Synthesis and Biological Evaluation of Novel Synthetic Indolone Derivatives as Anti-Tumor Agents Targeting p53-MDM2 and p53-MDMX
Abstract
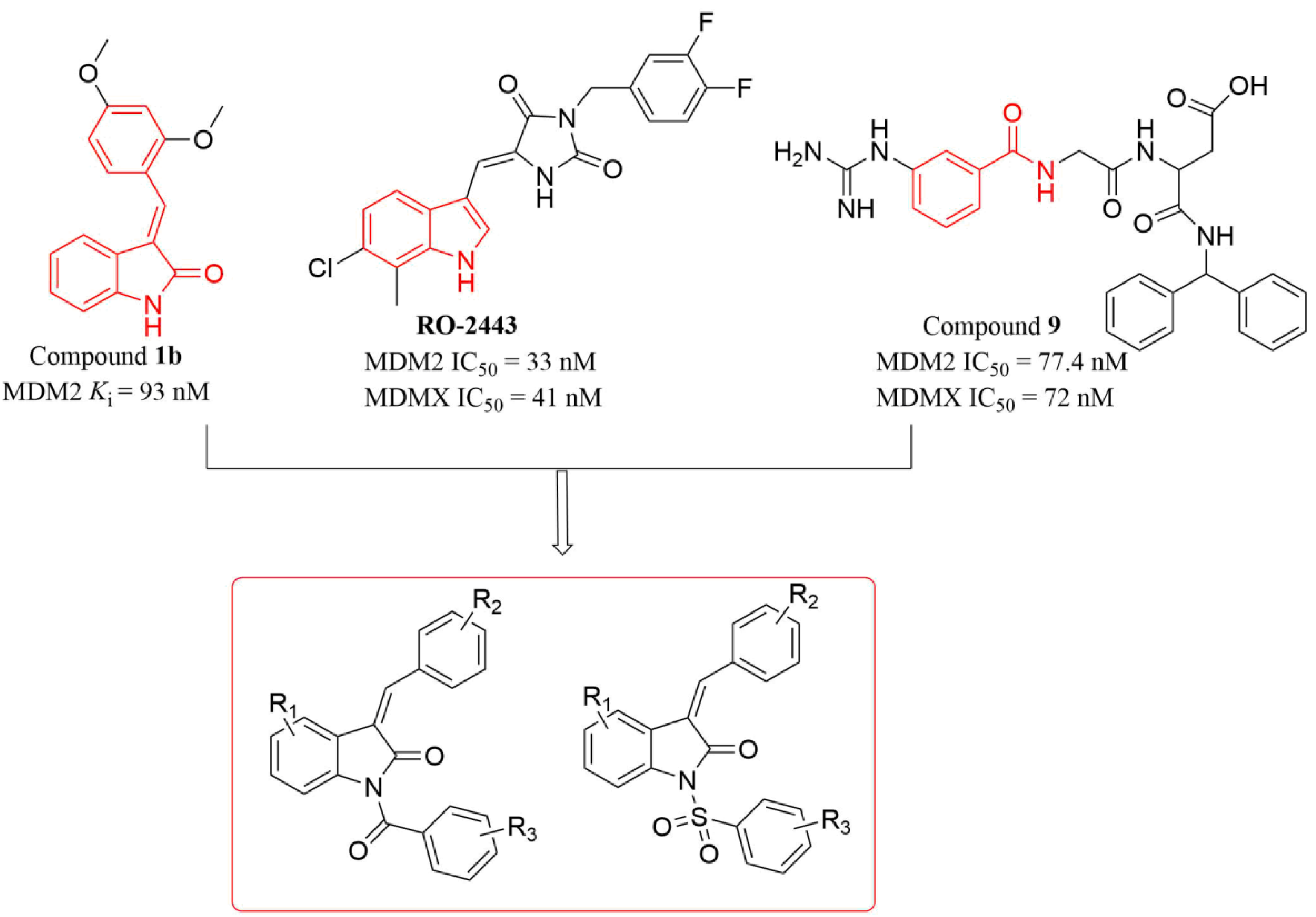
:1. Introduction
2. Results and Discussion
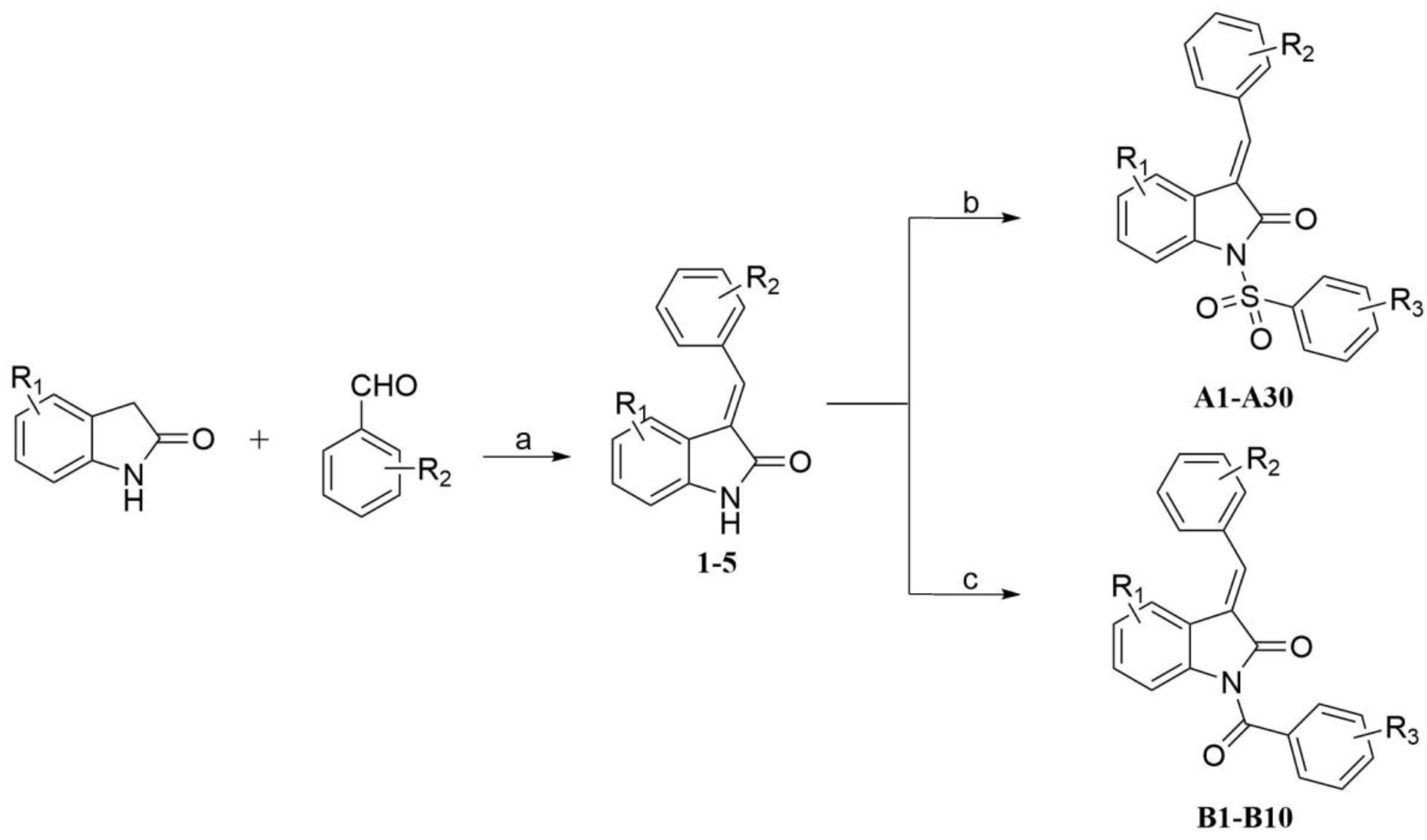
2.1. Chemistry
2.2. In Vitro Enzymatic Assays and Structure–Activity Relationships
2.3. In Vitro Anti-Tumor Activities
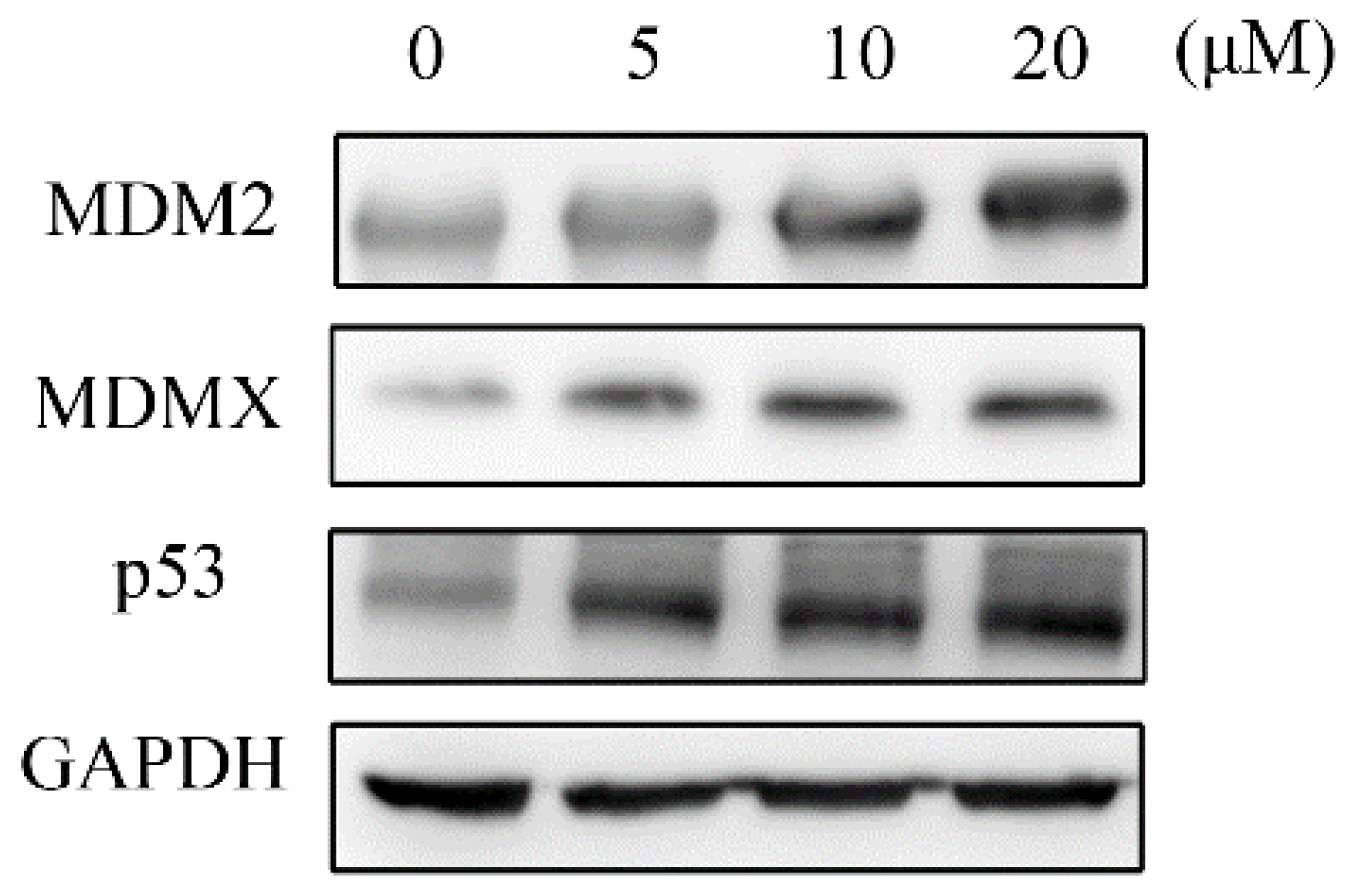
2.4. Western Blotting Analyses
2.5. Molecular Docking Analyses
3. Experimental Section
3.1. General Procedures
3.2. General Procedure for Preparation of Intermediates (1–5)
3.3. General Procedure for Preparation of Target Compounds (A1–A30)
3.4. General Procedure for Preparation of Target Compounds B1–B10
3.5. In Vitro Enzymatic Assays
3.6. MTT Assay in Vitro
3.7. Western Blotting Assay
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhuang, C.; Miao, Z.; Zhu, L.; Dong, G.; Guo, Z.; Wang, S.; Zhang, Y.; Wu, Y.; Yao, J.; Sheng, C.; et al. Discovery, synthesis, and biological evaluation of orally active pyrrolidone derivatives as novel inhibitors of p53-MDM2 protein-protein interaction. J. Med. Chem. 2012, 55, 9630–9642. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Wang, G.; Qiu, S.; Shangary, S.; Gao, W.; Qin, D.; Stuckey, J.; Krajewski, K.; et al. Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. J. Med. Chem. 2006, 49, 3432–3435. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Krzanik, S.; Kubica, K.; Guzik, K.; Labuzek, B.; Neochoritis, C.G.; Khoury, K.; Kowalska, K.; Czub, M.; Dubin, G.; et al. 1,4,5-Trisubstituted Imidazole-Based p53-MDM2/MDMX Antagonists with Aliphatic Linkers for Conjugation with Biological Carriers. J. Med. Chem. 2017, 60, 4234–4244. [Google Scholar] [CrossRef]
- Rew, Y.; Sun, D. Discovery of a small molecule MDM2 inhibitor (AMG 232) for treating cancer. J. Med. Chem. 2014, 57, 6332–6341. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.C.; Emerson, S.D.; Palme, S.; Vu, B.T.; Liu, C.M.; Podlaski, F. NMR structure of a complex between MDM2 and a small molecule inhibitor. J. Biomol. NMR 2004, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef]
