Poly(o-methoxyaniline) Chain Degradation Based on a Heat Treatment (HT) Process: Combined Experimental and Theoretical Evaluation
Abstract
1. Introduction
2. Results
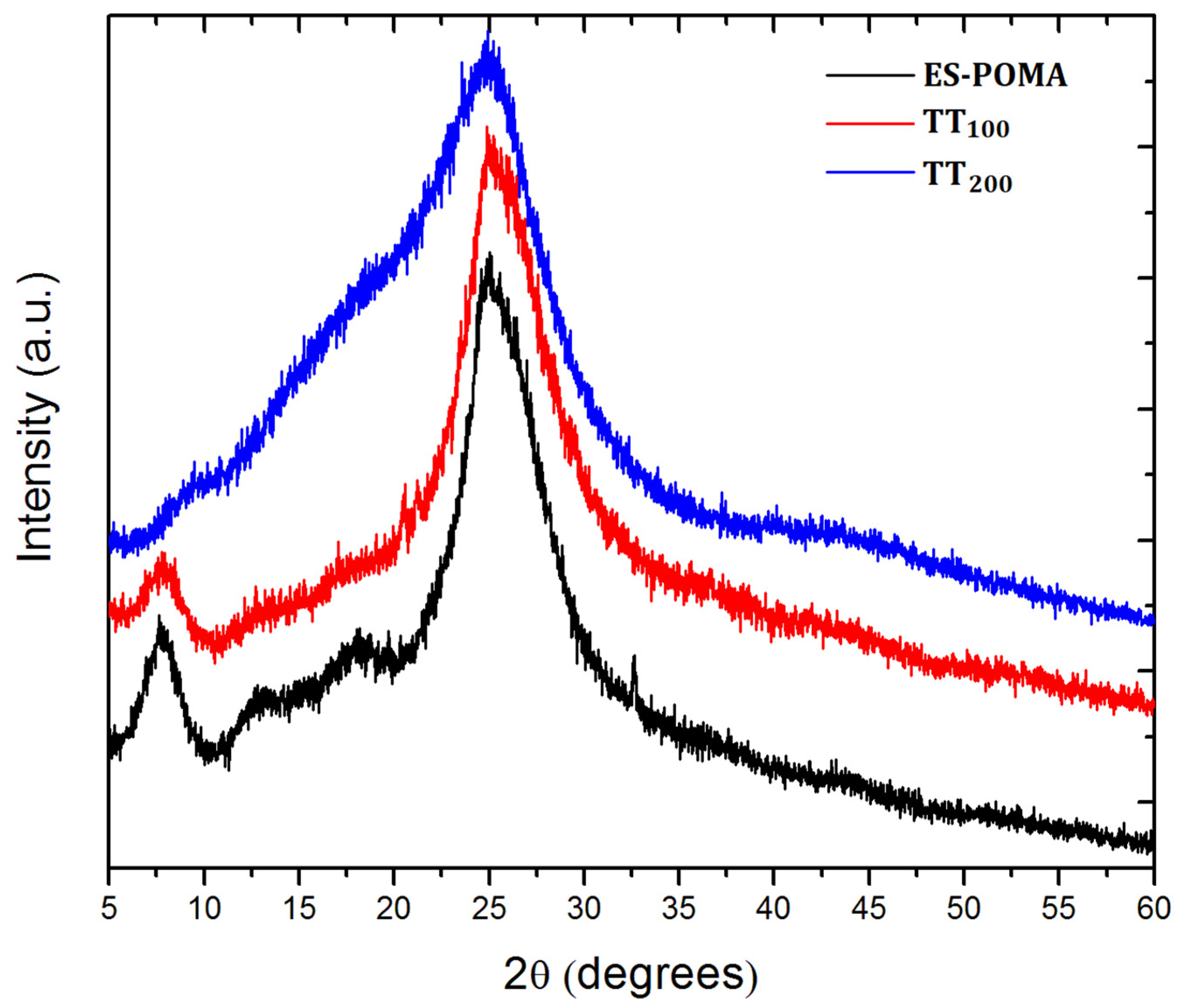
2.1. XRD Analysis and Percentage of Crystallinity
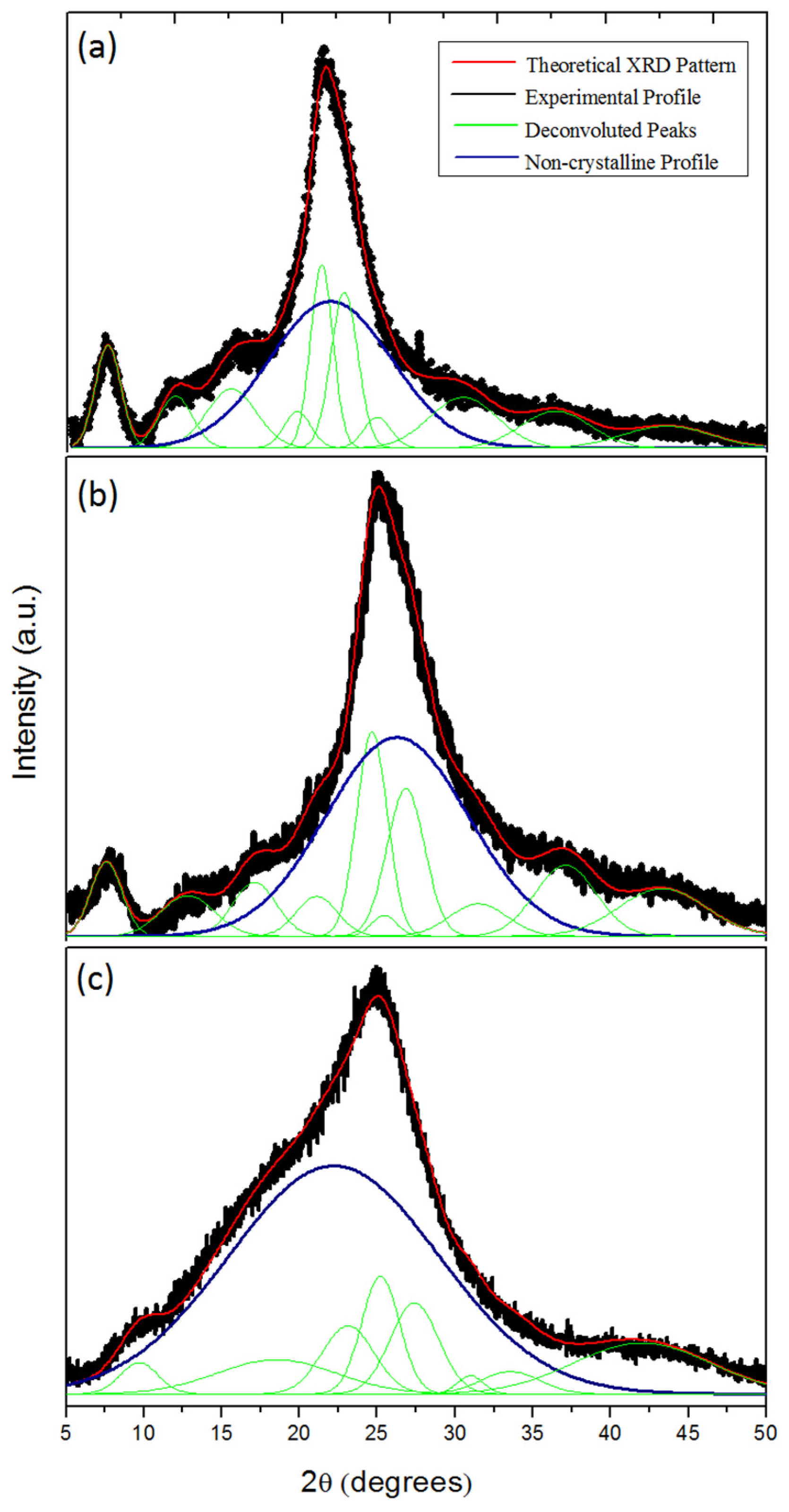
2.2. Le Bail Method
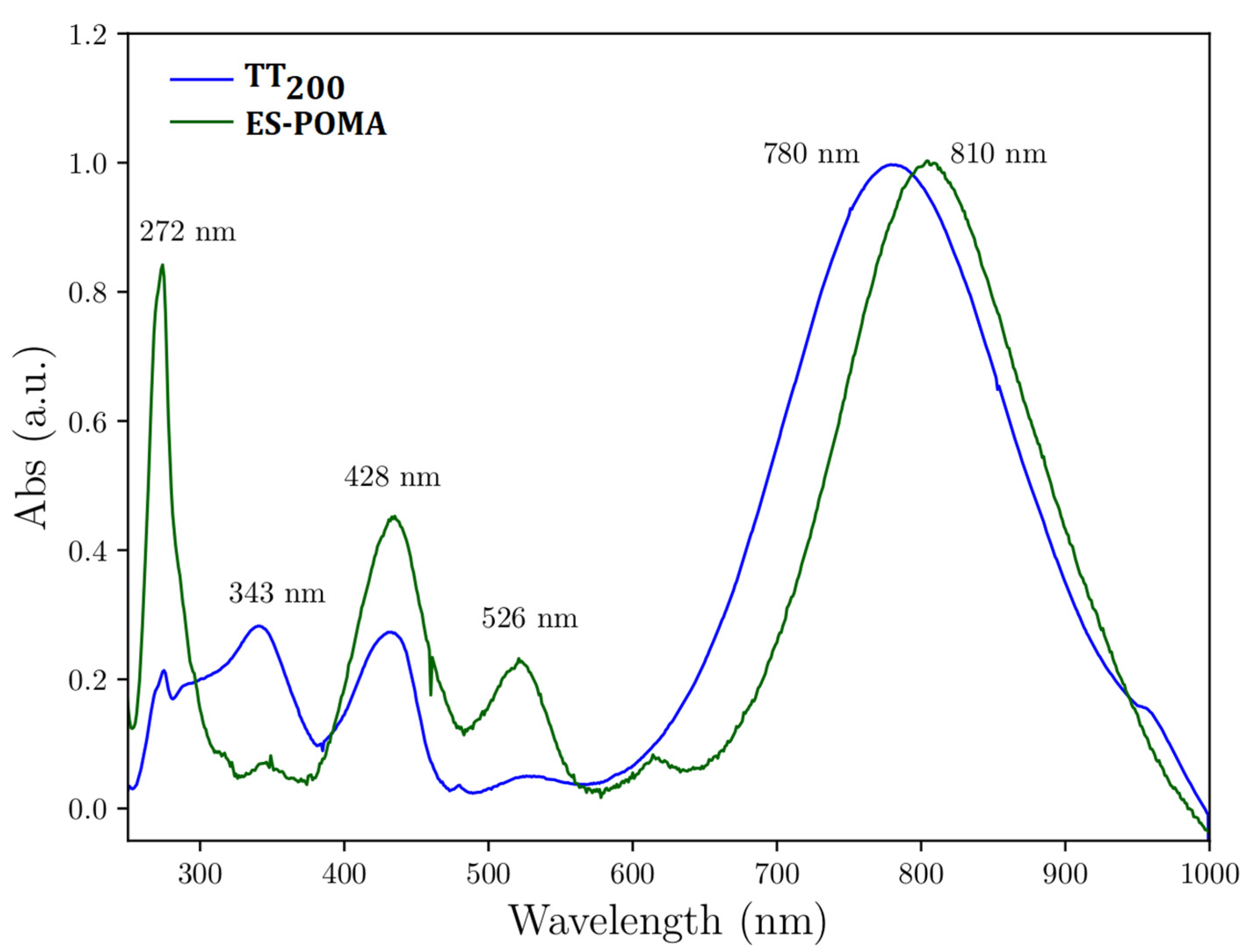
2.3. Experimental UV-VIS Analysis
2.4. SEM Analysis
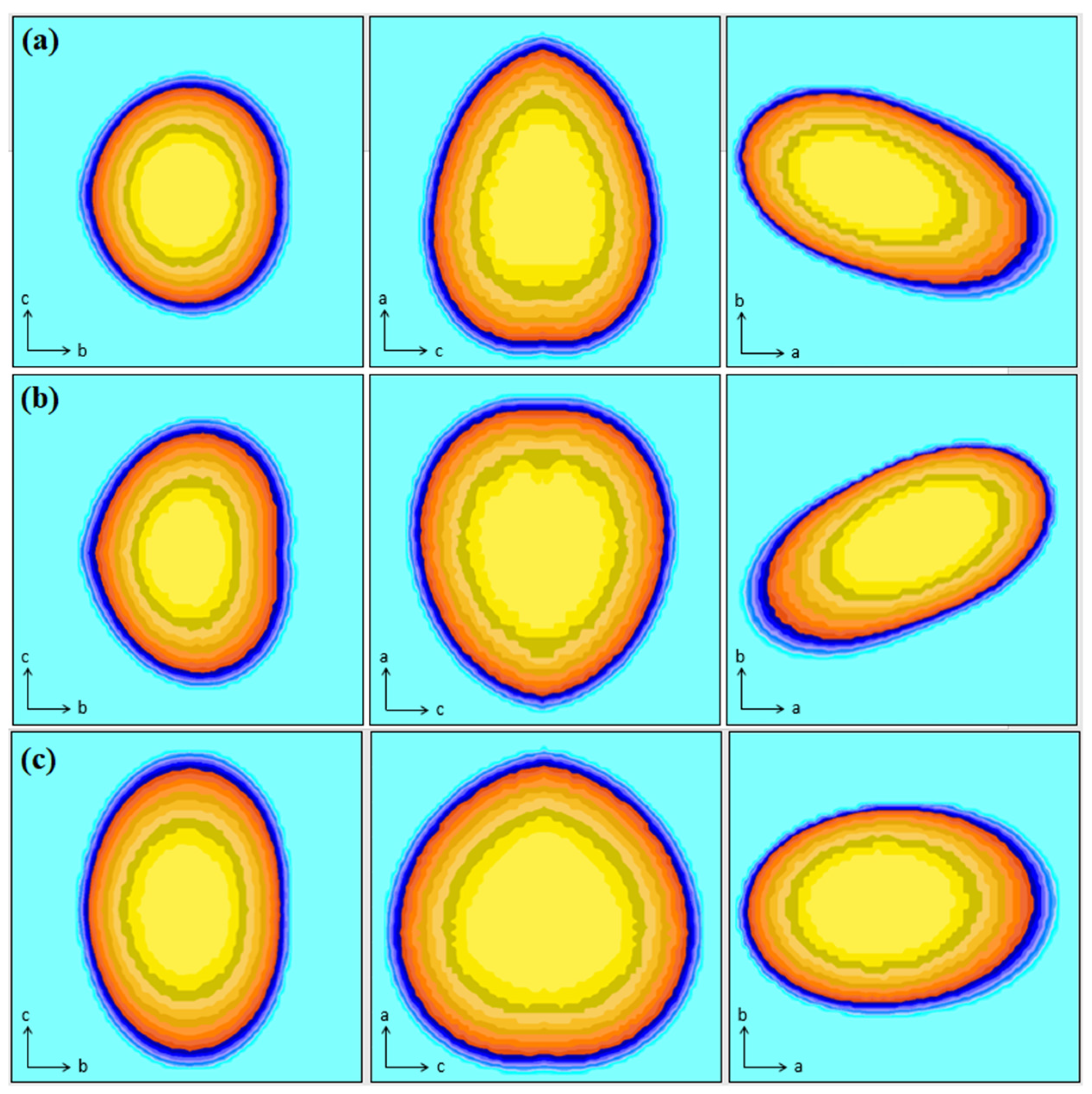
2.5. Geometric Optimization
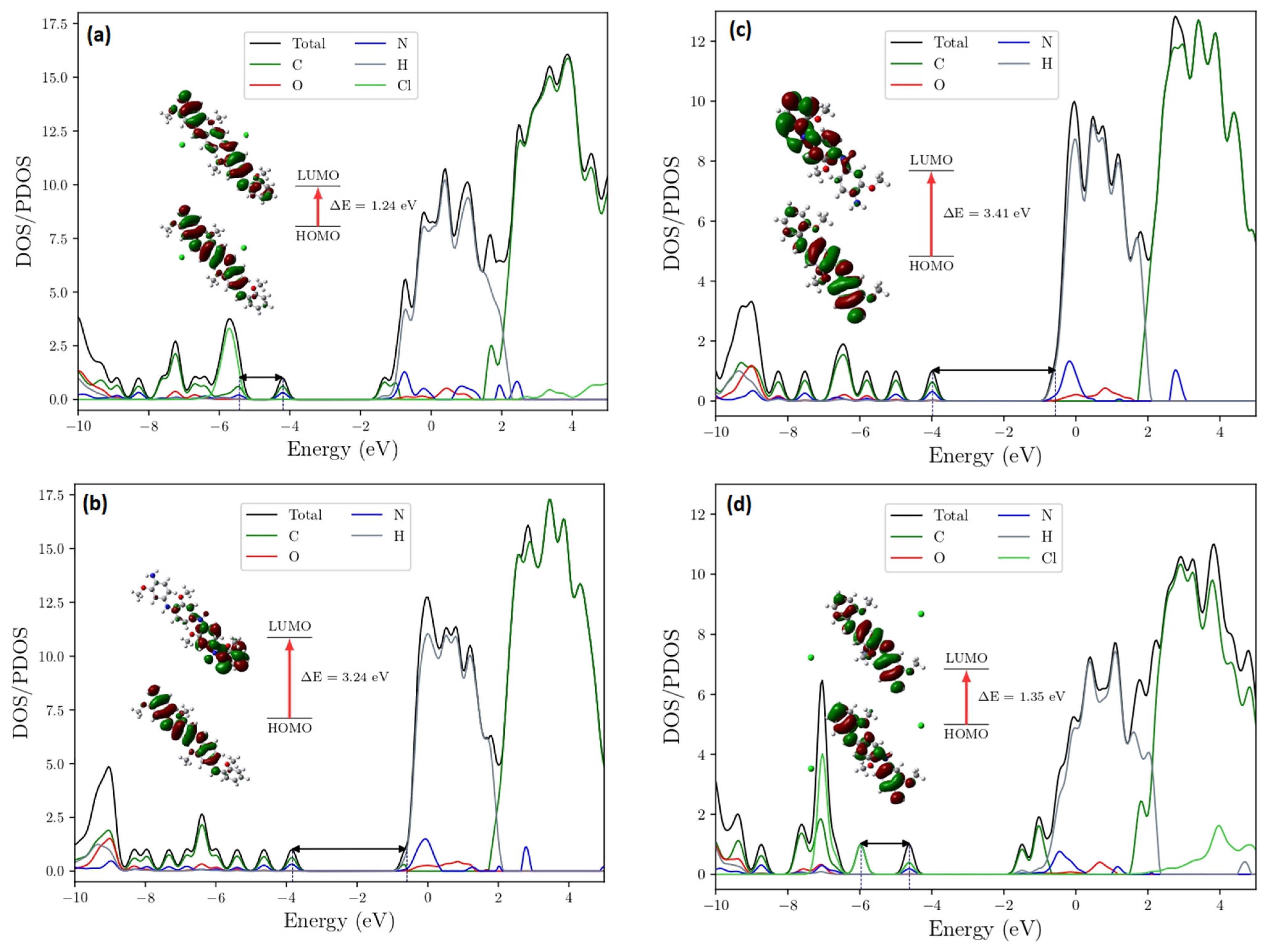
2.6. Theoretical UV-VIS Analysis
2.7. Electrical Conductivity
3. Discussion
3.1. XRD Analysis
3.2. Le Bail Method
3.3. Experimental UV-VIS Analysis
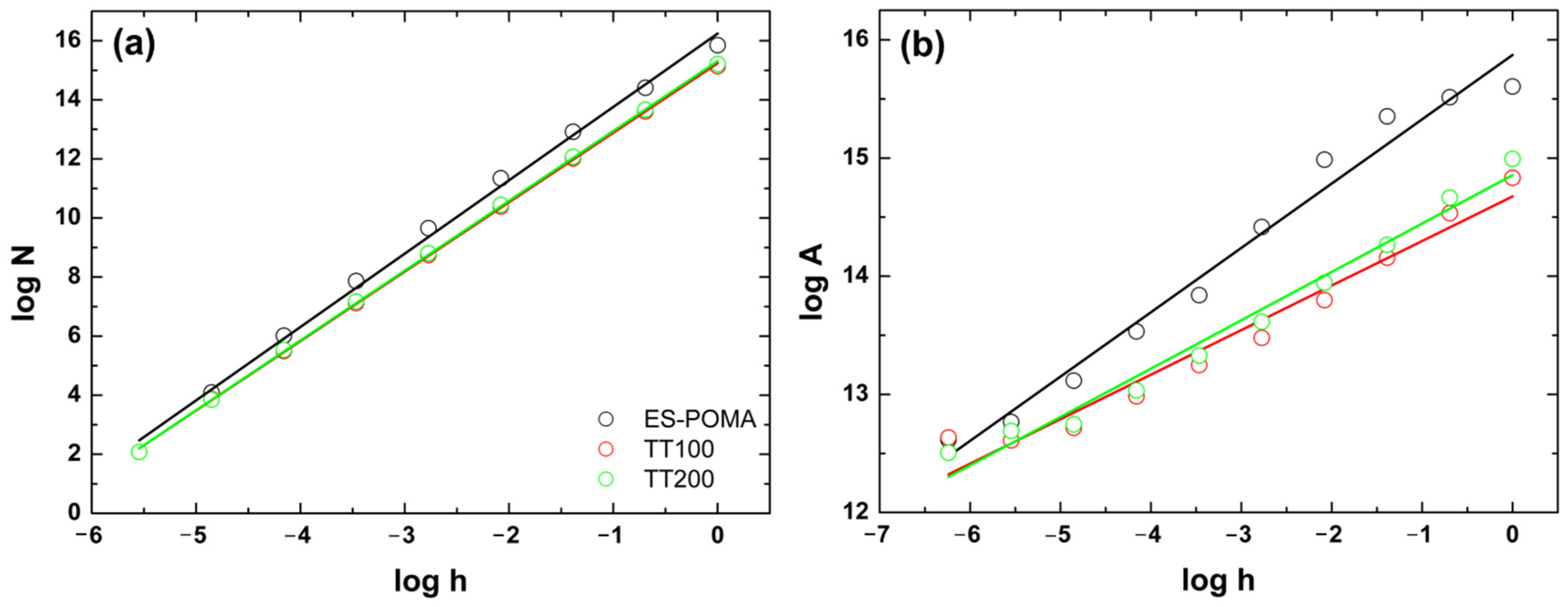
3.4. SEM Analysis


3.5. Geometric Optimization
3.6. Theoretical UV-VIS Analysis
| Experimental | Theoretical | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trimer | Tetramer Cl2 | Trimer | Trimer Cl2 | Tetramer | Tetramer Cl2 | ||||
| UV-VIS (nm) | UV-VIS (nm) | Trans. | UV-VIS (nm) | Trans. | UV-VIS (nm) | Trans. | UV-VIS (nm) | Trans. | |
| 272 | 272 | 290 | H − 1 > L + 2 (60%) | 280 | HOMO > L + 2 (71%) | 317 | HOMO > L + 18 (27%) | 310 | HOMO > L+2 (83%) |
| 343 | 343 | 317 | HOMO > L + 11 (43%) | 304 | HOMO > L + 1 (77%) | 345 | HOMO > L + 12 (37%) | 334 | HOMO > L + 1 (83%) |
| 428 | 433 | 354 | HOMO > L + 5 (60%) | 464 | H − 10 > LUMO (74%) | 369 | HOMO > L + 4 (70%) | 452 | H − 11 > LUMO (85%) |
| 526 | 526 | 419 | HOMO > LUMO (90%) | 586 | H − 8 > LUMO (84%) | 438 | HOMO > LUMO (87%) | 525 | H − 3 > LUMO (80%) |
