Abstract
Compromised activity is a common impediment for biologics requiring endosome trafficking into target cells. In cancer cells, antibody-drug conjugates (ADCs) are trapped in endosomes or subsequently pumped extracellularly, leading to a reduction in intracellular accumulation. In subsets of dendritic cells (DCs), endosome-engulfed antigens face non-specific proteolysis and collateral damage to epitope immunogenicity before proteasomal processing and subsequent surface presentation. To bypass these shortcomings, we devised Accum™, a conjugable biotechnology harboring cholic acid (ChAc) and a nuclear localization signal (NLS) sequence for endosome escape and prompt nuclear targeting. Combined, these mechanisms culminate in enhanced intracellular accumulation and functionalization of coupled biologics. As proof-of-principle, we have biochemically characterized Accum, demonstrating its adaptability to ADCs or antigens in different cancer settings. Additionally, we have validated that endosome escape and nuclear routing are indispensable for effective intracellular accumulation and guaranteed target cell selectivity. Importantly, we have demonstrated that the unique mechanism of action of Accum translates into enhanced tumor cytotoxicity when coupled to ADCs, and durable therapeutic and prophylactic anti-cancer immunogenicity when coupled to tumor antigens. As more pre-clinical evidence accumulates, the adaptability, unique mechanism of action, and high therapeutic potency of Accum signal a promising transition into clinical investigations in the context of onco-immunotherapy.
1. Introduction
As medical demand and technological advancements balloon, the biotechnology market is foreseen to expand at a compound annual growth rate of 12.66%, reaching USD 106.75 billion by 2028 [1]. Oncology is the disease area that responsible for the largest share of revenue—almost half—from the development of biologics [2]. Biologics for cancer treatment chiefly include immunotherapies [3], such as monoclonal antibodies (mAbs), vaccines, and oncolytic viruses, which have transformed cancer care, providing patients with significant survival and quality of life benefits [4]. These therapeutic modalities, however, remain logistically, mechanistically, and biologically challenged, with disparate limitations hindering further clinical development and licensing [5]. Bypassing these challenges in cancer settings has thus become, and remains, the mission of multitudinous technology platforms that incorporate exosomes, chimeric antigen receptors (CARs), organoids, viruses, stem cells, cellular vaccines, antigen vaccines, mAbs, and ADCs, among others [6,7,8,9].
Accum™ is a novel biotechnology exemplifying, in its initial concept, the need to circumvent the biological challenges of ADCs [10,11,12,13,14,15]. Despite their targeted therapy nature (a receptor-specific mAb linked to a cytotoxic payload), ADCs become captives of the cellular transport mechanisms they were designed to exploit to deposit their cargo in a trojan horse-like fashion [9]. Cellular transport pathways (primarily receptor-mediated internalization followed by endosomal-lysosomal trafficking [16]) have been shown to develop resistance to ADCs, reducing their intracellular deposition and ultimately compromising their activity [9,17]. Indeed, imaging data show that only a minimal fraction of ADCs reaches its target [18,19,20]. In addition, off-target toxicities are largely seen even with FDA-approved ADCs [21,22]. This has prompted vigorous research on ADC modifications that could keep endosome entrapment in check. ADCs equipped with cell-penetrating peptides (CPPs) were effective, yet their nontarget tissue accumulation manifested a drawback [23,24,25,26,27,28]. Contrarily, ADCs equipped with pH-sensitive polymers maintained both endosome escape and target cell selectivity [29,30]. However, whether endosome escape corresponded to elevated intracellular payload accumulation—the centroid maintaining clinical significance—still begged answers. Other modification attempts consequently emerged, including ADCs equipped with peptides containing compartment-localizing amino acids, such as the NLS sequence, or segments thereof [31], that directs protein transport into the nucleus [32,33,34]. Nevertheless, entrapment in endosomes or other recycling pathways resurfaced [35,36,37].
Accum™ employs a ChAc-NLS fusion compound that enables its conjugate to override endosome entrapment and accumulate intracellularly in target cells [12,15]. The basis for exploiting ChAc, the bile acid moiety of Accum™, is that it allows nonenveloped viruses to escape endosomes [38] by inducing the metabolism of endosomal membrane sphingomyelin into ceramide, appending subsequent structural and dynamic membrane changes that promote endosome-to-cytoplasm protein traversal without killing cells [39,40,41,42]. On the other hand, the NLS moiety of Accum™ redirects trafficking of the conjugate to the nucleus; ultimately, both Accum moieties increase intracellular retention in target cells [12]. The concept of the ChAc-NLS fusion compound serving as a cell accumulator (Accum for short) has been shown to be pliable, with the potential for coupling to different molecular conjugates for use in different cancer settings [10,11,12,13,14,15]. In this review article, we discuss the genesis of Accum™ and highlight its docility in producing context-specific therapeutic strategies. Furthermore, we recapitulate the data reported hitherto on the validity and efficacy of Accum™ as a functionalizing technology that elevates the therapeutic potency of various molecular conjugates. Finally, we underline the advantages of Accum over other conjugable molecules in the framework of previous literature reports.
2. Accum™: Genesis of a Primer
Assimilating their previous studies [31,35,36,37,43], wherein a synthetic peptide harboring the classical NLS (cNLS) sequence amply dictated the nuclear translocation of a mAb conjugate, Beaudoin and colleagues [12] coupled ChAc to the non-CPP, CGYGPKKKRKVGG [44], generated by an automated peptide synthesizer, then processed and characterized the conjugate via mass spectroscopy and ultra-performance liquid chromatography. PKKKRKV represents the cNLS sequence taken from simian virus 40 (SV-40) large T antigen, while GYG and GG residues serve as N- and C-terminus spacers, respectively [12]. Conjugation of Accum to protein can be performed by using any conjugation technique, such as the cross-linker sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC) reacting with the amine of lysine and the sulfhydryl moiety of cysteine via its NHS-ester and the maleimide reactive group of the cross-linker (Figure 1) [12,14,15]. ChAcNLS, or Accum, is water-soluble; has a positive net charge of +5 and a molecular weight of 1.8 kDa; and is synthetically recovered with ≥94% purity and a molecular mass of 1768.5 g/mol [12,14]. Accum is added to conjugates in the same, or higher, molar excess ratio to yield Accum-modified conjugates [15]. During the modification process, free cross-linker and free Accum are eliminated by multiple centricon filtration and a Sephadex column, while Accum-modified conjugates are subsequently processed in ultrafiltration tubes, concentrated, and biochemically characterized with SDS-PAGE, turbidity assays, differential scanning fluorimetry, and protein concentration assays, followed by functional evaluation with conjugate-receptor affinity assays against naked or non-Accum-modified conjugates [14]. The number of Accum per conjugate is proportional to the number of accessible lysine residues [15] and the intracellular accumulation of conjugates [12,14]. The rate of conjugation is estimated by SDS-PAGE gel [12,14].