- Koblish, H.K.; Zhao, S.; Franks, C.F.; Donatelli, R.R.; Tominovich, R.M.; LaFrance, L.V.; Leonard, K.A.; Gushue, J.M.; Parks, D.J.; Calvo, R.R.; et al. Benzodiazepinedione inhibitors of the Hdm2:p53 complex suppress human tumor cell proliferation in vitro and sensitize tumors to doxorubicin in vivo. Mol. Cancer Ther. 2006, 5, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Rothweiler, U.; Czarna, A.; Krajewski, M.; Ciombor, J.; Kalinski, C.; Khazak, V.; Ross, G.; Skobeleva, N.; Weber, L.; Holak, T.A. Isoquinolin-1-one inhibitors of the MDM2-p53 interaction. Chem. Med. Chem. 2008, 3, 1118–1128. [Google Scholar] [CrossRef]
- Allen, J.G.; Bourbeau, M.P.; Wohlhieter, G.E.; Bartberger, M.D.; Michelsen, K.; Hungate, R.; Gadwood, R.C.; Gaston, R.D.; Evans, B.; Mann, L.W.; et al. Discovery and optimization of chromenotriazolopyrimidines as potent inhibitors of the mouse double minute 2-tumor protein 53 protein-protein interaction. J. Med. Chem. 2009, 52, 7044–7053. [Google Scholar] [CrossRef]
- Furet, P.; Chene, P.; De Pover, A.; Valat, T.S.; Lisztwan, J.H.; Kallen, J.; Masuya, K. The central valine concept provides an entry in a new class of non peptide inhibitors of the p53-MDM2 interaction. Bioorg. Med. Chem. Lett. 2012, 22, 3498–3502. [Google Scholar] [CrossRef]
- Yang, M.C.; Peng, C.; Huang, H.; Yang, L.; He, X.H.; Huang, W.; Cui, H.L.; He, G.; Han, B. Organocatalytic Asymmetric Synthesis of Spiro-oxindole Piperidine Derivatives That Reduce Cancer Cell Proliferation by Inhibiting MDM2-p53 Interaction. Org. Lett. 2017, 19, 6752–6755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Hager, E.; Pettit, C.; Gurulingappa, H.; Davidson, N.E.; Khan, S.R. Design, Synthesis, and Evaluation of Novel Boronic-Chalcone Derivatives as Antitumor Agents. J. Med. Chem. 2003, 46, 2813–2815. