The Intervention of Prebiotics on Depression via the Gut–Brain Axis
Abstract
:1. Introduction
2. The Mechanisms of Intestinal Microbiota and GBA on Depression
2.1. The Composition of Intestinal Microbiota and Its Effects on the Brain
2.2. The Bidirectional Regulation Mechanism of GBA
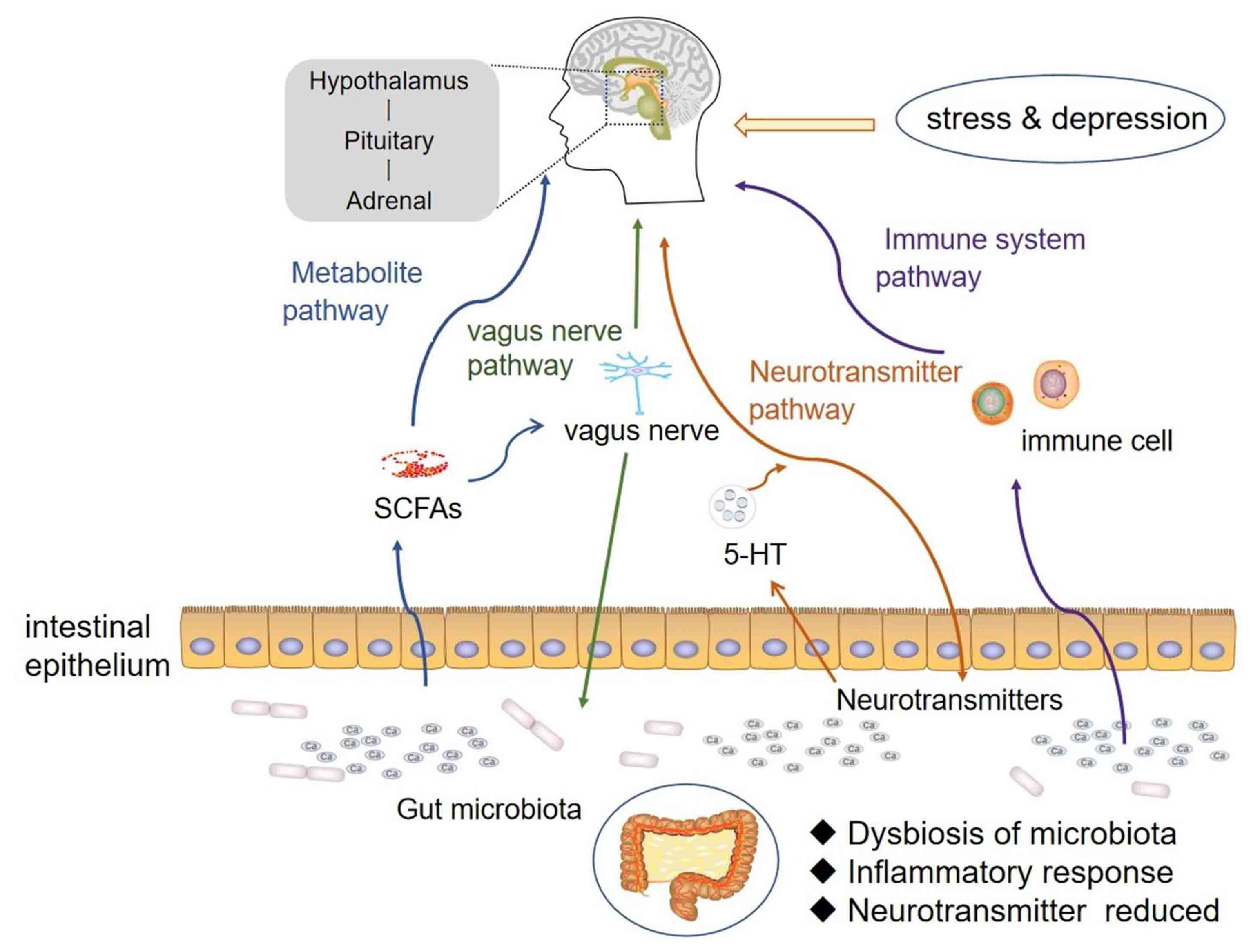
3. GBA on the Pathogenesis of Depression
3.1. Neurotransmitter Pathway
3.2. Metabolite Pathway
3.3. The Vagus Nerve Pathway
3.4. Immune System Pathway
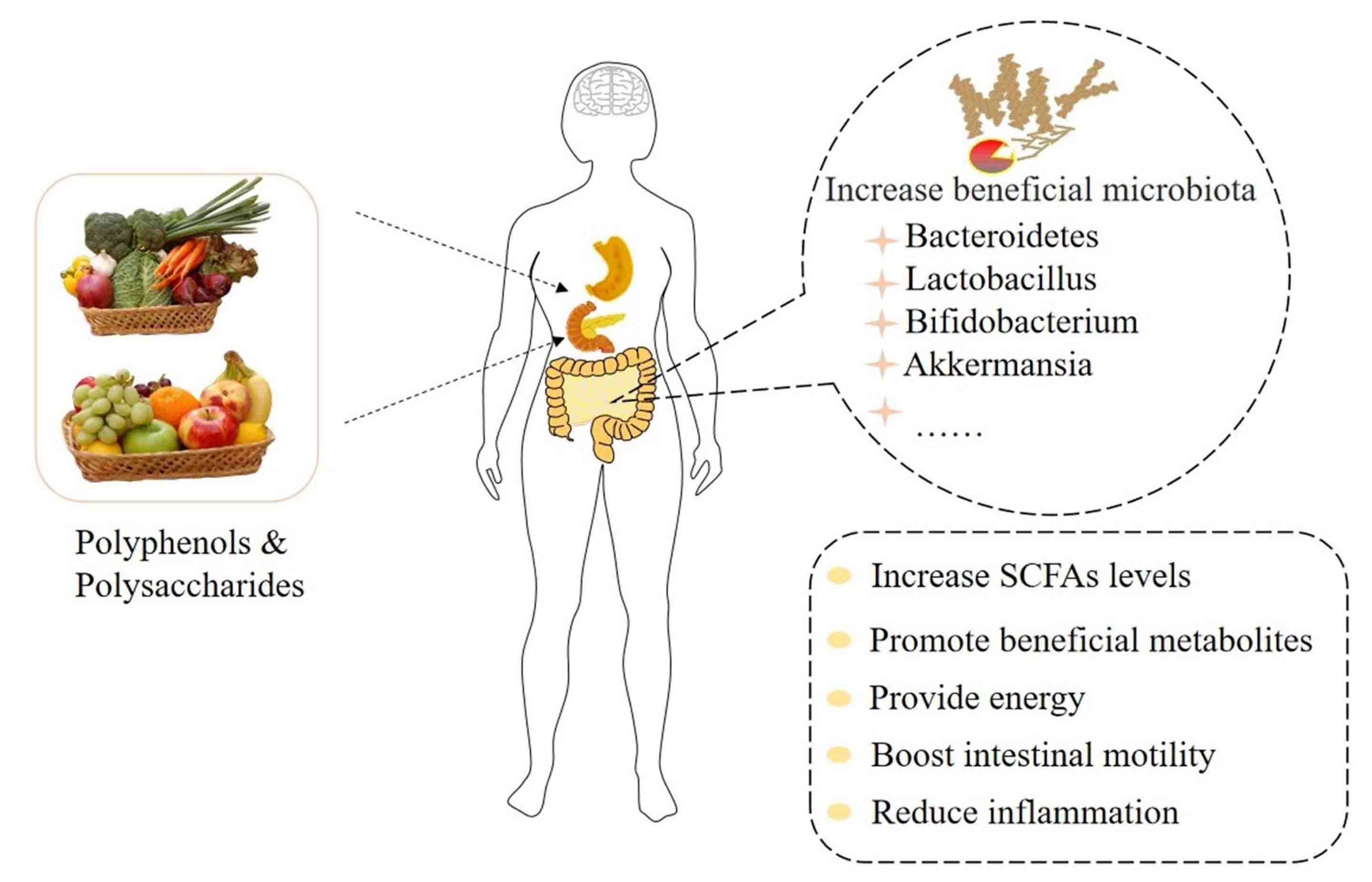
4. Prebiotics: Improve the Imbalance of Intestinal Microbiota to Ameliorate Depression
4.1. The Regulation of Depression by Plant Polysaccharides through Intestinal Microbiota
4.2. The Regulation of Depression by Plant Polyphenols through Intestinal Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastro. Hepat. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Banks, W.A. Evidence for a cholecystokinin gut-brain axis with modulation by bombesin. Peptides 1980, 1, 347–351. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 1310, 701–712. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 1253, 926–938. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Anuar, F.; Zadjali, F.; Rafter, J.; Pettersson, S. Gut microbial communities modulating brain development and function. Gut Microbes 2012, 3, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.; Kim, M.; Yun, C. Regulation of gastrointestinal immunity by metabolites. Nutrients 2021, 13, 167. [Google Scholar] [CrossRef]
- Oleskin, A.V. Microbial Communication and Microbiota-Host Interactivity: Neurophysiological, Biotechnological, and Biopolitical Implications; Nova Science Publishers: Hauppauge, NY, USA, 2020; 256p. [Google Scholar]
- Wouw, M.V.D.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Psychophysiol. 2018, 596, 4923–4944. [Google Scholar]
- Teichman, E.M.; O’Riordan, K.J.; Gahan, C.G.M.; Dinan, T.G.; Cryan, J.F. When rhythms meet the blues: Circadian interactions with the microbiota-gut-brain axis. Cell Metab. 2020, 31, 448–471. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and mechanisms of gut microbiota in patients with Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 650047–650064. [Google Scholar] [CrossRef]
- Douglas-Escobar, M.; Elliott, E.; Neu, J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013, 167, 374–379. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut-brain axis in 2016: Brain-gut-microbiota axis -mood, metabolism and behaviour. Nat. Rev. Gastro. Hepat. 2017, 14, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Alina, W.; Lukasz, P.; Wieslaw, J. Gut microbiota in depression: A focus on ketamine. Front. Behav. Neurosci. 2021, 15, 693362. [Google Scholar]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Glaciol. 2015, 28, 203–209. [Google Scholar]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 5490–15496. [Google Scholar] [CrossRef] [Green Version]