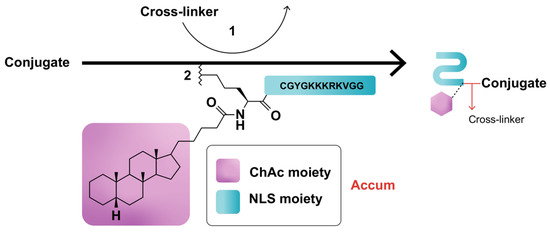
Figure 1.
Schematic representation of Accum-modification of protein conjugates. In step (1), the protein reacts with a cross-linker, yielding a maleimide-activated conjugate. In step (2), the sulfhydryl group of the N-terminus cysteine cap of the NLS moiety of Accum reacts with the maleimide-activated conjugate, yielding an Accum-conjugate.
Accum can be likened to a polymath primer: it is well-informed about the biological limitations of CPP- and NLS-equipped conjugates [10] and highly adaptable for functionalizing different conjugates for different therapeutic purposes [10,11,12,13,14,15]. For instance, Beaudoin et al. [12] designed 7G3-Accum—Accum coupled to 7G3—a mAb specifically targeting interleukin-3 receptor-α (IL-3Rα), a cell-surface antigen expressed by leukemic cells. In the context of muscle invasive bladder cancer (MIBC), the same team [13,14] designed A14-Accum, where A14 is a mAb specific for interleukin-5 receptor α-subunit (IL-5Rα). In another therapeutic context, Accum was coupled to the clinically approved ADC trastuzumab-emtansine (T-DM1) [10]. Recently, Bikorimana et al. [15] introduced Accum to the setting of cellular vaccines, coupling ChAcNLS to the xenoantigen ovalbumin (OVA) with the rationale of triggering potent antigen presentation by DCs. The same group further linked Accum to lymphoma tumor lysate proteins to instill potent anti-tumor immunity in DCs. Overall, these studies attest to the pliability of Accum as a biotechnology that primes the function of various molecular conjugates, thereby enhancing therapeutic outcomes.
3. What Has Accum™ Demonstrated So Far? Characterization, Validation, and Efficacy Data
Most ADCs undergo receptor-mediated intracellular transport to lysosomes, where they exploit pH-sensitive proteases for the catabolism, release, and intracellular diffusion of their cytotoxic payloads [9]. However, this transport system faces a backlash from cancer cell mechanisms, including receptor downregulation/recycling and increased expression of multidrug resistance pumps, that either entrap drug payloads in endosomes or counteract their intracellular accumulation [45,46]. On another note, several new ADC modifications, including ADCs coupled to synthetic CPPs or pH-sensitive polymers, have been effective in evading endosome entrapment or routing to alternative cellular compartments; however, they have failed to either produce intracellular retention [29,30,47] or achieve target cell specificity and sufficient tumor uptake [23,24,25,26,27,28]; otherwise, they have induced high toxicity by indiscriminately penetrating off-target cells [24,47]. The Accum fusion compound ChAcNLS was thus devised with the aim of enabling ADCs to (i) dodge entrapment in the endosomal-lysosomal pathway, (ii) partake in nuclear routing, and (iii) accumulate intracellularly while retaining target cell selectivity and evading off-target payload effects [12]. Optimum endosome escape and intracellular accumulation was hypothesized to be the result of synergism between (a) the ChAc moiety, which selectively disrupts endosomal membranes by triggering ceramide formation while leaving plasma membranes intact [12] in the same way it allows nonenveloped viruses to escape from endosomes into the cytoplasm [38,39,40,41,42]; and (b) the NLS moiety, which directs molecular conjugates toward the nucleus [12]. To validate these hypotheses, different in vitro and in vivo studies were performed using different Accum-modified conjugates. Additional studies were also conducted to evaluate whether the distinctive cellular delivery mechanism of Accum provides superior therapeutic advantages compared to control counterparts.
3.1. 7G3-Accum
Beaudoin et al. [12,13] initially designed 7G3-Accum, an Accum-modified mAb specific for IL-3Rα. In characterization assays, the number of Accum copies per antibody was shown to correspond with the sulfo-SMCC:antibody ratio, reaching a 50:1 ratio with an average of 8.5 and 2.6 copies per heavy and light chain of 7G3, respectively [12,13,15]. In proof-of-concept experiments, IL-3Rα-expressing TF-1a leukemic cells were used to study the intracellular trafficking of 7G3-ChAcNLS against different controls, including unmodified 7G3, 7G3-ChAc, 7G3-NLS, 7G3-ChAcLeu, and 7G3-ChAcDap (ChAcLeu and ChAcDap enable endosome escape—but with less efficacity—without nuclear trafficking, and possess, respectively, structural and net charge similarities to ChAcNLS). Indeed, confocal microscopy revealed a distinctive cellular distribution pattern for 7G3-ChAcNLS, which was centered and homogenously radiating throughout the target cell, suggesting intracellular antibody retention [12,13]. Contrarily, 7G3- and 7G3-NLS-treated cells both showed similar distribution patterns with clusters proximal to the intracellular surface of the plasma membrane, typical of endosome entrapment [12]. On the other hand, 7G3-ChAc was detected in the cytoplasm with a homogeneous distribution throughout, but not in the nucleus. In quantitative analyses, flow cytometry showed significantly higher intracellular accumulation of 7G3-ChAcNLS compared to all controls [12]. Intracellular accumulation levels compared to unmodified 7G3 were significantly higher for 7G3-ChAcNLS (3-fold) and 7G3-ChAc (1.8-fold), whereas no significant change was detected for 7G3-NLS. The higher intracellular accumulation of 7G3-ChAcNLS compared to 7G3-ChAc, 7G3-ChAcLeu, and 7G3-ChAcDap revealed that the combination of the ChAc moiety with the NLS moiety is responsible for the endosome escape and intracellular accumulation of ChAcNLS [12]. In fact, if ChAc had only endosome escape activity and NLS only nuclear trafficking activity, the level of intracellular accumulation should have been the same as 7G3-ChAc, 7G3-ChAcLeu, or 7G3-ChAcDap [12]. To further evaluate target cell specificity, intracellular and nuclear accumulation of 7G3-ChAcNLS versus unmodified 7G3 were imaged with confocal microscopy in IL-3Rα-positive and -negative cells. The data revealed that ChAcNLS modification resulted in ample specific accumulation of 7G3 in target cells and nuclei, with a margin of 10–13% nonspecific localization. Similarly, the authors used flow cytometry, western blot, and radioactivity assays to demonstrate that Accum could enhance the intracellular and nuclear accumulation of its conjugate by a margin of 21.1-fold [12,13]. These data showed that the complementary functions of the Accum moieties—ChAc and NLS—are equally indispensable for maintaining effective intracellular conjugate accumulation and target cell selectivity.