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Quant, M.; Min, J.; Iconaru, L.; Kriwacki, R.W.; Waddell, M.B.; Guy, R.K.; Luthman, K.; Grotli, M. Design, Synthesis and Evaluation of 2,5-Diketopiperazines as Inhibitors of the MDM2-p53 Interaction. PLoS ONE 2015, 10, e0137867. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.Z.; Eksterowicz, J.; Bartberger, M.D.; Beck, H.P.; Canon, J.; Chen, A.; Chow, D.; Duquette, J.; Fox, B.M.; Fu, J.; et al. Selective and potent morpholinone inhibitors of the MDM2-p53 protein-protein interaction. J. Med. Chem. 2014, 57, 2472–2488. [Google Scholar] [CrossRef]
- Surmiak, E.; Twarda-Clapa, A.; Zak, K.M.; Musielak, B.; Tomala, M.D.; Kubica, K.; Grudnik, P.; Madej, M.; Jablonski, M.; Potempa, J.; et al. A Unique Mdm2-Binding Mode of the 3-Pyrrolin-2-one- and 2-Furanone-Based Antagonists of the p53-Mdm2 Interaction. ACS Chem. Biol. 2016, 11, 3310–3318. [Google Scholar] [CrossRef]
- Andreeff, M.; Kelly, K.R.; Yee, K.; Assouline, S.; Strair, R.; Popplewell, L.; Bowen, D.; Martinelli, G.; Drummond, M.W.; Vyas, P.; et al. Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia. Clin. Cancer Res. 2016, 22, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Zanjirband, M.; Edmondson, R.J.; Lunec, J. Pre-clinical efficacy and synergistic potential of the MDM2-p53 antagonists, Nutlin-3 and RG7388, as single agents and in combined treatment with cisplatin in ovarian cancer. Oncotarget 2016, 7, 40115–40134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jonge, M.; de Weger, V.A.; Dickson, M.A.; Langenberg, M.; Le Cesne, A.; Wagner, A.J.; Hsu, K.; Zheng, W.; Mace, S.; Tuffal, G.; et al. A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur. J. Cancer 2017, 76, 144–151. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z.; Rew, Y.; Gribble, M.; Bartberger, M.D.; Beck, H.P.; Canon, J.; Chen, A.; Chen, X.; Chow, D.; et al. Discovery of AMG 232, a potent, selective, and orally bioavailable MDM2-p53 inhibitor in clinical development. J. Med. Chem. 2014, 57, 1454–1472. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. Study of Safety and Efficacy of HDM201 in Combination With LEE011 in Patients With Liposarcoma. ClinicalTrials. Gov Identifier: NCT02343172. 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02343172 (accessed on 15 May 2022).
- Aguilar, A.; Lu, J.; Liu, L.; Du, D.; Bernard, D.; McEachern, D.; Przybranowski, S.; Li, X.; Luo, R.; Wen, B.; et al. Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017, 60, 2819–2839. [Google Scholar]
- Reed, D.; Shen, Y.; Shelat, A.A.; Arnold, L.A.; Ferreira, A.M.; Zhu, F.; Mills, N.; Smithson, D.C.; Regni, C.A.; Bashford, D.; et al. Identification and characterization of the first small molecule inhibitor of MDMX. J. Biol. Chem. 2010, 285, 10786–10796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ma, X.; Ren, S.; Buolamwini, J.K.; Yan, C. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Mol. Cancer Ther. 2011, 10, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yu, G.; Yang, Y.; Wang, Y.; Guo, M.; Yin, Q.; Yan, C.; Tian, J.; Fu, F.; Wang, H. A small-molecule inhibitor of MDMX suppresses cervical cancer cells via the inhibition of E6-E6AP-p53 axis. Pharmacol. Res. 2022, 177, 106128. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yang, F.; Zhou, C.; Chen, Y.; Zhang, H.; Su, Z. Efficient reactivation of p53 in cancer cells by a dual MdmX/Mdm2 inhibitor. J. Am. Chem. Soc. 2014, 136, 18023–18033. [Google Scholar] [CrossRef]
- Golestanian, S.; Sharifi, A.; Popowicz, G.M.; Azizian, H.; Foroumadi, A.; Szwagierczak, A.; Holak, T.A.; Amanlou, M. Discovery of novel dual inhibitors against Mdm2 and Mdmx proteins by in silico approaches and binding assay. Life Sci. 2016, 145, 240–246. [Google Scholar] [CrossRef]
- Lee, X.A.; Verma, C.; Sim, A.Y.L. Designing dual inhibitors of Mdm2/MdmX: Unexpected coupling of water with gatekeeper Y100. Proteins 2017, 85, 1493–1506. [Google Scholar] [CrossRef]