| 780 | 810 | - | 710 | H − 1 > LUMO (63%) | - | 684 | H − 1 > LUMO (70%) | ||
| - | - | - | 847 | HOMO > LUMO (46%) | - | 936 | HOMO > LUMO (50%) | ||
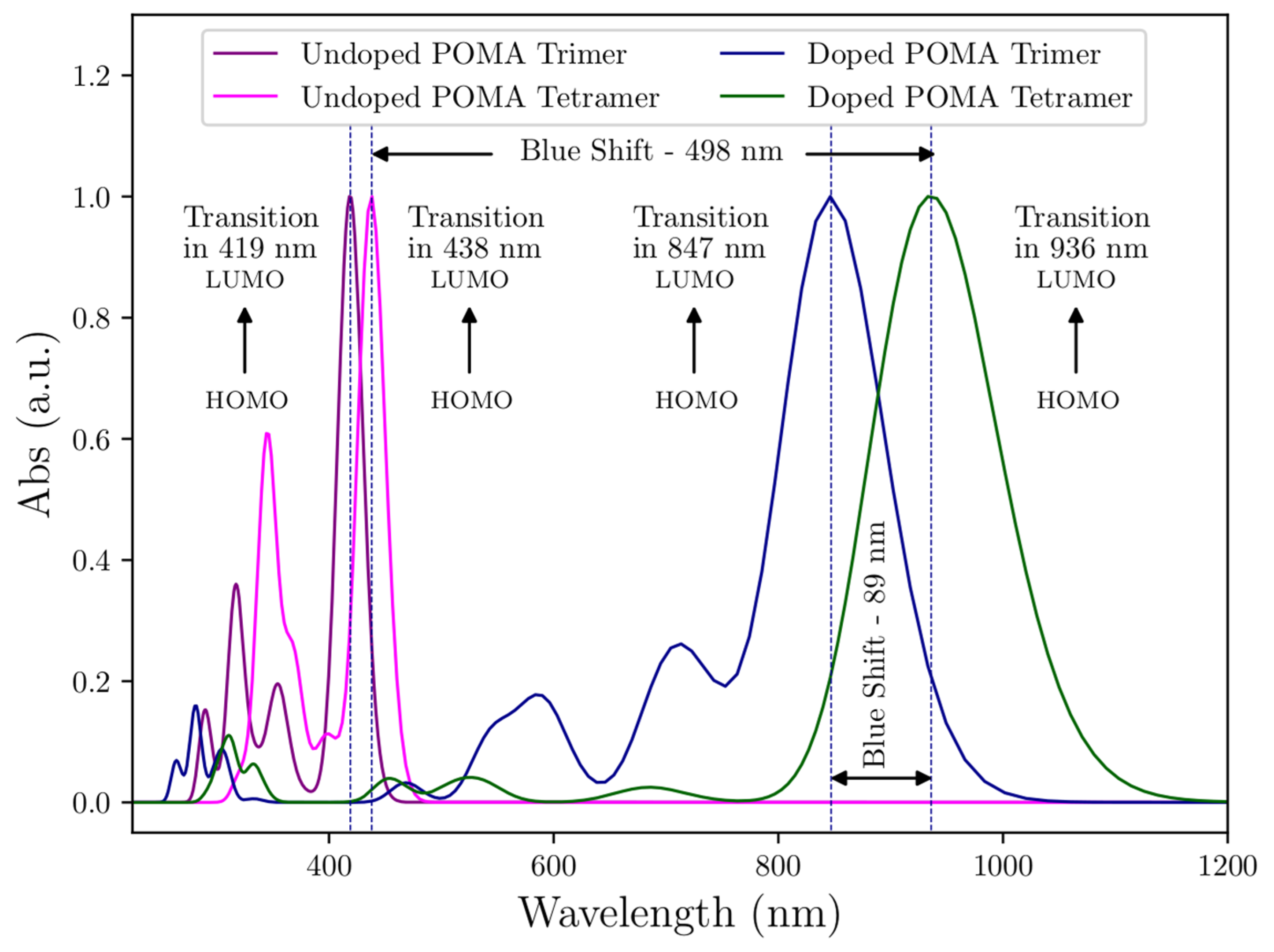
HOMO-LUMO Analysis
3.7. DC Electrical Conductivity
4. Methods and Materials
4.1. Synthesis of ES-POMA and Heat Treatment (HT)
4.2. XRD Analysis and Percentage of Crystallinity
4.3. Le Bail Whole Powder Pattern Decomposition Method
4.4. Ultraviolet-Visible (UV-VIS) Spectroscopy
4.5. SEM Analysis
4.6. Theoretical and Computational Methods
4.7. DC Electrical Conductivity Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ferreira, A.A.; Sanches, E.A. Multimorphologies of hydrochloride polyaniline synthesized by conventional and interfacial polymerization. J. Mol. Struct. 2017, 1143, 294–305. [Google Scholar] [CrossRef]
- de Oliveira, L.R.; Manzato, L.; Mascarenhas, Y.P.; Sanches, E.A. The influence of heat treatment on the semi-crystalline structure of polyaniline Emeraldine-salt form. J. Mol. Struct. 2017, 1128, 707–717. [Google Scholar] [CrossRef]
- Casas-Cabanas, M.; Palacin, M.R.; Rodriguez-Carvajal, J. Microstructural analysis of nickel hydroxide: Anisotropic size versus stacking faults. Powder Diffr. 2005, 20, 334–344. [Google Scholar] [CrossRef]
- Tsai, C.H.; Huang, W.C.; Hsu, Y.C.; Shih, C.J.; Teng, I.J.; Yu, Y.H. Poly(o-methoxyaniline) doped with an organic acid as cost-efficient counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2016, 213, 791–801. [Google Scholar] [CrossRef]