3.2. A14-Accum
Paquette et al. [11] have previously shown that the rapid internalization of IL-5Rα, whose levels are preferentially elevated in MIBC tumors, renders the use of ADCs with specificity for IL-5Rα strategic for the intracellular accumulation of payloads, including the positron emitter copper-64 (64Cu) used for imaging. Therefrom, the same group [14] designed A14-Accum, an Accum-modified mAb against IL-5Rα, to characterize its use as a tumor cell accumulator. To further assess its use as an efficient drug delivery system, A14-Accum was coupled to the radioactive diagnostic agent 64Cu [13]. 64Cu-A14-Accum indeed revealed a purity of preparation within the 95–99% range and had an affinity for IL-5Rα in the nanomolar spectrum [14]. Unpublished data also revealed that Accum augmented the kinetics of receptor internalization. When incubated with MIBC cell lines and assessed with flow cytometry (for quantifying ceramide levels) and confocal microscopy (for visualizing endosome disruption and quantifying nuclear localization), A14-ChAcNLS demonstrated marked endosome escape with nuclear routing compared to A14 [14]. In radioactivity assays investigating cargo deposition, A14-ChAcNLS significantly increased the intracellular accumulation of 64Cu cargo by 3.3–9.4- and 3.2–4.6-fold compared to A14 and A14-NLS, respectively, in high- and low-density IL-5Rα-expressing MIBC cell lines [14]. In the same cell lines, A14-ChAcNLS also increased the nuclear accumulation of 64Cu cargo by 1.7–5.3- and 2.5–2.6-fold compared to A14 and A14-NLS, respectively. When target selectivity was analyzed against an IgG-ChAcNLS control showed, A14-ChAcNLS showed only trace non-specificity for intracellular 64Cu cargo accumulation [14]. Beaudoin et al. [13] also showed that Accum resulted in significant intracellular and nuclear specificity and accumulation (≥3-fold) of 64Cu in IL-5Rα-positive invasive bladder cancer cells. These in vitro data demonstrated that both Accum moieties are necessary for increasing the intracellular accumulation of cargo-bearing conjugates and maintaining target specificity.
Paquette and colleagues [14] further characterized the pharmacokinetic (PK) profile of 64Cu-A14-ChAcNLS injected into mouse models of human high- or low-density IL-5Rα MIBC. Blood sampling revealed an estimated half-life (t1/2) of 54.4 h, and a study of the organs indicated major hepatic metabolism and clearance with early transient glomerular accumulation. In comparative analyses, the t1/2 of 64Cu-A14-ChAcNLS was 45% lower compared to 64Cu-A14 (t1/2 = 99.5 h), indicating increased blood clearance. Nonetheless, ChAcNLS maintained the levels of 64Cu-A14 tumor uptake. Moreover, organ dissection 96 h post-injection showed that the biodistribution of 64Cu-A14-ChAcNLS was reduced in most healthy organs compared to 64Cu-A14 with significant reductions in the liver and heart [14]. Biodistribution of 64Cu-A14-ChAcNLS was also visualized with positron emission tomography (PET) and revealed equivalent tumor uptake with a higher tumor/adjacent muscle uptake ratio compared to 64Cu-A14 [13,14]. These preclinical PK and PET analyses demonstrate that Accum technology provides ACs with (i) comparable high rates of tumor uptake, (ii) elevated intracellular accumulation, and (iii) intermediate clearance rates from healthy tissues, for an overall improvement in selective tumor targeting.
3.3. T-DM1-Accum
T-DM1 clinically hinders breast cancer growth, yet several patients still experience disease progression [48] due to insufficient intracellular accumulation [17,49]. Lacasse et al. [10] thus investigated whether Accum-modification enhanced intracellular accumulation of T-DM1 by using the human epidermal growth factor receptor 2 (HER2)-positive breast cancer cell line SKBR3. Confocal microscopy with temporal imaging revealed that T-DM1-Accum first undergoes endosome entrapment, then diffuses throughout the cytoplasm before slowly localizing in the nucleus, where it is proteolyzed and T-DM1 is released [10]. In subsequent analyses, nuclear transport of T-DM1-Accum was shown to be mediated by the nuclear transport receptor importin-7 (IPO7) based on electrostatic interactions fueled by the cationic charge build-up on NLS moieties [10]. Contrarily, unmodified T-DM1 localized near the plasma membrane [10] due to the rapid recycling of HER2 back to the cell surface of SKBR3 [17,50,51]. Additionally, T-DM1-Accum had stronger cytotoxic potency compared to T-DM1-NLS and unmodified T-DM1 by several-fold. Importantly, the authors showed that the specificity of T-DM1 for HER2 was not altered by Accum modification, and Accum did not perturb HER2 binding and its internalization processes [10]. These data validated that Accum modifications prime the cytotoxicity of molecular payloads by overriding endosome entrapment and directing nuclear localization, all while retaining target cell specificity. Next, the same group [10] investigated the therapeutic potency of T-DM1-Accum in HER2-positive SKBR3 cells. T-DM1-Accum demonstrated marked cytotoxicity, achieving 50% tumor growth inhibition at concentrations >9- and >3.2-fold lower compared to T-DM1 and T-DM1-NLS, respectively [10]. In gene knockdown studies, this cytotoxicity relied on IPO7-mediated nuclear transport, likely driven by electrostatic interactions between the NLS moiety of Accum (positive charge) and IPO7 nuclear receptor (negative charge) [10]. Notably, T-DM1-NLS demonstrated >1.8-fold more cytotoxicity compared to T-DM1, and Accum was not cytotoxic itself, but rather enhanced the cytotoxicity of T-DM1, since Trastuzumab (Tmab)-Accum was as equally non-toxic as Tmab. Overall, these data corroborate that both Accum moieties operate with a functional synergism that results in enhanced cellular accumulation and therapeutic efficacy [10].