- Mrkvova, Z.; Uldrijan, S.; Pombinho, A.; Bartunek, P.; Slaninova, I. Benzimidazoles Downregulate Mdm2 and MdmX and Activate p53 in MdmX Overexpressing Tumor Cells. Molecules 2019, 24, 2152. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, I.; Rahman, N.; Nayab, G.E.; Niaz, S.; Shah, M.; Afridi, S.G.; Khan, H.; Daglia, M.; Capanoglu, E. The Molecular Docking of Flavonoids Isolated from Daucus carota as a Dual Inhibitor of MDM2 and MDMX. Recent Pat. Anticancer Drug Discov. 2020, 15, 154–164. [Google Scholar] [CrossRef]
- Pairawan, S.; Zhao, M.; Yuca, E.; Annis, A.; Evans, K.; Sutton, D.; Carvajal, L.; Ren, J.G.; Santiago, S.; Guerlavais, V.; et al. First in class dual MDM2/MDMX inhibitor ALRN-6924 enhances antitumor efficacy of chemotherapy in TP53 wild-type hormone receptor-positive breast cancer models. Breast Cancer Res. 2021, 23, 29. [Google Scholar] [CrossRef]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef] [PubMed]
- Cilibrasi, V.; Spano, V.; Bortolozzi, R.; Barreca, M.; Raimondi, M.V.; Rocca, R.; Maruca, A.; Montalbano, A.; Alcaro, S.; Ronca, R.; et al. Synthesis of 2H-Imidazo[2′,1′:2,3] [1,3]thiazolo[4,5-e]isoindol-8-yl-phenylureas with promising therapeutic features for the treatment of acute myeloid leukemia (AML) with FLT3/ITD mutations. Eur. J. Med. Chem. 2022, 235, 114292. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, Z.; Su, Q.; Ye, S.; Yuan, H.; Kuai, M.; Lv, M.; Tu, Z.; Yang, X.; Liu, R.; et al. Dual inhibitors of RAF-MEK-ERK and PI3K-PDK1-AKT pathways: Design, synthesis and preliminary anticancer activity studies of 3-substituted-5-(phenylamino) indolone derivatives. Bioorg. Med. Chem. 2019, 27, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Spano, V.; Barreca, M.; Rocca, R.; Bortolozzi, R.; Bai, R.; Carbone, A.; Raimondi, M.V.; Piccionello, A.P.; Montalbano, A.; Alcaro, S.; et al. Insight on [1,3]thiazolo[4,5-e]isoindoles as tubulin polymerization inhibitors. Eur. J. Med. Chem. 2021, 212, 