Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans
Abstract
1. Introduction
2. Effects of Different Extraction Methods on Biological Properties
2.1. Aqueous, Polar, and Semi-Polar Extractions
2.2. Non-Polar Extraction
2.3. Microwave-Assisted and Carbon Dioxide-Assisted Extraction
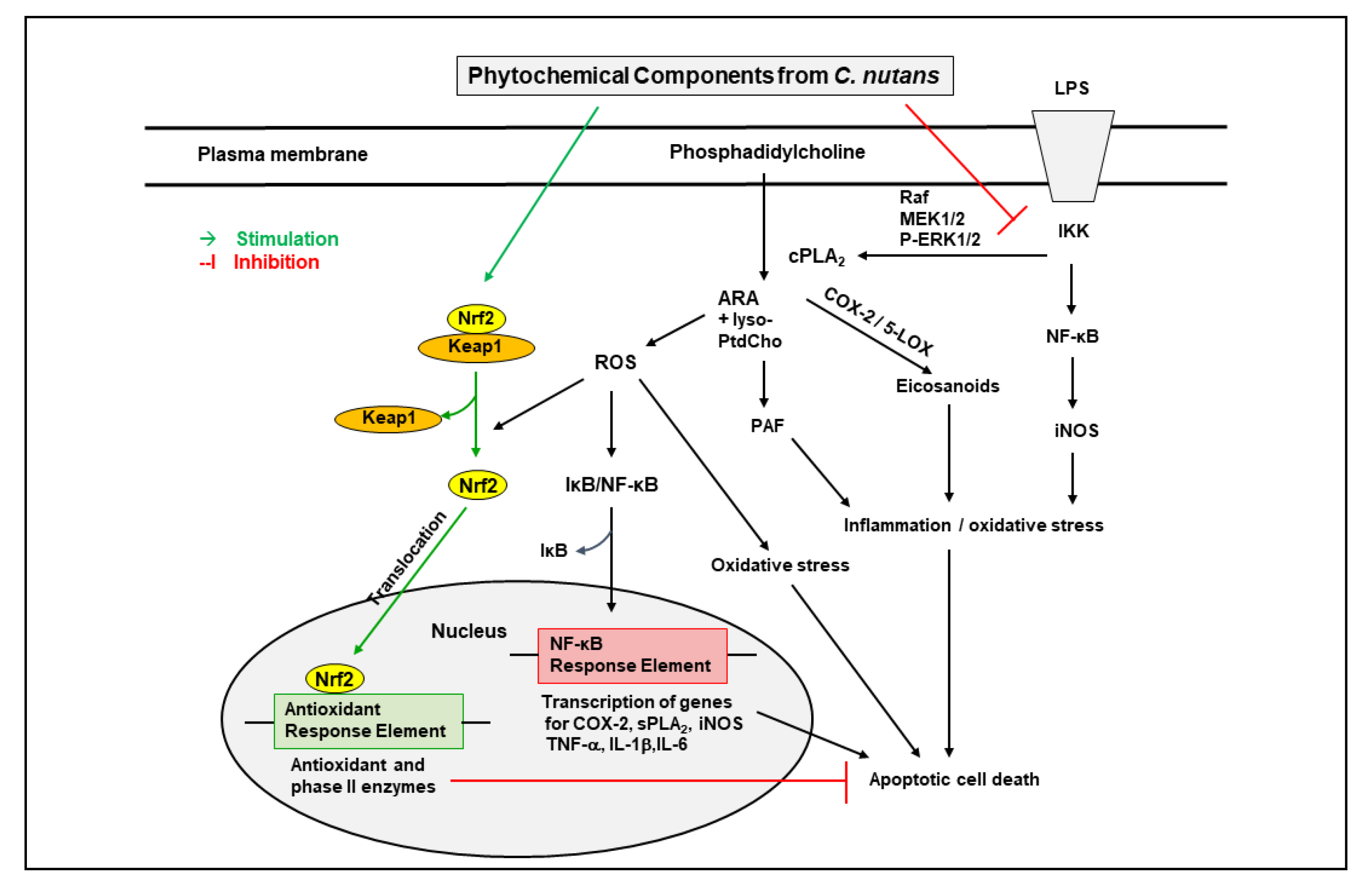
3. Effects of Individual Phytochemical Components of C. nutans
3.1. Schaftoside
3.2. Vitexin
3.2.1. NF-kB and Nrf2 Pathways in Cells and Peripheral Tissues
3.2.2. Cells and Vessels in the CNS
3.3. Isovitexin
3.3.1. Peripheral Organs
3.3.2. Blood Vessels and the CNS
3.4. Orientin
3.4.1. Peripheral Organs
3.4.2. Blood Vessels and the CNS
3.5. Isoorientin
3.5.1. Peripheral Organs
3.5.2. Blood Vessels and the CNS
4. Clinacanthus nutans Extracts on Inflammatory Pathways in Cerebral Ischemia and Neurovascular Systems
4.1. Clinacanthus nutans on Cerebral Ischemia
4.2. Clinacanthus nutans on Neurovascular Systems
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Flora and Fauna Web. Clinacanthus nutans (Burm. F.) Lindau. National Parks: Singapore, 2022. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/5/6/5655 (accessed on 4 May 2022).