3.4. OVA- and Lymphoma Lysate Proteins-Accum
Antigen presenting cells such as DCs engulf soluble antigens and sort them into endosomes for limited degradation before exportation to the cytosol [52]. Therein, the proteasome further processes antigen fragments into short amino acid sequences that are presented on the cell surface by Major Histocompatibility Complex (MHC) class I molecules for T-cell activation, a process termed cross-presentation [53,54]. However, non-specific endosomal degradation of antigens in certain subsets of DCs, such as monocyte-derived CD8− DCs, can compromise antigen immunogenicity and reduce anti-tumoral immunity [55]. Drawing on Accum’s innovative mechanism of action validated with 7G3 [12], A14 [14], and T-DM1 [10], Bikorimana and colleagues [15] conjugated Accum to soluble antigens to investigate whether its ADC-functionalizing biotechnology could prime the antigen cross-presentation properties of CD8− DCs. Accum modification increased the resistance of OVA to thermal denaturation [15]. Unlike OVA, OVA-Accum uptake by DCs induced endosome rupture, as revealed by confocal microscopy. Using primary bone-marrow derived DCs, it was further shown that OVA-Accum was processed in the cytosol, as per flow cytometry analysis [15]. Complementary antigen presentation assays using primary DCs co-cultured with T cells also showed that OVA-Accum induced marked immune cell activation, resulting in heightened inflammatory cytokine secretion [15]. Similarly, Accum-modified proteins derived from lymphoma lysate significantly enhanced the immunogenicity of allogeneic DCs in tumor-bearing mice [15]. Combined, these data validate the peculiarity of endosome-to-cytosol translocation, where Accum enables antigens to escape endosomal damage, undergo efficient proteasomal processing into immunogenic peptides, and arrive at the cell surface for potent antigen cross-presentation and subsequent T-cell activation.
Deploying these proof-of-principle data, Bikorimana and colleagues investigated whether the enhanced immunogenicity of OVA-Accum in DCs translates to a potent cellular vaccine [15]. In the context of prophylactic vaccination, two doses of OVA-Accum-pulsed primary DCs were administered subcutaneously 2 weeks apart in mice challenged thereafter with three ascending doses of an OVA-expressing T-cell lymphoma. Compared to OVA-pulsed DCs, OVA-Accum-pulsed DCs completely counteracted tumor growth, maintaining full and durable survival of all mice at 96 days post-immunization. Concomitantly, serum analysis showed higher antibody titers, and immunophenotyping revealed markedly higher levels of CD4 effector and CD8 central and effector memory T cells. Pro-inflammatory mediators were also more heightened [15]. As for therapeutic vaccination, lymphoma-bearing mice were administered two subcutaneous injections of OVA-Accum-pulsed syngeneic or allogeneic DCs one week apart. Compared to OVA-pulsed DCs, OVA-Accum-pulsed DCs delayed tumor growth and prolonged survival. Co-administration of the immune-checkpoint inhibitor anti-PD-1 over 2 weeks further enhanced these outcomes, producing at 54 days post-immunization 50% overall survival (OS), 30–40% partial response, and 10–20% complete response (CR) [15]. Allogeneic DCs pulsed with Accum-modified non-OVA-expressing lymphoma lysate proteins were also examined as a broader-spectrum vaccine for therapeutic efficacy. In lymphoma-bearing mice, there was only a minor delay in tumor growth. However, combined with anti-PD-1, this treatment resulted in marked tumor growth delays resulting in 70% OS and 30% CR compared to standard lysate-pulsed DCs co-administered with anti-PD-1 [15]. With Accum-lysate-pulsed DCs/anti-PD-1, immunophenotyping also showed elevated levels of immune effector cells and diminished levels of regulatory CD4 T cells [15].
Interestingly, the authors went beyond cellular vaccination to assess Accum for its benefits in protein vaccination for prophylaxis. Indeed, two doses of OVA-Accum were administered subcutaneously 2 weeks apart in mice challenged thereafter with an OVA-expressing T-cell lymphoma. The authors found that OVA-Accum injections significantly delayed tumor growth, maintaining 30% animal survival beyond 1 month post-immunization [15]. The addition of adjuvants to the OVA-Accum formulation further elevated anti-tumor immunity, producing up to 100% survival at 42 days post-immunization. Concurrently, serum analysis showed significantly higher anti-OVA IgG antibody titers [15].
Overall, these animal experiments showed that Accum-modification of antigens generates prophylactically and therapeutically potent cellular and protein antigen vaccines for cancer treatment.
4. Discussion
Accum presents advantages over other conjugable primers, including NLS, TPP-9476, CPPs, and targeting peptides.
When coupled to 7G3-NLS, the Auger electron-emitting indium-111 (111In) did not induce marked cytotoxicity compared to 111In-7G3 that was likely due to meager nuclear localization [35,36,37,56]. Contrarily, ChAcNLS increased intracellular accumulation of 7G3 by factors of 4.1–21.1 due to the combined properties of endosome escape and nuclear localization with a matching increase in intracellularly deposited 64Cu radioactivity [12]. A report has previously shown that the internalization of TPP-9476, an ADC targeting IL-3Rα, was 3.5-fold higher compared to isotype mAb [57]. Our data show that internalization is 8.6-fold higher for 7G3-Accum compared to isotype mAb and up to 9.4-fold higher for A14-Accum compared to A14 [14].
Additionally, the peak of accumulation for ADCs functionalized with Accum [12] has either exceeded [58] or shown to be comparable to [25] that for ADCs functionalized with the trans-activator of transcription (TAT) sequence [58] and the membrane transport sequence (MTS) [25], respectively. TAT and MTS are both examples of CPPs, short sequences of natural or synthetic origin that have been preclinically evaluated for cancer diagnosis and therapy due to their ability to translocate cargo complexes across the plasma membrane via direct translocation or endocytosis [59,60,61]. Despite being numerous and showing promise for delivering different types of drugs, CPPs have yet to attain regulatory approvals due, in part, to pharmacokinetic handicaps hindering translation into clinical settings [62]. For instance, TAT-coupled ADCs have failed to achieve adequate tumor uptake in vivo due to rapid washout and sequestration in the spleen and liver preventing sufficient biodistribution [27,28,47]. Other CPPs have also been degraded in circulation before reaching their target [63]. With Accum on the other hand, 64Cu-A14 has shown intermediate clearance from blood and healthy tissues with good tumor uptake [13,14]. Other clinical limitations for CPPs, which appear to be forestalled by Accum, include non-target tissue uptake and subsequent systemic toxicity [23,24,25,26,27,28,64,65], low specificity [62], and immunogenicity [66]. It has also been shown that CPPs undergo endosomal entrapment after translocating into the cytosol [67,68], which ultimately compromises the function of coupled drugs due to inevitable targeting to either the lysosomes for degradation or the plasma membrane for recycling and exportation [69,70]; therefore, effective endosome escape and homing into specific cellular organelles have since been sought to achieve therapeutic efficacy [71,72,73].