113122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.H.; Shen, J.J.; Zhan, Y.C.; Yi, H.; Xue, S.T.; Wang, Z.; Ji, X.Y.; Li, Z.R. Design, synthesis and in vitro and in vivo antitumour activity of 3-benzylideneindolin-2-one derivatives, a novel class of small-molecule inhibitors of the MDM2-p53 interaction. Eur. J. Med. Chem. 2014, 81, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, M.; Spanò, V.; Raimondi, M.V.; Montalbano, A.; Bai, R.; Gaudio, E.; Alcaro, S.; Hamel, E.; Bertoni, F.; Barraja, P. Evaluation of [1,2]oxazolo[5,4-e]isoindoles in lymphoma cells. Eur. J. Cancer 2020, 138, 35–36. [Google Scholar] [CrossRef]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spano, V.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; Montalbano, A. Insight on pyrimido[5,4-g]indolizine and pyrimido[4,5-c]pyrrolo[1,2-a]azepine systems as promising photosensitizers on malignant cells. Eur. J. Med. Chem. 2022, 237, 114399. [Google Scholar] [CrossRef]
- Zhang, S.; Lou, J.; Li, Y.; Zhou, F.; Yan, Z.; Lyu, X.; Zhao, Y. Recent Progress and Clinical Development of Inhibitors that Block MDM4/p53 Protein-Protein Interactions. J. Med. Chem. 2021, 64, 10621–10640. [Google Scholar] [CrossRef]
- Popowicz, G.M.; Czarna, A.; Wolf, S.; Wang, K.; Wang, W.; Domling, A.; Holak, T.A. Structures of low molecular weight inhibitors bound to MDMX and MDM2 reveal new approaches for p53-MDMX/MDM2 antagonist drug discovery. Cell Cycle 2010, 9, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Nikolovska-Coleska, Z.; Wang, R.; Fang, X.; Pan, H.; Tomita, Y.; Li, P.; Roller, P.P.; Krajewski, K.; Saito, N.G.; Stuckey, J.A.; et al. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 2004, 332, 261–273. [Google Scholar] [CrossRef]


| Compd. | R1 | R2 | R3 | Ki (μM) ± SD | |
|---|---|---|---|---|---|
| MDM2 | MDMX | ||||
| A1 | H | 2-Cl | H | 4.39 ± 0.82 | >50 |
| A2 | H | 2-Cl | 2-CH3 | 7.23 ± 1.03 | >50 |
| A3 | H | 2-Cl | 3-CH3 | 5.58 ± 0.99 | >50 |
| A4 | H | 2-Cl | 4-CH3 | 10.16 ± 1.45 | >50 |