- Goehler, L.E.; Gaykema, R.P.A.; Opitz, N.; Reddaway, R.; Badr, N.; Lyte, M. Activation in vagal afferents and central autonomic pathways: Early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005, 19, 334–344. [Google Scholar] [CrossRef]
- Tracey, B.; Julie, D.; Jane, C.; Nicole, R.; Christine, B.; Pramod, G. The microbiome-gut-brain axis and resilience to developing anxiety or depression under stress. Microorganisms 2021, 9, 723. [Google Scholar]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Dietz, C.; Dekker, M. Effect of green tea phytochemicals on mood and cognition. Curr. Pharm. Des. 2017, 23, 2876–2905. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut microbiota in autism and mood disorders. World J. Gastroentero. 2016, 22, 361–368. [Google Scholar] [CrossRef]
- Shashidhar, G.M.; Giridhar, P.; Manohar, B. Functional polysaccharides from medicinal mushroom Cordyceps sinensis as a potent food supplement: Extraction, characterization and therapeutic potentials—A systematic review. RSC Adv. 2015, 5, 16050–16066. [Google Scholar] [CrossRef]
- Ji, X.L.; Hou, C.Y.; Guo, X.D. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): A review. Int. J. Biol. Macromol. 2019, 135, 637–646. [Google Scholar] [CrossRef]
- Yuan, L.; Zhong, Z.C.; Liu, Y. Structural characterisation and immunomodulatory activity of a neutral polysaccharide from Sambucus adnata Wall. Int. J. Biol. Macromol. 2020, 154, 1400–1407. [Google Scholar] [CrossRef]
- Rajesh, J.; Pravin, M. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechno. 2021, 12, 51–67. [Google Scholar]
- Pyryeva, E.A.; Safronova, A.I. The role of dietary fibers in the nutrition of the population. Vopr. Pitan. 2019, 88, 5–11. [Google Scholar]
- Dinan, T.G.; Cryan, J.F. Microbes, immunity, and behavior: Psychoneuroimmunology meets the microbiome. Neuropsychopharmacology 2017, 42, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Mao, J.; Ding, K.; Zhou, Y.; Zeng, X.L.; Yang, W.J.; Wang, P.P.; Zhao, C.; Yao, J.; Xia, P.; et al. Polysaccharides from Ganoderma lucidum promote cognitive function and neural progenitor proliferation in mouse model of Alzheimer’s disease. Stem Cell Rep. 2017, 8, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.D.; Sang, S.M. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Ishida, N.; Iizuka, M.; Kataoka, K.; Okazaki, M.; Shiraishi, K.; Yagi, Y.; Jobu, K.; Yokota, J.; Oishi, M.; Moriyama, H.; et al. Improvement of blood lipid profiles by Goishi tea polyphenols in a randomised, double-blind, placebo-controlled clinical study. Int. J. Food Sci. Nutr. 2018, 69, 598–607. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.W.; Hu, Y.Z.; Wang, J.; Liu, J.M.; Qui, R.B.; Lv, S.W.; Wang, S. Correlation analysis of intestinal redox state with the gut microbiota reveals the positive intervention of tea polyphenols on hyperlipidemia in high fat diet fed mice. J. Agric. Food Chem. 2019, 67, 7325–7335. [Google Scholar] [CrossRef]