Besides CPPs, other targeting peptides, such as p28, TCP-1, and LyP-1, rely on receptor recognition to deliver therapeutic payloads [74]. p28 is an amino acid that enables the copper-containing redox protein azurin to target cancer cells, eventually inducing cell cycle arrest [75]. In tumor-bearing subjects, it has been shown that p28 induces 6.6% CR, 20% PR, and 20% OS [76]. In tumor-bearing mice, we have shown that an Accum-OVA vaccine maintains 30% OS alone and 100% OS in combination with adjuvants. Combined with anti-PD-1, Accum-DC vaccine also induced 10–20% CR, 30–40% PR, and 50% OS [15]. TCP-1, a peptide targeting the vasculature of gastric cancer cells, was conjugated to tumor necrosis factor α (TNFα) to deliver the anticancer agent 5-fluorouracil (5-FU). The results showed that TCP-1 modification did not enhance the bioactivity of TNFα because no differences in 5-FU-induced cancer cell death were detected [77]. Tumor volume also did not significantly change [77]. Accum-modification, however, was reported to enhance the bioactivity of T-DM1, markedly increasing its cytotoxic potency [10]. The tumor homing peptide LyP-1 was conjugated to abraxane, a clinically approved paclitaxel-albumin nanoparticle, then administered to mice bearing human cancer xenografts. The data showed statistically significant results with a 1.38-fold increase in cytotoxicity [78]. With Accum, however, the cytotoxicity of the clinically approved T-DM1 was enhanced by several fold [10].
Furthermore, NLS-modified ADCs, such the epidermal growth factor receptor-positive tumor-targeting 111In-nimotuzumab-NLS, are infamous for their rapid plasma clearance and increased sequestration in normal tissues, which translate to reduced tumor accumulation [31,79]. However, Accum demonstrates that the combination of ChAc with NLS delays the sequestration of ADCs in healthy tissues, thereby permitting targeted and good tumor uptake relative to unmodified ADCs [13,14]. Our data on T-DM1-Accum further provide strong evidence that Accum can prime the function of tumor-targeting ADCs by evading the resistance mechanisms that affect Tmab, T-DM1, and other biopharmaceuticals [49,80,81,82]. On another note, given that ChAc has been previously conjugated to liver-targeting drugs for enhanced delivery [83,84], ChAcNLS could similarly be exploited in targeted cancer therapy to decrease cellular exportation of drugs and promote intracellular retention. Other applications for ChAcNLS-modified ADCs could include phenotype-specific tumor imaging and chemotherapeutic drug transport to tumors to achieve more specific uptake and higher therapeutic efficacy.
With Accum-modified antigens [15], endosome-to-cytosol translocation was suggested to be crucial for antigen cross-presentation, a property unattained by certain tumor associated antigen-based DC vaccines due to endosome entrapment that failed to elicit sufficient T-cell responses [85,86]. Our in vivo experiments with Accum-based vaccines also convey the potential for therapeutic application of i) Accum-linked antigens with add-on checkpoint blockers and ii) Accum-linked tumor lysate proteins, which permit the development of personalized cancer vaccines without requiring specific epitope identification.
Per scientific integrity, we stand aware of the small yet growing number of published reports investigating Accum as a conjugable primer [10,11,12,13,14,15] and the conclusion biases that might arise thereof. However, the uniqueness of this biotechnology merits early communication to the research community as it continues to unfold with further pre-clinical studies underway.
5. Conclusions
Accum is a novel biotechnology harnessing the properties of two moieties for enhanced intracellular accumulation. By promoting endosome escape and nuclear routing, Accum has been demonstrated to make for an effective conjugation moiety that functionalizes different molecular conjugates. When coupled to ADCs, Accum maintains target cell selectivity, shows high tumor uptake rate with intermediate clearance rates from healthy tissues, and enhances tumor cytotoxicity while minimizing off-target effects. When coupled to antigens, Accum triggers durable immunogenicity in mice and has proven strategic for creating prophylactic and therapeutic anti-cancer cellular and protein vaccines. As its properties continue to emerge with further studies for planification and execution phases, Accum is a promising candidate for clinical investigations in onco-immunotherapy settings.
6. Patents
The Accum™ technology is protected by 10 different patents (US 63/127,731, US 63/202,047, PCT/CA2021/051543, US 17/516,161, US 63/201,620, US 63/260,648, US 63/264,126, US 63/265,125, US 16/085,141 and US 63/256,726).
Author Contributions
Original draft preparation, A.E.-H.E.-K.; writing—review, S.B. and S.P.; writing—review and editing, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank J.-P. Bikorimana for his critical assessment of the review.
Conflicts of Interest
S.B. and S.P. are currently employed by Defence Therapeutics and declare competing financial interest. S.B. and S.P. had a role in the review of the manuscript.
Sample Availability
Samples of the compounds are not available from the authors.
Abbreviations
| 5-FU | 5-fluorouracil |
| 64Cu | copper-64 |
| 111In | indium-111 |
| ADC(s) | antibody-drug conjugate(s) |
| CARs | chimeric antigen receptors |
| ChAc | cholic acid |
| cNLS | classical nuclear localization signal |
| CPP(s) | cell-penetrating peptide(s) |
| CR | complete response |
| DCs | dendritic cells |
| HER2 | human epidermal growth factor receptor 2 |
| IL-3Rα | interleukin-3 receptor-α |
| IL-5Rα | interleukin-5 receptor-α |
| IPO7 | importin-7 |
| mAb(s) | monoclonal antibodies |
| MHC | Major Histocompatibility Complex |
| MIBC | muscle invasive bladder cancer |
| NLS | nuclear localization signal |
| OS | overall survival |
| OVA | ovalbumin |
| PET | positron emission tomography |
| PK | pharmacokinetic |
| Sulfo-SMCC | sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate |
| SV-40 | simian virus 40 |
| T1/2 | half-life |
| T-DM1 | trastuzumab-emtansine |
| Tmab | Trastuzumab |
| TNFα | tumor necrosis factor α |
References
- Biotechnology Instruments Market Report, 2021–2028; Grand View Research, Inc.: San Francisco, CA, USA, 2021; p. 227.