- Alam, A.; Ferdosh, S.; Ghafoor, K.; Hakim, A.; Juraimi, A.S.; Khatib, A.; Sarker, Z.I. Clinacanthus nutans: A review of the medicinal uses, pharmacology and phytochemistry. Asian Pac. J. Trop. Med. 2016, 9, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Siew, Y.Y.; Zareisedehizadeh, S.; Seetoh, W.G.; Neo, S.Y.; Tan, C.H.; Koh, H.L. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J. Ethnopharmacol. 2014, 155, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.Y.; Gan, C.Y.; Murugaiyah, V.; Hashmi, S.F.; Fatima, T.; Ibrahim, L.; Abdulla, M.H.; Alswailmi, F.K.; Johns, E.J.; Ahmad, A.A. Narrative Review on the Phytochemistry, Pharmacology and Therapeutic Potentials of Clinacanthus nutans (Burm. f.) Lindau Leaves as an Alternative Source of Future Medicine. Molecules 2021, 27, 139. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.; Baghdadi, A.; Ahmad, I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef]
- Abd Samat, N.M.A.; Ahmad, S.; Awang, Y.; Bakar, R.A.H.; Hakiman, M. Alterations in Herbage Yield, Antioxidant Activities, Phytochemical Contents, and Bioactive Compounds of Sabah Snake Grass (Clinacanthus Nutans L.) with Regards to Harvesting Age and Harvesting Frequency. Molecules 2020, 25, 2833. [Google Scholar] [CrossRef]
- Fong, S.Y.; Piva, T.; Dekiwadia, C.; Urban, S.; Huynh, T. Comparison of cytotoxicity between extracts of Clinacanthus nutans (Burm. f.) Lindau leaves from different locations and the induction of apoptosis by the crude methanol leaf extract in D24 human melanoma cells. BMC Complementary Altern. Med. 2016, 16, 368. [Google Scholar] [CrossRef]
- Farsi, E.; Esmailli, K.; Shafaei, A.; Moradi Khaniabadi, P.; Al Hindi, B.; Khadeer Ahamed, M.B.; Sandai, D.; Abdul Sattar, M.; Ismail, Z.; Abdul Majid, A.M.; et al. Mutagenicity and preclinical safety assessment of the aqueous extract of Clinacanthus nutans leaves. Drug Chem. Toxicol. 2016, 39, 461–473. [Google Scholar] [CrossRef]
- Khoo, L.W.; Foong Kow, A.S.; Maulidiani, M.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. Hematological, Biochemical, Histopathological and (1)H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract. Molecules 2018, 23, 2172. [Google Scholar] [CrossRef]
- Aliyu, A.; Shaari, M.R.; Ahmad Sayuti, N.S.; Reduan, M.F.H.; Sithambaram, S.; Noordin, M.M.; Shaari, K.; Hamzah, H. Subacute Oral Administration of Clinacanthus nutans Ethanolic Leaf Extract Induced Liver and Kidney Toxicities in ICR Mice. Molecules 2020, 25, 2631. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Khaw, K.Y.; Ong, Y.S.; Khan, T.M.; Lee, L.-H.; Lee, W.-L.; Goh, B.-H. An Overview of Clinacanthus nutans (Burm. f.) Lindau as a Medicinal Plant with Diverse Pharmacological Values. Plant Deriv. Bioact. 2020, 461–491. [Google Scholar]
- Khoo, L.W.; Audrey Kow, S.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. A Comprehensive Review on Phytochemistry and Pharmacological Activities of Clinacanthus nutans (Burm.f.) Lindau. Evid. Based Complementary Altern. Med. eCAM 2018, 2018, 9276260. [Google Scholar] [CrossRef] [PubMed]
- Tuntiwachwuttikul, P.; Pootaeng-On, Y.; Phansa, P.; Taylor, W.C. Cerebrosides and a monoacylmonogalactosylglycerol from Clinacanthus nutans. Chem. Pharm. Bull. 2004, 52, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Teshima, K.-I.; Kaneko, T.; Ohtani, K. C-glycosyl flavones from Clinacanthus nutans. Nat. Med. 1997, 51, 557. [Google Scholar]
- Chelyn, J.L.; Omar, M.H.; Mohd Yousof, N.S.; Ranggasamy, R.; Wasiman, M.I.; Ismail, Z. Analysis of flavone C-glycosides in the leaves of Clinacanthus nutans (Burm. f.) Lindau by HPTLC and HPLC-UV/DAD. Sci. World J. 2014, 2014, 724267. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.F.; Liu, R.H.; Cheng, Y.B.; Hsu, Y.M.; Du, Y.C.; El-Shazly, M.; Wu, Y.C.; Chang, F.R. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules 2014, 19, 20382–20390. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef]