- Barres, B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Ronzano, R. Astrocytes and microglia: Active players in synaptic plasticity. M S-Med. Sci. 2017, 33, 1071–1078. [Google Scholar]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef]
- Yu, X.; Chu, S.; Hagerman, A.E.; Lorigan, G.A. Probing the interaction of polyphenols with lipid bilayers by solidstate NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 6783–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, L.J.; Zhang, H.; Qi, R.L.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Scholl, C.; Lepper, A.; Steffens, M.; Von, M.D.; Brockmoller, J.; Stingl, J. Individual variability in the pharmacokinetic of tea polyphenols and gene expression after oral intake of green tea extract. Clin. Ther. 2015, 37, 116. [Google Scholar] [CrossRef]
- Aktas, O.; Prozorovski, T.; Smorodchenko, A.; Savaskan, N.E.; Lauster, R.; Kloetzel, P.M.; Infante-Duarte, C.; Brocke, S.; Zipp, F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004, 173, 5794–5800. [Google Scholar] [CrossRef] [Green Version]
- Soffietti, R.; Pellerino, A.; Rudà, R. Neuro-oncology perspective of treatment options in metastatic breast cancer. Future Oncol. 2018, 14, 1765–1774. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. 2020, 60, 2691–2709. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Farahani, M.S.; Rahimi, R. Phytochemical constituents as future antidepressants: Acomprehensive review. Rev. Neurosci. 2015, 26, 699–719. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Weng, P. The intervention of unique plant polysaccharides-dietary fiber on depression from the gut-brain axis. Int. J. Boil. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef]
- Yan, T.X.; Nian, T.T.; Liao, Z.Z.; Xiao, F.; Wu, B.; Bi, K.S.; He, B.; Jia, Y. Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int. J. Biol. Macromol. 2020, 144, 427–440. [Google Scholar] [CrossRef]
- Putignani, L.; Chierico, F.D.; Petrucca, A.; Vernocchi, P.; Dallapiccola, B. The human gut microbiota: A dynamic interplay with the host from birth to senescence settled during childhood. Pediatr. Res. 2014, 76, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennisi, E. Evidence mounts that gut bacteria can influence mood, prevent depression. Science 2019. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y.; et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety-and depressive-like behavior following social defeat in mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biot. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104, 132–142. [Google Scholar] [CrossRef]
- Filipe, D.V.; Petia, K.D.; Daisy, G.; Jennifer, V.; Carine, Z.; Adeline, D.; Fredrik, B.; Gilles, M. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar]
- Elaine, Y.H.; Sara, W.M.; Sophia, H.; Gil, S.; Embriette, R.H.; Tyler, M.; Julian, A.C.; Janet, C.; Sarah, E.R.; Joseph, F.P.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar]