- Cherpak, V.; Stakhira, P.; Hotra, Z.; Aksimentyeva, O.; Tsizh, B.; Volynyuk, D.; Bordun, I. Vacuum-deposited poly(o-methoxyaniline) thin films: Structure and electronic properties. J. Non-Cryst. Solids 2008, 354, 4282–4286. [Google Scholar] [CrossRef]
- Nogueira, J.S.; Mattoso, L.H.c.; Lepienski, C.M.; Faria, R.M. AC conduction of poly(o-methoxyaniline). Synth. Met. 1995, 69, 259–260. [Google Scholar] [CrossRef]
- Ribeiro, P.A.; Steitz, R.; Lopis, I.E.; Haas, H.; Souza, N.C.; Oliveira, O.N.; Raposo, M. Thermal Stability of Poly(o-Methoxyaniline) Layer-by-Layer Films Investigated by Neutron Reflectivity and UV-VIS Spectroscopy. J. Nanosci. Nanotechnol. 2006, 6, 1396–1404. [Google Scholar] [CrossRef]
- Silva, A.S.; Soares, J.C.; Carolina, A.; De Souza, S.M.; Fernandes, E.G.R.; Mascarenhas, Y.P.; Sanches, E.A. Structural and morphological characterization of Poly(o-ethoxyaniline) Emeraldine-salt form using FTIR, XRD, LeBail Method and SEM. J. Mol. Struct. 2014, 1071, 1–5. [Google Scholar] [CrossRef]
- Sanches, E.A.; Carolino, A.S.; Santos, A.L.; Fernandes, E.G.R.; Trichês, D.M.; Mascarenhas, Y.P. The use of Le Bail Method to analyze the semicrystalline pattern of a nanocomposite based on polyaniline emeraldine-salt form and α-Al2O3. Adv. Mater. Sci. Eng. 2015, 2015, 375312. [Google Scholar] [CrossRef]
- Sanches, E.A.; Alves, S.F.; Soares, J.C.; Da Silva, A.M.; Da Silva, C.G.; De Souza, S.M.; Da Frota, H.O. Nanostructured polypyrrole powder: A structural and morphological characterization. J. Nanomater. 2015, 2015, 129678. [Google Scholar] [CrossRef]