- Biologics Contract Development Market Report, 2020–2027; Grand View Research, Inc.: San Francisco, CA, USA, 2020; p. 144.
- NCI Immunotherapy to Treat Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy (accessed on 1 January 2022).
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Nguyen, T.T.; Tien, N.L.B.; Tran, D.K.; Jeong, J.H.; Anh, P.G.; Thanh, V.V.; Truong, D.T.; Dinh, T.C. Recent Progress of Stem Cell Therapy in Cancer Treatment: Molecular Mechanisms and Potential Applications. Cells 2020, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Hu, Q.; Kasagi, Y.; Oki, E.; Mori, M. Recent developments in cancer research: Expectations for a new remedy. Ann. Gastroenterol. Surg. 2021, 5, 419–426. [Google Scholar] [CrossRef]
- El-Kadiry, A.E.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Lacasse, V.; Beaudoin, S.; Jean, S.; Leyton, J.V. A Novel Proteomic Method Reveals NLS Tagging of T-DM1 Contravenes Classical Nuclear Transport in a Model of HER2-Positive Breast Cancer. Mol. Ther Methods Clin. Dev. 2020, 19, 99–119. [Google Scholar] [CrossRef]
- Paquette, M.; Vilera-Perez, L.G.; Beaudoin, S.; Ekindi-Ndongo, N.; Boudreaut, P.L.; Bonin, M.A.; Battista, M.C.; Bentourkia, M.; Lopez, A.F.; Lecomte, R.; et al. Targeting IL-5Ralpha with antibody-conjugates reveals a strategy for imaging and therapy for invasive bladder cancer. Oncoimmunology 2017, 6, e1331195. [Google Scholar] [CrossRef]
- Beaudoin, S.; Rondeau, A.; Martel, O.; Bonin, M.A.; van Lier, J.E.; Leyton, J.V. ChAcNLS, a Novel Modification to Antibody-Conjugates Permitting Target Cell-Specific Endosomal Escape, Localization to the Nucleus, and Enhanced Total Intracellular Accumulation. Mol. Pharm. 2016, 13, 1915–1926. [Google Scholar] [CrossRef]
- Beaudoin, S.; Paquette, M.; Fafard-Couture, L.; Tremblay, M.A.; Lecomte, R.; Guerin, B.; Leyton, J.V. Initial Evaluation of Antibody-Conjugates Modified with Viral-Derived Peptides for Increasing Cellular Accumulation and Improving Tumor Targeting. J. Vis. Exp. 2018, 133, e55440. [Google Scholar] [CrossRef]
- Paquette, M.; Beaudoin, S.; Tremblay, M.A.; Jean, S.; Lopez, A.F.; Lecomte, R.; Guerin, B.; Bentourkia, M.; Sabbagh, R.; Leyton, J.V. NLS-Cholic Acid Conjugation to IL-5Ralpha-Specific Antibody Improves Cellular Accumulation and In Vivo Tumor-Targeting Properties in a Bladder Cancer Model. Bioconjugate Chem. 2018, 29, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Bikorimana, J.-P.; Salame, N.; Beaudoin, S.; Balood, M.; Crosson, T.; Abusarah, J.; Talbot, S.; Löbenberg, R.; Plouffe, S.; Rafei, M. Promoting antigen escape from dendritic cell endosomes potentiates anti-tumoral immunity. Cell Rep. Med. 2022, 3, 100534. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.D.; De Maziere, A.M.; Pisacane, P.I.; van Dijk, S.M.; Eigenbrot, C.; Sliwkowski, M.X.; Klumperman, J.; Scheller, R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 2004, 15, 5268–5282. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Chari, R.V. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Mach, J.P.; Carrel, S.; Forni, M.; Ritschard, J.; Donath, A.; Alberto, P. Tumor localization of radiolabeled antibodies against carcinoembryonic antigen in patients with carcinoma: A critical evaluation. N. Engl. J. Med. 1980, 303, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Thurber, G.M.; Dane Wittrup, K. A mechanistic compartmental model for total antibody uptake in tumors. J. Theor. Biol. 2012, 314, 57–68. [Google Scholar] [CrossRef]
- Donaghy, H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs 2016, 8, 659–671. [Google Scholar] [CrossRef]
- Saber, H.; Leighton, J.K. An FDA oncology analysis of antibody-drug conjugates. Regul. Toxicol. Pharmacol. 2015, 71, 444–452. [Google Scholar] [CrossRef]
- Kameyama, S.; Horie, M.; Kikuchi, T.; Omura, T.; Takeuchi, T.; Nakase, I.; Sugiura, Y.; Futaki, S. Effects of cell-permeating peptide binding on the distribution of 125I-labeled Fab fragment in rats. Bioconjugate Chem. 2006, 17, 597–602. [Google Scholar] [CrossRef]
- Kameyama, S.; Okada, R.; Kikuchi, T.; Omura, T.; Nakase, I.; Takeuchi, T.; Sugiura, Y.; Futaki, S. Distribution of immunoglobulin Fab fragment conjugated with HIV-1 REV peptide following intravenous administration in rats. Mol. Pharm. 2006, 3, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lou, D.; Burkett, J.; Kohler, H. Chemical engineering of cell penetrating antibodies. J. Immunol. Methods 2001, 254, 137–145. [Google Scholar] [CrossRef]
- Anderson, D.C.; Nichols, E.; Manger, R.; Woodle, D.; Barry, M.; Fritzberg, A.R. Tumor cell retention of antibody Fab fragments is enhanced by an attached HIV TAT protein-derived peptide. Biochem. Biophys. Res. Commun. 1993, 194, 876–884. [Google Scholar] [CrossRef]
- Cornelissen, B.; Hu, M.; McLarty, K.; Costantini, D.; Reilly, R.M. Cellular penetration and nuclear importation properties of 111In-labeled and 123I-labeled HIV-1 tat peptide immunoconjugates in BT-474 human breast cancer cells. Nucl. Med. Biol. 2007, 34, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, P.; Wang, J.; Chan, C.; Scollard, D.A.; Reilly, R.M. Site-specific conjugation of HIV-1 tat peptides to IgG: A potential route to construct radioimmunoconjugates for targeting intracellular and nuclear epitopes in cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 301–310. [Google Scholar] [CrossRef]
- Berguig, G.Y.; Convertine, A.J.; Shi, J.; Palanca-Wessels, M.C.; Duvall, C.L.; Pun, S.H.; Press, O.W.; Stayton, P.S. Intracellular delivery and trafficking dynamics of a lymphoma-targeting antibody-polymer conjugate. Mol. Pharm. 2012, 9, 3506–3514. [Google Scholar] [CrossRef]
- Lackey, C.A.; Press, O.W.; Hoffman, A.S.; Stayton, P.S. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjugate Chem. 2002, 13, 996–1001. [Google Scholar] [CrossRef]