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory effects of gut microbiota on the central nervous system: How gut could play a role in neuropsychiatric health and diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Mi, W.L.; Li, Q.; Zhang, M.T.; Han, P.; Hu, S.; Mao-Ying, Q.L.; Wang, Y.Q. Spinal IL-33/ST2 signaling contributes to neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3 cascades in mice. Anesthesiology 2015, 123, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotech. 2015, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The neuroendocrinology of the microbiota-gut-brain axis: A behavioural perspective. Front. Neuroendocrin. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroentero. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Emeran, A.M. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 2, 453–466. [Google Scholar]
- Karakan, T.; Ozkul, C.; Akkol, E.K.; Bilici, S.; SobarzoSánchez, E.; Capasso, R. Gut-Brain-Microbiota axis: Antibiotics and functional gastrointestinal disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- Chase, M.; Matt, V. The fragility of probiotic Bifidobacterium longum NCC3001 use for depression in patients with irritable bowel syndrome. Gastroenterology 2018, 154, 764–769. [Google Scholar]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety-and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Afifa, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar]
- Frye, R.E.; Rose, S.; Slattery, J.; MacFabe, D.F. Gastrointestinal dysfunction in autism spectrum disorder: The role of the mitochondria and the enteric microbiome. Microb. Ecol. Health Dis. 2015, 26, 27458. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A. Bacterial metabolites of human gut microbiota correlating with depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Koren, O.; Elliott, E. Role of tryptophan in microbiota-induced depressive-like behavior: Evidence from tryptophan depletion study. Front. Behav. Neurosci. 2019, 13, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Qin, X.; Gui, Z. The effect of Bailemian on neurotransmitters and gut microbiota in p-chlorophenylalanine induced insomnia mice. Microb. Pathog. 2020, 148, 104474. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The gut microbiome in human neurological disease: A review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Erny, D.; Angelis, A.L.H.D.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatr. 2016, 21, 738–748. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.G.; Milani, C.; De Giori, G.S.; Sesma, F.; Van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Wang, S.-M.; Han, C.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. A review of current evidence for acetyl-l-carnitine in the treatment of depression. J. Psychiatr. Res. 2014, 53, 30–37. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroent. Motil. 2011, 23, e1132–e1544. [Google Scholar] [CrossRef] [Green Version]
- Almeida, D.L.M.V.; Funchal, C.; Gottfried, C.; Wajner, M.; Pessoa-Pureur, R. Propionic acid induces cytoskeletal alterations in cultured astrocytes from rat cerebral cortex. Metab. Brain Dis. 2006, 21, 51–62. [Google Scholar] [CrossRef]
