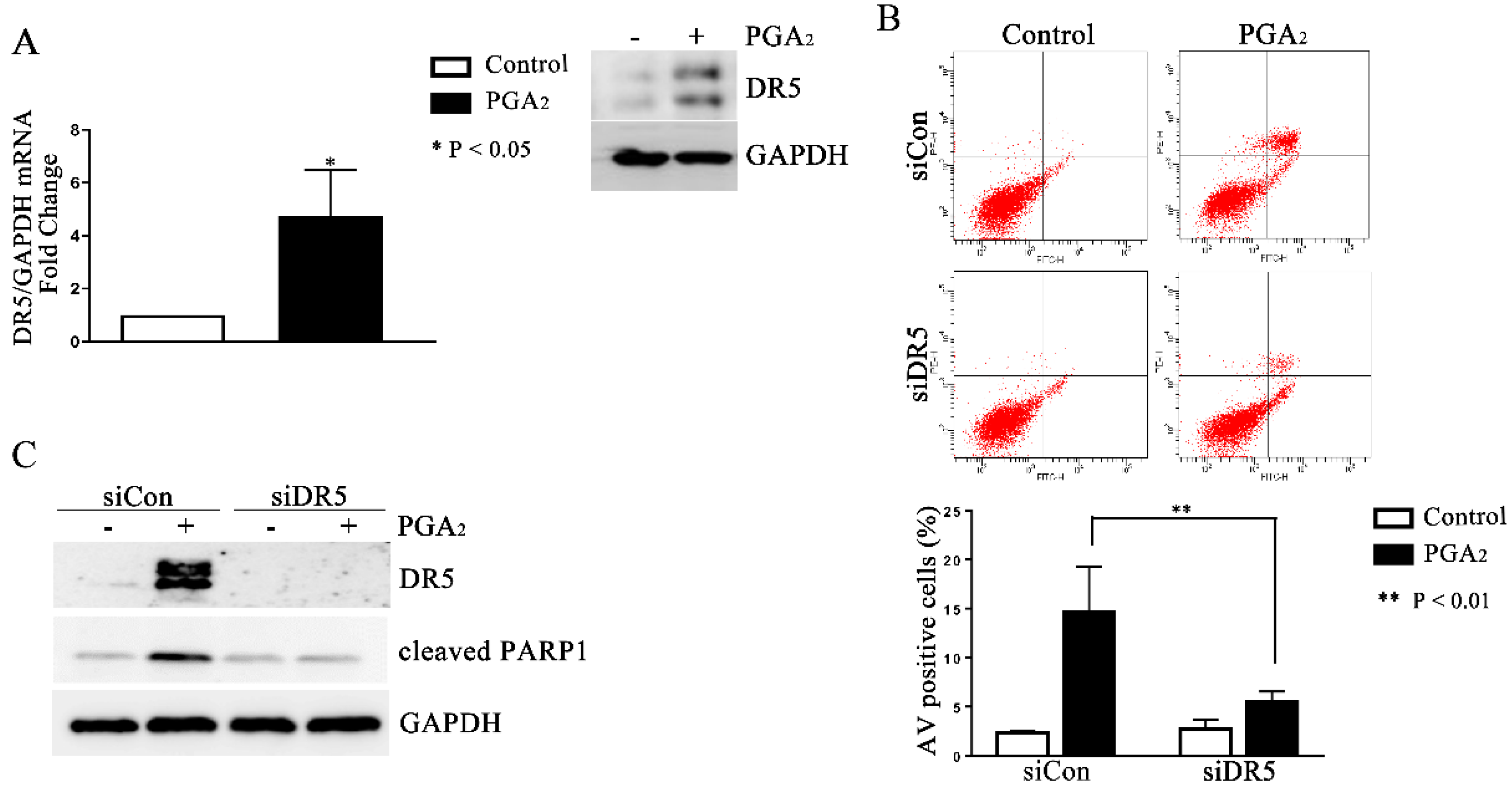
2.2. Induction of DR5-Dependent Apoptosis in HCT116 p53 −/− Cells by PGA2
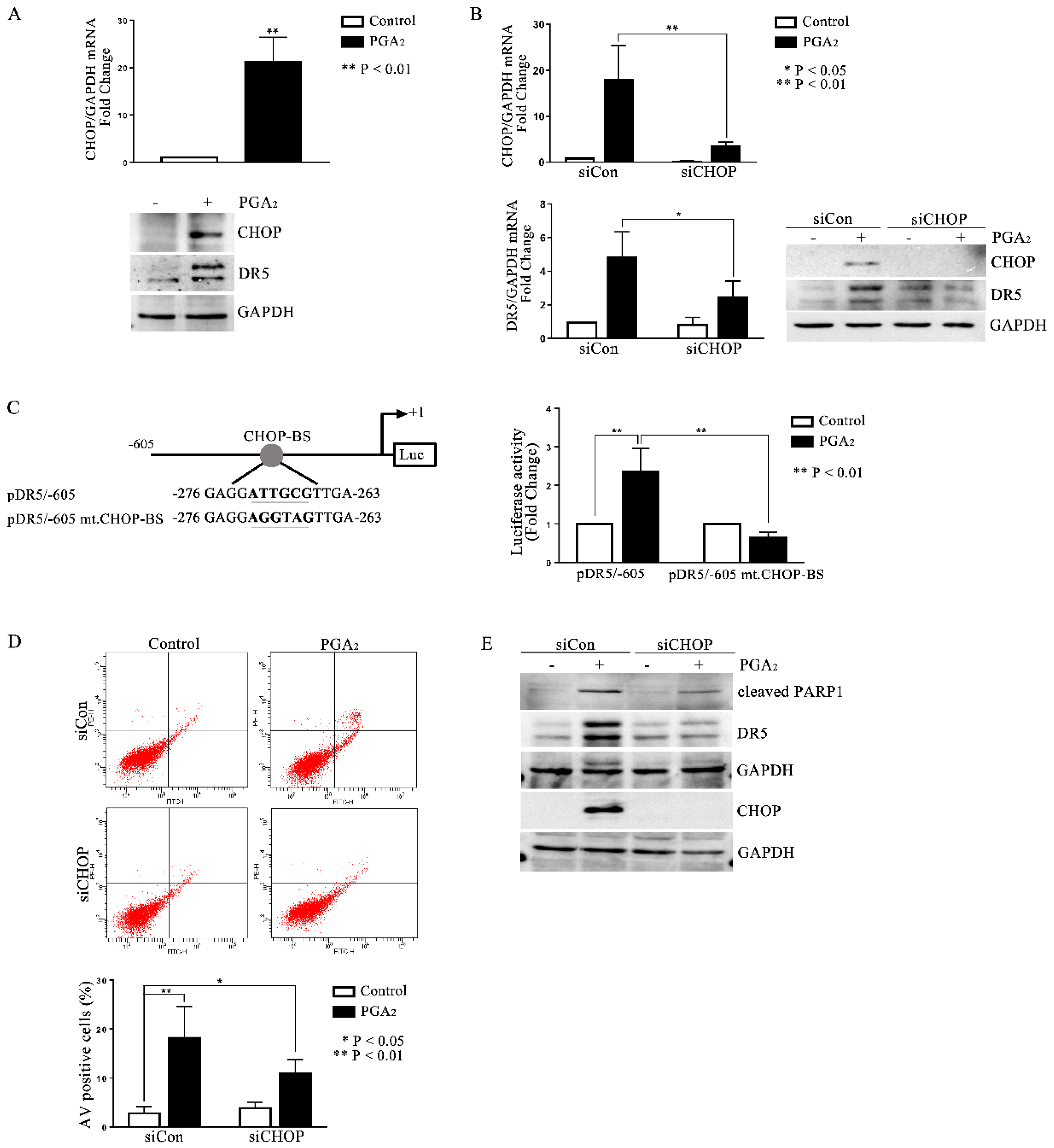
2.3. Involvement of CHOP in PGA2-Induced Apoptosis and Expression of DR5
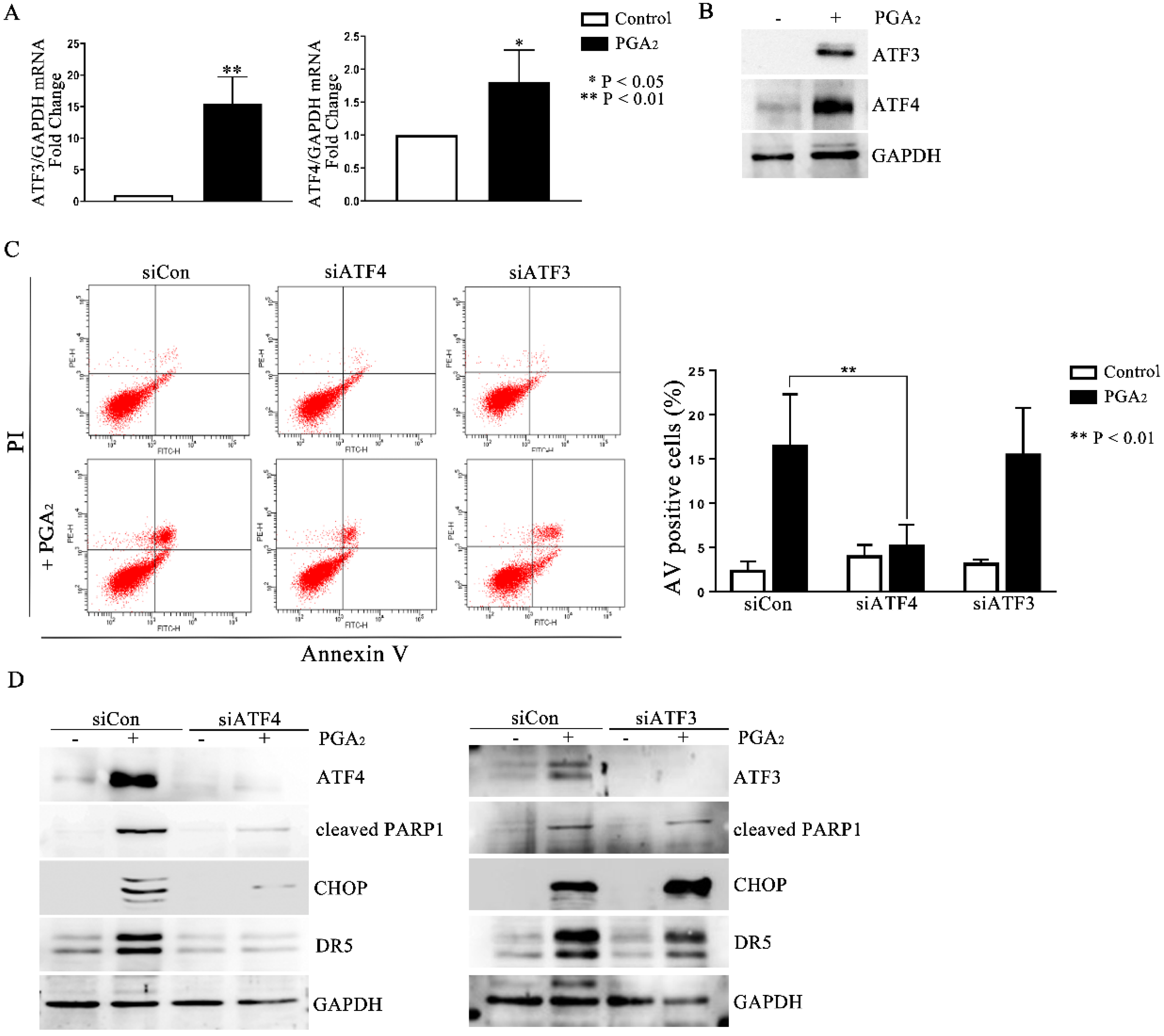
2.4. The Role of ATF4 in PGA2-Induced Apoptosis and Activation of CHOP-DR5 Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Transfection with Small Interfering RNA (siRNA)
4.4. Apoptosis Assay
4.5. Immunoblot Analysis and Antibodies
4.6. Quantitative Real-Time PCR (qPCR)
4.7. Luciferase Reporter Gene Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díez-Dacal, B.; Pérez-Sala, D. A-class prostaglandins: Early findings and new perspectives for overcoming tumor chemoresistance. Cancer Lett. 2012, 320, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Joubert, A.; Maritz, C.; Joubert, F. Influence of prostaglandin A2 and 2-methoxyestradiol on Bax and Bcl-2 expression levels in cervical carcinoma cells. Biomed. Res. 2005, 26, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Joubert, A.M.; Panzer, A.; Bianchi, P.C.; Lottering, M.L. The effects of prostaglandin A2 on cell growth, cell cycle status and apoptosis induction in HeLa and MCF-7 cells. Cancer Lett. 2003, 191, 203–209. [Google Scholar] [CrossRef]