- Sanches, E.A.; Soares, J.C.; Iost, R.M.; Marangoni, V.S.; Trovati, G.; Batista, T.; Mafud, A.C.; Zucolotto, V.; Mascarenhas, Y.P. Structural characterization of emeraldine-salt polyaniline/gold nanoparticles complexes. J. Nanomater. 2011, 2011, 697071. [Google Scholar] [CrossRef]
- Hopkins, A.R.; Lipeles, R.A.; Hwang, S.J. Morphology characterization of polyaniline nano- and microstructures. Synth. Met. 2008, 158, 594–601. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D. Determination of crystal structure of polyaniline and substituted polyanilines through powder X-ray diffraction analysis. Polym. Test. 2008, 27, 851–857. [Google Scholar] [CrossRef]
- Rodríquez-Carvajal, J.; Roisnel, T. Line Broadening Analysis Using FullProf*: Determination of Microstructural Properties. Mater. Sci. Forum 2004, 443–444, 123–126. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Solovyev, I.V.; Korotin, M.A.; Czyyk, M.T.; Sawatzky, G.A. Density-functional theory and NiO photoemission spectra. Phys. Rev. B 1993, 48, 16929–16934. [Google Scholar] [CrossRef]
- Van Caillie, C.; Amos, R.D. Geometric derivatives of excitation energies using SCF and DFT. Chem. Phys. Lett. 1999, 308, 249–255. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Sanches, E.A.; Soares, J.C.; Mafud, A.C.; Trovati, G.; Fernandes, E.G.; Mascarenhas, Y.P. Structural and morphological characterization of chloride salt of conducting poly(o-methoxyaniline) obtained at different time synthesis. J. Mol. Struct. 2013, 1039, 167–173. [Google Scholar] [CrossRef]
- De Souza, N.C.; Silva, J.R.; Giacometti, J.A.; Oliveira, O.N. H-bonding in entrapped water in poly(o-methoxyaniline): Results from a differential scanning calorimetry study. Thermochim. Acta 2006, 441, 124–126. [Google Scholar] [CrossRef]