- Perez-Burgos, A.; Wang, B.; Mao, Y.K.; Mistry, B.; Neufeld, K.A.M.; Bienenstock, J.; Kunze, W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, g211–g220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Yu, Q.Q.; Zhang, X.N.; Wang, X.Y.; Su, Y.S.; He, W.; Li, J.; Wan, H.Y.; Jing, X.H. Electroacupuncture regulates inflammatory cytokines by activating the vagus nerve to enhance antitumor immunity in mice with breast tumors. Life Sci. 2021, 272, 119259. [Google Scholar] [CrossRef] [PubMed]
- Pomara, N.; Bruno, D.; Plaska, C.R.; Pillai, A.; Ramos-Cejudo, J.; Osorio, R.; Imbimbo, B.P.; Heslegrave, A.; Zetterberg, H.; Blennow, K. Evidence of upregulation of the cholinergic anti-inflammatory pathway in late-life depression. J. Affect. Disord. 2021, 286, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Oleskin, A.V.; Shenderov, B.A. Interaction of the microbiota with the host’s gastro-intestinal, nervous and immune system in terms of network organization. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–16. [Google Scholar] [CrossRef]
- Kochalska, K.; Oakden, W.; Słowik, T.; Chudzik, A.; Pankowska, A.; Łazorczyk, A.; Kozioł, P.; Andres-Mach, M.; Pietura, R.; Rola, R.; et al. Dietary supplementation with Lactobacillus rhamnosus JB-1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr. Res. 2020, 82, 44–57. [Google Scholar] [CrossRef]
- Rahman, S.; Alzarea, S. Glial mechanisms underlying major depressive disorder: Potential therapeutic opportunities. Prog. Mol. Biol. Transl. 2019, 167, 159–178. [Google Scholar]
- Kosiewicz, M.M.; Dryden, G.W.; Chhabra, A.; Alard, P. Relationship between gut microbiota and development of T cell associated disease. FEBS Lett. 2014, 588, 4195–4206. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem. Res. 2014, 39, 624–644. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Hornig, M.; Serge, R.; Miranda, J.D.; Baghban, A.; Villar, G.; Lipkin, W.I. Passive transfer of Streptococcus induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with Streptococcal infection. Mol. Psychiatr. 2010, 15, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Mossad, O.; Erny, D. The microbiota-microglia axis in central nervous system disorders. Brain Pathol. 2020, 30, 1159–1177. [Google Scholar] [CrossRef]
- Jin, M.; Zhu, Y.; Shao, D.; Zhao, K.; Xu, C.; Li, Q.; Yang, H.; Huang, Q.; Shi, J. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int. J. Biol. Macromol. 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.M.; Peng, Y.J.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.Y.; Zeng, X.X.; Cao, Y.L. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.J.; Chen, C.X.; Zeng, X.X.; Ye, H. Prebiotics effects in vitro of polysaccharides from tea flowers on gut microbiota of healthy persons and patients with inflammatory bowel disease. Int. J. Biol. Macromol. 2020, 158, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, G.; Chen, D.; Ye, H.; Sun, L.; Zeng, X.X.; Liu, Z.H. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. Int. J. Boil. Macromol. 2019, 140, 858–870. [Google Scholar] [CrossRef]