- Fasih, A.; Fonge, H.; Cai, Z.; Leyton, J.V.; Tikhomirov, I.; Done, S.J.; Reilly, R.M. 111In-Bn-DTPA-nimotuzumab with/without modification with nuclear translocation sequence (NLS) peptides: An Auger electron-emitting radioimmunotherapeutic agent for EGFR-positive and trastuzumab (Herceptin)-resistant breast cancer. Breast Cancer Res. Treat. 2012, 135, 189–200. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J.; Hope, K.; Jin, L.; Dick, J.; Cameron, R.; Brandwein, J.; Minden, M.; Reilly, R.M. Nuclear localizing sequences promote nuclear translocation and enhance the radiotoxicity of the anti-CD33 monoclonal antibody HuM195 labeled with 111In in human myeloid leukemia cells. J. Nucl. Med. 2006, 47, 827–836. [Google Scholar]
- Marks, A.J.; Cooper, M.S.; Anderson, R.J.; Orchard, K.H.; Hale, G.; North, J.M.; Ganeshaguru, K.; Steele, A.J.; Mehta, A.B.; Lowdell, M.W.; et al. Selective apoptotic killing of malignant hemopoietic cells by antibody-targeted delivery of an amphipathic peptide. Cancer Res. 2005, 65, 2373–2377. [Google Scholar] [CrossRef][Green Version]
- Sturzu, A.; Regenbogen, M.; Klose, U.; Echner, H.; Gharabaghi, A.; Heckl, S. Novel dual labelled nucleus-directed conjugates containing correct and mutant nuclear localisation sequences. Eur. J. Pharm. Sci. 2008, 33, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.V.; Gao, C.; Williams, B.; Keating, A.; Minden, M.; Reilly, R.M. A radiolabeled antibody targeting CD123(+) leukemia stem cells-initial radioimmunotherapy studies in NOD/SCID mice engrafted with primary human AML. Leuk Res. Rep. 2015, 4, 55–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leyton, J.V.; Hu, M.; Gao, C.; Turner, P.V.; Dick, J.E.; Minden, M.; Reilly, R.M. Auger electron radioimmunotherapeutic agent specific for the CD123+/CD131− phenotype of the leukemia stem cell population. J. Nucl. Med. 2011, 52, 1465–1473. [Google Scholar] [CrossRef]
- Zereshkian, A.; Leyton, J.V.; Cai, Z.; Bergstrom, D.; Weinfeld, M.; Reilly, R.M. The human polynucleotide kinase/phosphatase (hPNKP) inhibitor A12B4C3 radiosensitizes human myeloid leukemia cells to Auger electron-emitting anti-CD123 111In-NLS-7G3 radioimmunoconjugates. Nucl Med. Biol 2014, 41, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, V.; Kim, Y.; Chang, K.O. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology 2014, 456–457, 268–278. [Google Scholar] [CrossRef]
- Basanez, G.; Ruiz-Arguello, M.B.; Alonso, A.; Goni, F.M.; Karlsson, G.; Edwards, K. Morphological changes induced by phospholipase C and by sphingomyelinase on large unilamellar vesicles: A cryo-transmission electron microscopy study of liposome fusion. Biophys. J. 1997, 72, 2630–2637. [Google Scholar] [CrossRef][Green Version]
- Gulbins, E.; Dreschers, S.; Wilker, B.; Grassme, H. Ceramide, membrane rafts and infections. J. Mol. Med. 2004, 82, 357–363. [Google Scholar] [CrossRef]
- Montes, L.R.; Ruiz-Arguello, M.B.; Goni, F.M.; Alonso, A. Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol. Chem. 2002, 277, 11788–11794. [Google Scholar] [CrossRef]
- Siskind, L.J.; Colombini, M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 2000, 275, 38640–38644. [Google Scholar] [CrossRef]
- Leyton, J.V.; Williams, B.; Gao, C.; Keating, A.; Minden, M.; Reilly, R.M. MicroSPECT/CT imaging of primary human AML engrafted into the bone marrow and spleen of NOD/SCID mice using 111In-DTPA-NLS-CSL360 radioimmunoconjugates recognizing the CD123+/CD131− epitope expressed by leukemia stem cells. Leuk Res. 2014, 38, 1367–1373. [Google Scholar] [CrossRef]
- Sarko, D.; Beijer, B.; Garcia Boy, R.; Nothelfer, E.M.; Leotta, K.; Eisenhut, M.; Altmann, A.; Haberkorn, U.; Mier, W. The pharmacokinetics of cell-penetrating peptides. Mol. Pharm. 2010, 7, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hou, J.; Newman, E.; Kim, Y.; Donohue, C.; Liu, X.; Thomas, S.H.; Forman, S.J.; Kane, S.E. CD30 Downregulation, MMAE Resistance, and MDR1 Upregulation Are All Associated with Resistance to Brentuximab Vedotin. Mol. Cancer Ther. 2015, 14, 1376–1384. [Google Scholar] [CrossRef]
- Tolstikov, V.V.; Cole, R.; Fang, H.; Pincus, S.H. Influence of endosome-destabilizing peptides on efficacy of anti-HIV immunotoxins. Bioconjugate Chem. 1997, 8, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R.; et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-DM1). Mol. Cancer Ther. 2018, 17, 243–253. [Google Scholar] [CrossRef]
- Hommelgaard, A.M.; Lerdrup, M.; van Deurs, B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol. Biol. Cell 2004, 15, 1557–1567. [Google Scholar] [CrossRef]
- Longva, K.E.; Pedersen, N.M.; Haslekas, C.; Stang, E.; Madshus, I.H. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int. J. Cancer 2005, 116, 359–367. [Google Scholar] [CrossRef]
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976, 143, 1283–1288. [Google Scholar] [CrossRef]
- Carbone, F.R.; Heath, W.R. Cross-priming: Its beginnings. J. Immunol 2010, 185, 1353–1354. [Google Scholar] [CrossRef]
- Trombetta, E.S.; Ebersold, M.; Garrett, W.; Pypaert, M.; Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 2003, 299, 1400–1403. [Google Scholar] [CrossRef]
- Savina, A.; Peres, A.; Cebrian, I.; Carmo, N.; Moita, C.; Hacohen, N.; Moita, L.F.; Amigorena, S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009, 30, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. The amazing world of auger electrons. Int. J. Radiat. Biol. 2004, 80, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, D.; Stelte-Ludwig, B.; Lerchen, H.G.; Wengner, A.M.; Ahsen, O.V.; Buchmann, P.; Marsch, S.; Mahlert, C.; Greven, S.; Dietz, L.; et al. IL3RA-Targeting Antibody-Drug Conjugate BAY-943 with a Kinesin Spindle Protein Inhibitor Payload Shows Efficacy in Preclinical Models of Hematologic Malignancies. Cancers 2020, 12, 3464. [Google Scholar] [CrossRef] [PubMed]