- Evain, M.; Quillard, S.; Corraze, B.; Wang, W.; MacDiarmid, A.G. A phenyl-end-capped tetramer of aniline. Acta Crystallogr. Sect. E Struct. Rep. Online 2002, 58, o343–o344. [Google Scholar] [CrossRef]
- Mattoso, L.H.C.; Bulhões, L.O.S. Synthesis and characterization of poly(o-anisidine) films. Synth. Met. 1992, 52, 171–181. [Google Scholar] [CrossRef]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-Ray diffraction (WAXD): Comparison between measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar] [CrossRef][Green Version]
- Trovati, G.; Sanches, E.A.; De Souza, S.M.; Dos Santos, A.L.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Rigid and semi rigid polyurethane resins: A structural investigation using DMA, SAXS and Le Bail method. J. Mol. Struct. 2014, 1075, 589–593. [Google Scholar] [CrossRef]
- Le Bail, A. Whole powder pattern decomposition methods and applications: A retrospection. Powder Diffr. 2005, 20, 316–326. [Google Scholar] [CrossRef]
- Ţălu, Ş. Characterization of surface roughness of unworn hydrogel contact lenses at a nanometric scale using methods of modern metrology. Polym. Eng. Sci. 2013, 53, 2141–2150. [Google Scholar] [CrossRef]
- Mwema, F.M.; Akinlabi, E.T.; Oladijo, O.P. Effect of Substrate Type on the Fractal Characteristics of AFM Images of Sputtered Aluminium Thin Films. Mater. Sci. 2019, 26, 49–57. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Nikola, P.; Sobola, D.; Achour, A.; Solaymani, S. Micromorphology investigation of GaAs solar cells: Case study on statistical surface roughness parameters. J. Mater. Sci. Mater. Electron. 