- Shang, Q.S.; Song, G.R.; Zhang, M.F.; Shi, J.J.; Xu, C.Y.; Hao, J.J.; Li, G.Y.; Yu, G.L. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Chen, P.; Hei, M.; Kong, L.; Liu, Y.; Yang, Y.; Mu, H.; Zhang, X.; Zhao, S.; Duan, J. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019, 10, 8161–8171. [Google Scholar] [CrossRef]
- Liu, P.; Bai, X.; Zhang, T.; Zhou, L.; Li, J.; Zhang, L. The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann. Transl. Med. 2019, 7, 811. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Sun, L.; Zhu, Y. Lentinan produces a robust antidepressant-like effect via enhancing the prefrontal Dectin-1/AMPA receptor signaling pathway. Behav. Brain Res. 2017, 317, 263–271. [Google Scholar] [CrossRef]
- Li, B.; Hou, Y.; Zhu, M. 3’-deoxyadenosine (cordycepin) produces a rapid and robust antidepressant effect via enhancing prefrontal AMPA receptor signaling pathway. Int. J. Neuropsychoph. 2015, 19, pyv112. [Google Scholar] [CrossRef] [Green Version]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Design. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stantona, C.; Dinana, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis. Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Mage, I.; Rud, I.; Aas, A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef]
- Parkar, S.G.; Redgate, E.L.; Wibisono, R.; Luo, X.X.; Koh, E.T.H.; Schroder, R. Gut health benefits of kiwifruit pectins: Comparison with commercial functional polysaccharides. J. Funct. Foods 2010, 2, 210–218. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Kan, J.; Gou, Y.R.; Liu, J.; Zhang, X.; Wu, X.N.; Tang, S.X.; Sun, R.; Qian, C.L.; et al. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Boil. Macromol. 2020, 153, 708–722. [Google Scholar] [CrossRef]
- Shohei, A.; Yuko, A.; Yoko, N.; Mitsuru, Y.; Sohsaku, Y.; Takahisa, K.; Kazushige, C.; Taiga, T.; Masaki, H.; Atsushi, A.; et al. Fiber-rich barley increases butyric acid-producing bacteria in the human gut microbiota. Metabolites 2021, 11, 559–607. [Google Scholar]
- Mark, L.; Ashley, C.; Joshua, L.; Ai, Y.; Alexandra, P.; Jay, J.; Gregory, P.; Mihai, C. Resistant starch alters the microbiota-gut brain axis: Implications for dietary modulation of behavior. PLoS ONE 2016, 11, e0146406. [Google Scholar]
- Alfa, M.J.; Strang, D.; Tappia, P.S. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 2018, 37, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celestine, W.; Philip, H.; Lynnette, F. Potential benefits of dietary fiber intervention in inflammatory bowel disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Coussa-Charley, M.; Kahouli, I.; Jones, M.L.; Labbe, A.; Prakash, S. Probiotic ferulic acid esterase active Lactobacillus fermentum NCIMB 5221 APA microcapsules for oral delivery: Dreparation and in vitro characterization. Pharmaceuticals 2012, 5, 236–248. [Google Scholar] [CrossRef]