- Mie, M.; Takahashi, F.; Funabashi, H.; Yanagida, Y.; Aizawa, M.; Kobatake, E. Intracellular delivery of antibodies using TAT fusion protein A. Biochem. Biophys. Res. Commun. 2003, 310, 730–734. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Gupta, B.; Levchenko, T.S.; Torchilin, V.P. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv. Drug Deliv. Rev. 2005, 57, 637–651. [Google Scholar] [CrossRef]
- Tripathi, P.P.; Arami, H.; Banga, I.; Gupta, J.; Gandhi, S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 2018, 9, 37252–37267. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Pujals, S.; Giralt, E. Proline-rich, amphipathic cell-penetrating peptides. Adv. Drug Deliv. Rev. 2008, 60, 473–484. [Google Scholar] [CrossRef]
- Aguilera, T.A.; Olson, E.S.; Timmers, M.M.; Jiang, T.; Tsien, R.Y. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr. Biol. 2009, 1, 371–381. [Google Scholar] [CrossRef]
- Moutal, A.; Francois-Moutal, L.; Brittain, J.M.; Khanna, M.; Khanna, R. Differential neuroprotective potential of CRMP2 peptide aptamers conjugated to cationic, hydrophobic, and amphipathic cell penetrating peptides. Front. Cell. Neurosci. 2014, 8, 471. [Google Scholar] [CrossRef]
- Jarver, P.; Mager, I.; Langel, U. In vivo biodistribution and efficacy of peptide mediated delivery. Trends Pharmacol. Sci. 2010, 31, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Zaitseva, E.; Chernomordik, L.V.; Melikov, K. Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophys. J. 2010, 99, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Futaki, S.; Harashima, H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 2009, 11, 13–22. [Google Scholar] [CrossRef]
- Richard, J.P.; Melikov, K.; Vives, E.; Ramos, C.; Verbeure, B.; Gait, M.J.; Chernomordik, L.V.; Lebleu, B. Cell-penetrating peptides. A reevaluation of the mechanism of cel.l.llular uptake. J. Biol. Chem. 2003, 278, 585–590. [Google Scholar] [CrossRef]
- LeCher, J.C.; Nowak, S.J.; McMurry, J.L. Breaking in and busting out: Cell-penetrating peptides and the endosomal escape problem. Biomol. Concepts 2017, 8, 131–141. [Google Scholar] [CrossRef]
- Erazo-Oliveras, A.; Muthukrishnan, N.; Baker, R.; Wang, T.Y.; Pellois, J.P. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals 2012, 5, 1177–1209. [Google Scholar] [CrossRef]
- Horton, K.L.; Stewart, K.M.; Fonseca, S.B.; Guo, Q.; Kelley, S.O. Mitochondria-penetrating peptides. Chem. Biol. 2008, 15, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef]
- Timur, S.S.; Gursoy, R.N. The role of peptide-based therapeutics in oncotherapy. J. Drug Target. 2021, 29, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Mehta, R.R.; Lekmine, F.; Christov, K.; King, M.L.; Majumdar, D.; Shilkaitis, A.; Green, A.; Bratescu, L.; Beattie, C.W.; et al. A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol. Cancer Ther. 2009, 8, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Warso, M.A.; Richards, J.M.; Mehta, D.; Christov, K.; Schaeffer, C.; Rae Bressler, L.; Yamada, T.; Majumdar, D.; Kennedy, S.A.; Beattie, C.W.; et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br. J. Cancer 2013, 108, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, Z.J.; Li, L.F.; Shen, J.; Zhang, L.; Li, M.X.; Xiao, Z.G.; Wang, J.H.; Cho, C.H. A novel vascular-targeting peptide for gastric cancer delivers low-dose TNFalpha to normalize the blood vessels and improve the anti-cancer efficiency of 5-fluorouracil. Peptides 2017, 97, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Kotamraju, V.R.; Kastantin, M.; Black, M.; Missirlis, D.; Tirrell, M.; Ruoslahti, E. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine 2009, 5, 73–82. [Google Scholar] [CrossRef]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.P.; Fielder, P.J.; Khawli, L.A. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjugate Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef]
- Liu, W.; Chang, J.; Liu, M.; Yuan, J.; Zhang, J.; Qin, J.; Xia, X.; Wang, Y. Quantitative proteomics profiling reveals activation of mTOR pathway in trastuzumab resistance. Oncotarget 2017, 8, 45793–45806. [Google Scholar] [CrossRef]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor cells chronically treated with a trastuzumab-maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef]
- Ramello, M.C.; Benzaid, I.; Kuenzi, B.M.; Lienlaf-Moreno, M.; Kandell, W.M.; Santiago, D.N.; Pabon-Saldana, M.; Darville, L.; Fang, B.; Rix, U.; et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci Signal. 2019, 12, eaap9777. [Google Scholar] [CrossRef]
- Dong, Z.; Li, Q.; Guo, D.; Shu, Y.; Polli, J.E. Synthesis and Evaluation of Bile Acid-Ribavirin Conjugates as Prodrugs to Target the Liver. J. Pharm. Sci. 2015, 104, 2864–2876. [Google Scholar] [CrossRef]
- Kramer, W.; Wess, G.; Schubert, G.; Bickel, M.; Girbig, F.; Gutjahr, U.; Kowalewski, S.; Baringhaus, K.H.; Enhsen, A.; Glombik, H.; et al. Liver-specific drug targeting by coupling to bile acids. J. Biol. Chem. 1992, 267, 18598–18604. [Google Scholar] [CrossRef]
- Hiltbold, E.M.; Alter, M.D.; Ciborowski, P.; Finn, O.J. Presentation of MUC1 tumor antigen by class I MHC and CTL function correlate with the glycosylation state of the protein taken Up by dendritic cells. Cell Immunol. 1999, 194, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hiltbold, E.M.; Vlad, A.M.; Ciborowski, P.; Watkins, S.C.; Finn, O.J. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J. Immunol. 2000, 165, 3730–3741. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).