2017, 28, 15370–15379. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. The Fractal Geometry of Nature/Revised and Enlarged Edition; WH Freeman and Co.: New York, NY, USA, 1983. [Google Scholar]
- Risović, D.; Pavlović, Ž. Performance assessment of methods for estimation of fractal dimension from scanning electron microscope images. Scanning 2013, 35, 402–411. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Yadav, R.P.; Agarwal, D.C.; Kumar, M.; Rajput, P.; Tomar, D.S.; Pandey, S.N.; Priya, P.K.; Mittal, A.K. Effect of angle of deposition on the Fractal properties of ZnO thin film surface. Appl. Surf. Sci. 2017, 416, 51–58. [Google Scholar] [CrossRef]
- Mazzeu, M.A.C.; Faria, L.K.; de Moura Cardoso, A.; Gama, A.M.; Baldan, M.R.; Gonçalves, E.S. Structural and morphological characteristics of polyaniline synthesized in pilot scale. J. Aerosp. Technol. Manag. 2017, 9, 39–47. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchová, M.; Nuzhnyy, D.; Bovtun, V.; Savinov, M.; Petzelt, J.; Proke, J. Interfaced conducting polymers. Synth. Met. 2017, 224, 109–115. [Google Scholar] [CrossRef]
- Behbahani, M.; Bide, Y.; Salarian, M.; Niknezhad, M.; Bagheri, S. The use of tetragonal star-like polyaniline nanostructures for efficient solid phase extraction and trace detection of Pb (II) and Cu (II) in agricultural products, sea foods, and water samples. Food Chem. 2014, 158, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Effect of aromatic substitution in aniline on the properties of polyaniline. Eur. Polym. J. 2008, 44, 1763–1770. [Google Scholar] [CrossRef]