- Ahn, S.G.; Kim, H.S.; Jeong, S.W.; Kim, B.E.; Rhim, H.; Shim, J.Y.; Kim, J.W.; Lee, J.H.; Kim, I.K. Sox-4 is a positive regulator of Hep3B and HepG2 cells’ apoptosis induced by prostaglandin (PG)A(2) and delta(12)-PGJ(2). Exp. Mol. Med. 2002, 34, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.; Wilson, N.S.; Ashkenazi, A. Proapoptotic DR4 and DR5 signaling in cancer cells: Toward clinical translation. Curr. Opin. Cell Biol. 2010, 22, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Woo, S.M.; Shahriyar, S.A.; Kwon, T.K. Elucidation for modulation of death receptor (DR) 5 to strengthen apoptotic signals in cancer cells. Arch. Pharm. Res. 2019, 42, 88–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.S.; Burns, T.F.; McDonald, E.R., III; Jiang, W.; Meng, R.; Krantz, I.D.; Kao, G.; Gan, D.D.; Zhou, J.Y.; Muschel, R.; et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 1997, 17, 141–143. [Google Scholar] [CrossRef]
- Takimoto, R.; El-Deiry, W.S. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene 2000, 19, 1735–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, H.; Wang, H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004, 279, 45495–45502. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, M.S.; Burns, T.F.; Huang, Y.; Wu, G.S.; Amundson, S.; Brooks, K.S.; Fornace, A.J.; El-Deiry, W.S. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998, 58, 1593–1598. [Google Scholar] [PubMed]
- Gupta, S.C.; Francis, S.K.; Nair, M.S.; Mo, Y.Y.; Aggarwal, B.B. Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: Evidence for the role of the ROS-ERK-CHOP-death receptor pathway. J. Biol. Chem. 2013, 288, 32343–32356. [Google Scholar] [PubMed] [Green Version]