- Zeni, A.L.; Zomkowski, A.D.; Maraschin, M.; Rodrigues, A.L.; Tasca, C.I. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur. J. Pharmacol. 2012, 679, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda Cazon, C.; Campos Munoz, L.; Conde Taboada, A.; Lopez Bran, E. Viral wart treatment with green tea sinecatechins. An. Pediatr. 2016, 84, 236–237. [Google Scholar] [CrossRef]
- Sun, H.Y.; Chen, Y.H.; Cheng, M.; Zhang, X.; Zheng, X.J.; Zhang, Z.C. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Cheng, L.; Zhang, X.; Yang, H. The role of the intestinal microbiota in the pathogenesis of host depression and mechanism of TPs relieving depression. Food Funct. 2021, 12, 7651. [Google Scholar] [CrossRef]
- Guo, T.; Song, D.; Ho, C.T.; Zhang, X.; Zhang, C.D.; Cao, J.X.; Wu, Z.F. Omics analyses of gut microbiota in a circadian rhythm disorder mouse model fed with oolong tea polyphenols. J. Agric. Food Chem. 2019, 67, 8847–8854. [Google Scholar] [CrossRef]
- Zhu, W.L.; Shi, H.S.; Wei, Y.M.; Wang, S.J.; Sun, C.Y.; Ding, Z.B.; Lu, L. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol. Res. 2012, 65, 74–80. [Google Scholar] [CrossRef]
- Park, K.S.; Han, J.Y.; Moon, D.C.; Hong, J.T.; Oh, K.W. (-)-epigallocatechin-3-O-gallate augments pentobarbital-induced sleeping behaviors through Cl-channel activation. J. Med. Food 2011, 14, 1456–1462. [Google Scholar] [CrossRef]
- Donoso, F.; Egerton, S.; Bastiaanssen, T.F.S.; Fitzgerald, P.; Cryan, J.F. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 2020, 116, 104673. [Google Scholar] [CrossRef]
- Anggreini, P.; Ardianto, C.; Rahmadi, M.; Khotib, J. Quercetin attenuates acute predator stress exposure-evoked innate fear and behavioral perturbation. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 181–214. [Google Scholar] [CrossRef]
- Mehta, V.; Singh, T.R.; Udayabanu, M. Quercetin ameliorates chronic unpredicted stress-induced behavioral dysfunction in male Swiss albino mice by modulating hippocampal insulin signaling pathway. Physiol. Behav. 2017, 182, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, G.B.; Jiménez-Ferrer, E.; Roman-Ramos, R.; Zamilpa, A.; Herrera-Ruiz, M. A mixture of quercetin 4’-O-rhamnoside and isoquercitrin from Tilia americana var. mexicana and its biotransformation products with antidepressant activity in mice. J. Ethnopharmacol. 2021, 267, 113619. [Google Scholar] [CrossRef]
- Guo, J.; Xue, C.; Duan, J.A.; Qian, D.; Tang, Y.; You, Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihotethanol extract and its potential active components in vivo. Phytomedicine 2011, 18, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Anjomshoa, M.; Boroujeni, S.N.; Ghasemi, S.; Lorigooini, Z.; Amiri, A.; Balali-Dehkordi, S.; Amini-Khoei, H. Rutin via increase in the CA3 diameter of the hippocampus exerted antidepressant-like effect in mouse model of maternal separation stress: Possible involvement of NMDA receptors. Behav. Neurol. 2020, 2020, 4813616. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xu, Y.; Jiang, X.; Wang, Z.; Hou, J. Avicularin relieves depressive-like behaviors induced by chronic unpredictable mild stressin mice. Med. Sci. Monit. Basic. 2019, 25, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