- Sanches, E.A.; Gomes, L.C.A.; Soares, J.C.; Da Silva, G.R.; Mascarenhas, Y.P. Characterization of Poly(o-methoxyaniline) Emeraldine-base form obtained at different time neutralization. J. Mol. Struct. 2014, 1063, 336–340. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Bahgat, A.A.; Abd El-Salam, H.M. Kinetic studies of the aqueous oxidative polymerization of 3-hydroxyaniline and characterization of the polymer obtained. Int. J. Polym. Mater. Polym. Biomater. 2002, 51, 291–314. [Google Scholar] [CrossRef]
- Koval’chuk, E.P.; Stratan, N.V.; Reshetnyak, O.V.; Błażejowski, J.; Whittingham, M.S. Synthesis and properties of the polyanisidines. Solid State Ion. 2001, 141, 217–224. [Google Scholar] [CrossRef]
- Veras, T.N.; Carolino, A.S.; Lima, S.X.; Biondo, M.M.; Santos, N.A.; Campelo, P.H.; Ruiz, Y.L.; Frota, H.O.; Sanches, E.A. Characterization and DFT calculation of poly(m-anisidine) synthesized with different dopant acids. J. Mol. Struct. 2020, 1201, 127182. [Google Scholar] [CrossRef]
- Dawn, A.; Nandi, A.K. Simple method for the preparation of DNA-poly(o-methoxyaniline) hybrid: Structure, morphology, and uncoiling of poly(o-methoxyaniline) on the DNA surface. Langmuir 2006, 22, 3273–3279. [Google Scholar] [CrossRef]
- Mokreva, P.; Tsocheva, D.; Ivanova, G.; Terlemezyan, L. Copolymers of aniline and o-methoxyaniline: Synthesis and characterization. J. Appl. Polym. Sci. 2006, 99, 75–81. [Google Scholar] [CrossRef]
- OriginLab Peak Fitting Module; Manual: Northampton, MA, USA, 2002.