- Nagata, S. Apoptosis by death factor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, J.P.; Marsters, S.A.; Pitti, R.M.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.L.; Baker, K.; Wood, W.I.; et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Lee, S.; Park, J.Y.; Lee, S.Y.; Kim, H.S. Induction of p53-Dependent Apoptosis by Prostaglandin A(2). Biomolecules 2020, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Odani, N.; Negishi, M.; Takahashi, S.; Kitano, Y.; Kozutsumi, Y.; Ichikawa, A. Regulation of BiP gene expression by cyclopentenone prostaglandins through unfolded protein response element. J. Biol. Chem. 1996, 271, 16609–16613. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Odani, N.; Tomokiyo, K.; Furuta, K.; Suzuki, M.; Ichikawa, A.; Negishi, M. Localization of a cyclopentenone prostaglandin to the endoplasmic reticulum and induction of BiP mRNA. Biochem. J. 1998, 335, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Su, L.; Hao, X.; Zhong, N.; Zhong, D.; Singhal, S.; Liu, X. Salermide up-regulates death receptor 5 expression through the ATF4-ATF3-CHOP axis and leads to apoptosis in human cancer cells. J. Cell. Mol. Med. 2012, 16, 1618–1628. [Google Scholar] [CrossRef]
- Joo, J.H.; Ueda, E.; Bortner, C.D.; Yang, X.P.; Liao, G.; Jetten, A.M. Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells. Biochem. Pharmacol. 2015, 97, 256–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straus, D.S.; Glass, C.K. Cyclopentenone prostaglandins: New insights on biological activities and cellular targets. Med. Res. Rev. 2001, 21, 185–210. [Google Scholar] [CrossRef]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The Antioxidant Defense System Keap1-Nrf2 Comprises a Multiple Sensing Mechanism for Responding to a Wide Range of Chemical Compounds. Mol. Cell. Biol. 2009, 29, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkB kinase. Nature 2000, 403, 103–108. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Ahn, J.-H.; Ko, K.; Kim, J.; Jeong, S.; Kim, I.-K.; Kim, J.; Kim, H.-S. Prostaglandin A2 activates intrinsic apoptotic pathway by direct interaction with mitochondria in HL-60 cells. Prostaglandins Other Lipid Mediat. 2010, 91, 23–37. [Google Scholar] [CrossRef]
- Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, J.; Xu, R. The molecular mechanisms of TRAIL resistance in cancer cells: Help in designing new drugs. Curr. Pharm. Des. 2014, 20, 6714–6722. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, D.; Schmid, J.O.; Beigl, T.B.; Mack, A.; Maichl, D.S.; Cao, K.; Budai, B.; Fullstone, G.; Kontermann, R.E.; Mürdter, T.E.; et al. Stress-induced TRAILR2 expression overcomes TRAIL resistance in cancer cell spheroids. Cell Death Differ. 2020, 27, 3037–3052. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhai, X.; Liang, P.; Cui, H. Overcoming TRAIL resistance for glioblastoma treatment. Biomolecules 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Joerger, A.C.; Fersht, A.R. The p53 pathway: Origins, inactivation in cancer, and emerging therapeutic approaches. Annu. Rev. Biochem. 2016, 85, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-E.; Pan, Y.-R.; Yeh, C.-N.; Lunec, J. Targeting P53 as a future strategy to overcome gemcitabine resistance in biliary tract cancers. Biomolecules 2020, 10, 1474. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-M.; Park, J.-Y.; Pyo, J.; Lee, S.-Y.; Kim, H.-S. Induction of DR5-Dependent Apoptosis by PGA2 through ATF4-CHOP Pathway. Molecules 2022, 27, 3804. https://doi.org/10.3390/molecules27123804
Park K-M, Park J-Y, Pyo J, Lee S-Y, Kim H-S. Induction of DR5-Dependent Apoptosis by PGA2 through ATF4-CHOP Pathway. Molecules. 2022; 27(12):3804. https://doi.org/10.3390/molecules27123804
Chicago/Turabian StylePark, Kyeong-Min, Ji-Young Park, Jaehyuk Pyo, Sun-Young Lee, and Ho-Shik Kim. 2022. "Induction of DR5-Dependent Apoptosis by PGA2 through ATF4-CHOP Pathway" Molecules 27, no. 12: 3804. https://doi.org/10.3390/molecules27123804
APA StylePark, K.-M., Park, J.-Y., Pyo, J., Lee, S.-Y., & Kim, H.-S. (2022). Induction of DR5-Dependent Apoptosis by PGA2 through ATF4-CHOP Pathway. Molecules, 27(12), 3804. https://doi.org/10.3390/molecules27123804