| A5 | H | 2-Cl | 2-Cl | 3.43 ± 0.57 | 42.1 ± 6.35 |
| A6 | H | 2-Cl | 3-Cl | 0.92 ± 0.11 | 17.43 ± 2.78 |
| A7 | H | 2-Cl | 4-Cl | 1.53 ± 0.19 | 20.38 ± 2.62 |
| A8 | H | 3-Cl | H | 1.47 ± 0.15 | 10.33 ± 1.45 |
| A9 | H | 3-Cl | 2-CH3 | 6.95 ± 0.97 | >50 |
| A10 | H | 3-Cl | 3-CH3 | 5.36 ± 1.06 | >50 |
| A11 | H | 3-Cl | 4-CH3 | 5.19 ± 1.25 | >50 |
| A12 | H | 3-Cl | 2-Cl | 0.19 ± 0.05 | 20.83 ± 2.01 |
| A13 | H | 3-Cl | 3-Cl | 0.031 ± 0.007 | 7.24 ± 0.78 |
| A14 | H | 3-Cl | 4-Cl | 0.082 ± 0.021 | 18.19 ± 1.39 |
| A15 | H | 3-Cl | 2-Br | 0.34 ± 0.12 | 24.37 ± 2.57 |
| A16 | H | 3-Cl | 3-Br | 0.079 ± 0.005 | 16.21 ± 1.94 |
| A17 | H | 3-Cl | 4-Br | 0.49 ± 0.15 | 43.18 ± 4.95 |
| A18 | H | 3-Cl | 2-F | 0.17 ± 0.03 | 17.69 ± 2.16 |
| A19 | H | 3-Cl | 3-F | 0.076 ± 0.005 | 16.89 ± 1.74 |
| A20 | H | 3-Cl | 4-F | 0.23 ± 0.02 | 29.16 ± 3.51 |
| A21 | H | 3-Cl | 3-CF3 | 0.092 ± 0.007 | 19.26 ± 2.69 |
| A22 | H | 3-Cl | 3-NO2 | 1.39 ± 0.21 | 13.57 ± 1.47 |
| A23 | H | 3-Cl | 4-OCH3 | 3.77 ± 0.45 | 42.17 ± 4.73 |
| A24 | H | 4-Cl | 2-CH3 | 3.17 ± 0.37 | 34.69 ± 4.02 |
| A25 | H | 4-Cl | 3-CH3 | 4.93 ± 0.47 | >50 |
| A26 | H | 4-Cl | 4-CH3 | 4.68 ± 0.39 | >50 |
| A27 | H | 4-Cl | 3-Cl | 0.31 ± 0.03 | 17.26 ± 1.91 |
| A28 | H | 4-Cl | H | 2.18 ± 0.29 | 37.43 ± 3.57 |
| A29 | 5-Cl | 3-Cl | 3-Cl | 0.28 ± 0.04 | 32.24 ± 3.24 |
| A30 | 6-Cl | 3-Cl | 3-Cl | 1.39 ± 0.15 | 28.96 ± 2.18 |
| B1 | H | 2-Cl | 2-Cl | 10.17 ± 2.01 | >50 |
| B2 | H | 2-Cl | 3-Cl | 8.43 ± 1.58 | >50 |
| B3 | H | 2-Cl | 4-Cl | 7.86 ± 1.34 | >50 |
| B4 | H | 2-Cl | 2-CH3 | 16.35 ± 2.77 | >50 |
| B5 | H | 2-Cl | 3-CH3 | 20.19 ± 3.05 | >50 |
| B6 | H | 2-Cl | 4-CH3 | 22.37 ± 3.64 | >50 |
| B7 | H | 3-Cl | 2-Cl | 14.87 ± 2.37 | >50 |
| B8 | H | 3-Cl | 3-Cl | 8.63 ± 1.55 | >50 |
| B9 | H | 3-Cl | 4-Cl | 15.92 ± 1.96 | >50 |
| B10 | H | 4-Cl | 3-Cl | 12.49 ± 1.04 | >50 |
| Nutlin-3a | - | - | - | 0.15 ± 0.02 | >50 |
| Compd. | IC50 (μM) | ||
|---|---|---|---|
| HCT116 | MCF7 | A549 | |