- Mydlová, J.; Krupčík, J.; Korytár, P.; Sandra, P. On the use of computer assisted resolution of non-separable peaks in a congener specific polybrominated diphenyl ether capillary gas chromatographic analysis. J. Chromatogr. A 2007, 1147, 95–104. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld Refinement of Debye-Scherrer Synchrotron X-ray Data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Popa, N.C. The (hkl) dependence of diffraction-line broadening caused by strain and size for all Laue groups in Rietveld refinement. J. Appl. Crystallogr. 1998, 31, 176–180. [Google Scholar] [CrossRef]
- Douketis, C.; Wang, Z.; Haslett, T.L.; Moskovits, M. Fractal character of cold-deposited silver films determined by low-temperature scanning tunneling microscopy. Phys. Rev. B 1995, 51, 11022–11031. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Gaussian, Inc.: Wallingford, CT, USA, 2003. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Chakraborty, S.; de Lima, B.C.; da Silva, A.M.; Chaudhuri, P. Effect of hydrogen-bonded interactions on the energetics and spectral properties of the astromolecule aminoacetonitrile. Int. J. Quantum Chem. 2018, 118, e25459. [Google Scholar] [CrossRef]
- Saielli, G. TD-DFT Prediction of the Intermolecular Charge-Transfer UV-Vis Spectra of Viologen Salts in Solution. Appl. Sci. 2020, 10, 8108. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]

| Refined Parameters | ES-POMA | TT100 | TT200 |
|---|---|---|---|
| a (Å) | 7.10275 | 7.06554 | 6.99176 |
| b (Å) | 11.39338 | 11.42507 | 10.87043 |
| c (Å) | 18.82489 | 15.58464 | 15.62562 |
| α (Å) | 82.27626 | 84.71527 | 83.43137 |
| β (Å) | 84.02425 | 85.33233 | 84.86502 |
| γ (Å) | 88.28839 | 90.64657 | 88.31005 |
| V (Å3) | 1501 | 1248 | 1175 |
| Average Crystallite Size (anisotr.) (Å) | 29 (4) | 27 (5) | 23 (3) |
| Crystallite Apparent Size [100] (Å) | 37 | 31 | 27 |
| Crystallite Apparent Size [010] (Å) | 23 | 20 | 17 |
| Crystallite Apparent Size [001] (Å) | 34 | 29 | 26 |
| RP (%) | 3.59 | 3.92 | 3.96 |
| RWP (%) | 4.66 | 4.98 | 3.73 |
| X2 | 1.13 | 1.11 | 1.12 |
| Bond Length | Doped-POMA Tetramer (Å) | Undoped-POMA Tetramer (Å) | Undoped-POMA Trimer (Å) | Doped-POMA Trimer (Å) |
|---|---|---|---|---|
| C–C | 1.39 | 1.40 | 1.40 | 1.40 |
| C–N | 1.36 | 1.39 | 1.41 | 1.36 |
| C–O | 1.39 | 1.39 | 1.39 | 1.39 |
| N–H | 1.04 | 1.00 | 1.01 | 1.04 |
| NH–Cl | 1.95 | - | - | 5.91 |
| Polymers | ΔEbinding (kcal.mol−1) | ΔGbinding (kcal.mol−1) |
|---|---|---|
| Doped (POMA tetramer/POMA trimer) | −186.37 | −178.90 |
| Undoped (POMA tetramer/POMA trimer) | −95.61 | −77.73 |
| Doped-POMA tetramer/Cl | −88.88 | −71.98 |
| Doped-POMA trimer/Cl | 6.82 | 25.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, J.M.S.; de Souza Carolino, A.; de Oliveira, L.R.; de Souza Gonçalves, D.; Biondo, M.M.; Campelo, P.H.; de Araújo Bezerra, J.; Ţălu, Ş.; da Fonseca Filho, H.D.; da Frota, H.O.; et al. Poly(o-methoxyaniline) Chain Degradation Based on a Heat Treatment (HT) Process: Combined Experimental and Theoretical Evaluation. Molecules 2022, 27, 3693. https://doi.org/10.3390/molecules27123693
da Silva JMS, de Souza Carolino A, de Oliveira LR, de Souza Gonçalves D, Biondo MM, Campelo PH, de Araújo Bezerra J, Ţălu Ş, da Fonseca Filho HD, da Frota HO, et al. Poly(o-methoxyaniline) Chain Degradation Based on a Heat Treatment (HT) Process: Combined Experimental and Theoretical Evaluation. Molecules. 2022; 27(12):3693. https://doi.org/10.3390/molecules27123693
Chicago/Turabian Styleda Silva, Jéssica Montenegro Santana, Adriano de Souza Carolino, Lilian Rodrigues de Oliveira, Douglas de Souza Gonçalves, Matheus Moraes Biondo, Pedro Henrique Campelo, Jaqueline de Araújo Bezerra, Ştefan Ţălu, Henrique Duarte da Fonseca Filho, Hidembergue Ordozgoith da Frota, and et al. 2022. "Poly(o-methoxyaniline) Chain Degradation Based on a Heat Treatment (HT) Process: Combined Experimental and Theoretical Evaluation" Molecules 27, no. 12: 3693. https://doi.org/10.3390/molecules27123693
APA Styleda Silva, J. M. S., de Souza Carolino, A., de Oliveira, L. R., de Souza Gonçalves, D., Biondo, M. M., Campelo, P. H., de Araújo Bezerra, J., Ţălu, Ş., da Fonseca Filho, H. D., da Frota, H. O., & Sanches, E. A. (2022). Poly(o-methoxyaniline) Chain Degradation Based on a Heat Treatment (HT) Process: Combined Experimental and Theoretical Evaluation. Molecules, 27(12), 3693. https://doi.org/10.3390/molecules27123693