| Active Substance | Microbiota Involved | Effects on Brain | Possible Mechanism |
|---|---|---|---|
| Tea polyphenols [40] | Bifidobacterium | protect nerve cells, restrain neuronal apoptosis, EGCG can reach brain parenchyma through BBB in vivo | increase intestinal barrier function, stimulate the immune system, inhibit ROS and NO contents in rat midbrain and striatum |
| Quercetin [41] | Firmicutes, Bacteroidetes | Improved melanin corticotropin and reduced cytokines | Firmicutes/Bacteroidetes ratio decreased |
| Pectin [42] | Lactobacillus | strengthen the mucus layer, prevent activation of inflammatory responses | stimulate diversity of microbiota, improving intestinal integrity and mucosal proliferation |
| Okra polysaccharide [43] | Bacteroides, Lactobacillus | promote goblet cells of the intestinal epithelium, increase the expression of tight junction proteins and repair the damaged intestinal barrier | regulate the imbalance of intestinal microbiota |
| Name | Active Substances (Prebiotics) | Possible Mechanism | Related Products |
|---|---|---|---|
| Hyperici Perforati Herba | Hypericin | Promote the expression of 5-HT and NE in the brain of stress-depressed rats | Extract of St. John’s Wort Tablets |
| Acanthopanax senticosus (Rupr.etMaxim.) SHarms | Syringin | Similar in potency to fluoxetine hydrochloride, overall improvement of depression-related somatic and core symptoms | Sultamicillin Tosylate Capsules |
| Bupleuri Radix | Saikosaponin | Decrease the amount of DA and 5-HT in the prefrontal lobe | Anle tablet, Jieyu Anshen tablet |
| Acorus tatarinowii | 9-aminoacridine, kaempferol and cycloatunol | promote the activity and expression of CREB protein and mRNA in the hippocampal region, and then reduced neuronal apoptosis | Puyu Capsule, Antiyu and tranquilizing granules |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Si, C.; Sun, Z.; Chen, Y.; Zhang, X. The Intervention of Prebiotics on Depression via the Gut–Brain Axis. Molecules 2022, 27, 3671. https://doi.org/10.3390/molecules27123671
He Q, Si C, Sun Z, Chen Y, Zhang X. The Intervention of Prebiotics on Depression via the Gut–Brain Axis. Molecules. 2022; 27(12):3671. https://doi.org/10.3390/molecules27123671
Chicago/Turabian StyleHe, Qinghui, Congcong Si, Zhenjiao Sun, Yuhui Chen, and Xin Zhang. 2022. "The Intervention of Prebiotics on Depression via the Gut–Brain Axis" Molecules 27, no. 12: 3671. https://doi.org/10.3390/molecules27123671
APA StyleHe, Q., Si, C., Sun, Z., Chen, Y., & Zhang, X. (2022). The Intervention of Prebiotics on Depression via the Gut–Brain Axis. Molecules, 27(12), 3671. https://doi.org/10.3390/molecules27123671