| A6 | 10.72 ± 1.47 | 25.47 ± 3.61 | 18.72 ± 2.49 |
| A12 | 15.36 ± 4.61 | 24.72 ± 2.53 | 17.83 ± 2.19 |
| A13 | 6.17 ± 0.93 | 11.21 ± 1.95 | 12.49 ± 2.01 |
| A14 | 15.18 ± 3.14 | 20.19 ± 2.86 | 20.33 ± 2.49 |
| A15 | 23.79 ± 2.69 | 31.08 ± 3.55 | 29.12 ± 3.31 |
| A16 | 21.73 ± 2.59 | 26.49 ± 3.40 | 25.44 ± 3.62 |
| A17 | 25.47 ± 3.26 | 37.70 ± 3.65 | 34.17 ± 3.39 |
| A18 | 17.88 ± 2.11 | 24.74 ± 3.63 | 22.58 ± 2.93 |
| A19 | 10.15 ± 1.37 | 21.03 ± 2.58 | 16.27 ± 1.82 |
| A20 | 16.32 ± 2.73 | 27.18 ± 2.83 | 22.19 ± 2.13 |
| A21 | 8.91 ± 1.35 | 15.69 ± 2.04 | 13.48 ± 2.73 |
| A27 | 17.49 ± 1.53 | 27.62 ± 3.81 | 20.36 ± 1.98 |
| A29 | 16.96 ± 2.37 | 27.43 ± 3.37 | 26.35 ± 4.05 |
| Nutlin-3a | 18.13 ± 2.17 | 32.18 ± 1.49 | 21.72 ± 2.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ji, B.; Cheng, Z.; Zhang, L.; Cheng, Y.; Li, Y.; Ren, J.; Liu, W.; Ma, Y. Synthesis and Biological Evaluation of Novel Synthetic Indolone Derivatives as Anti-Tumor Agents Targeting p53-MDM2 and p53-MDMX. Molecules 2022, 27, 3721. https://doi.org/10.3390/molecules27123721
Wang Y, Ji B, Cheng Z, Zhang L, Cheng Y, Li Y, Ren J, Liu W, Ma Y. Synthesis and Biological Evaluation of Novel Synthetic Indolone Derivatives as Anti-Tumor Agents Targeting p53-MDM2 and p53-MDMX. Molecules. 2022; 27(12):3721. https://doi.org/10.3390/molecules27123721
Chicago/Turabian StyleWang, Yali, Bo Ji, Zhongshui Cheng, Lianghui Zhang, Yingying Cheng, Yingying Li, Jin Ren, Wenbo Liu, and Yuanyuan Ma. 2022. "Synthesis and Biological Evaluation of Novel Synthetic Indolone Derivatives as Anti-Tumor Agents Targeting p53-MDM2 and p53-MDMX" Molecules 27, no. 12: 3721. https://doi.org/10.3390/molecules27123721
APA StyleWang, Y., Ji, B., Cheng, Z., Zhang, L., Cheng, Y., Li, Y., Ren, J., Liu, W., & Ma, Y. (2022). Synthesis and Biological Evaluation of Novel Synthetic Indolone Derivatives as Anti-Tumor Agents Targeting p53-MDM2 and p53-MDMX. Molecules, 27(12), 3721. https://doi.org/10.3390/molecules27123721