- Ban, W.K.; Fong, I.L.; Khong, H.Y.; Phung, J.H.Y. Wound Healing, Antimicrobial and Antioxidant Properties of Clinacanthus nutans (Burm.f.) Lindau and Strobilanthes crispus (L.) Blume Extracts. Molecules 2022, 27, 1722. [Google Scholar] [CrossRef]
- Nordin, F.J.; Pearanpan, L.; Chan, K.M.; Kumolosasi, E.; Yong, Y.K.; Shaari, K.; Rajab, N.F. Immunomodulatory potential of Clinacanthus nutans extracts in the co-culture of triple-negative breast cancer cells, MDA-MB-231, and THP-1 macrophages. PLoS ONE 2021, 16, e0256012. [Google Scholar] [CrossRef]
- Huang, D.; Li, Y.; Cui, F.; Chen, J.; Sun, J. Purification and characterization of a novel polysaccharide-peptide complex from Clinacanthus nutans Lindau leaves. Carbohydr. Polym. 2016, 137, 701–708. [Google Scholar] [CrossRef]
- Zakaria, K.N.; Amid, A.; Zakaria, Z.; Jamal, P.; Ismail, A. Anti-Proliferative Activity of Triterpenes Isolated from Clinicanthus nutans on Hep-G2 Liver Cancer Cells. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 563–567. [Google Scholar] [CrossRef][Green Version]
- Mutazah, R.; Hamid, H.A.; Mazila Ramli, A.N.; Fasihi Mohd Aluwi, M.F.; Yusoff, M.M. In vitro cytotoxicity of Clinacanthus nutans fractions on breast cancer cells and molecular docking study of sulphur containing compounds against caspase-3. Food Chem. Toxicol. 2020, 135, 110869. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Khatib, A.; Ahmed, Q.U.; Ibrahim, Z.; Uzir, B.F.; Benchoula, K.; Yusoff, N.I.N.; Perumal, V.; Alajmi, M.F.; Salamah, S.; et al. Toxicity study on Clinacanthus nutans leaf hexane fraction using Danio rerio embryos. Toxicol. Rep. 2019, 6, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Sakdarat, S.; Shuyprom, A.; Pientong, C.; Ekalaksananan, T.; Thongchai, S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorg. Med. Chem. 2009, 17, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Le, C.F.; Kailaivasan, T.H.; Chow, S.C.; Abdullah, Z.; Ling, S.K.; Fang, C.M. Phytosterols isolated from Clinacanthus nutans induce immunosuppressive activity in murine cells. Int. Immunopharmacol. 2017, 44, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, A.N.; Martin, A.; Mato, R.B.; Cocero, M.J. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind. Crops Prod. 2015, 74, 83–94. [Google Scholar] [CrossRef]
- Khoo, L.W.; Kow, A.S.F.; Maulidiani, M.; Ang, M.Y.; Chew, W.Y.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. (1) H-NMR metabolomics for evaluating the protective effect of Clinacanthus nutans (Burm. f) Lindau water extract against nitric oxide production in LPS-IFN-gamma activated RAW 264.7 macrophages. Phytochemic. Anal. PCA 2019, 30, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Nik Yusoff, N.I.; Uzir, B.F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of alpha-Glucosidase Inhibitors from Clinacanthus nutans Lindau Leaves by Gas Chromatography-Mass Spectrometry-Based Metabolomics and Molecular Docking Simulation. Molecules 2018, 23, 2402. [Google Scholar] [CrossRef]
- Teoh, P.L.; Cheng, A.Y.; Liau, M.; Lem, F.F.; Kaling, G.P.; Chua, F.N.; Cheong, B.E. Chemical composition and cytotoxic properties of Clinacanthus nutans root extracts. Pharm. Biol. 2017, 55, 394–401. [Google Scholar] [CrossRef]
- Panya, A.; Pundith, H.; Thongyim, S.; Kaewkod, T.; Chitov, T.; Bovonsombut, S.; Tragoolpua, Y. Antibiotic-Antiapoptotic Dual Function of Clinacanthus nutans (Burm. f.) Lindau Leaf Extracts against Bovine Mastitis. Antibiotics 2020, 9, 429. [Google Scholar] [CrossRef]
- Roeslan, M.O.; Ayudhya, T.D.N.; Yingyongnarongkul, B.E.; Koontongkaew, S. Anti-biofilm, nitric oxide inhibition and wound healing potential of purpurin-18 phytyl ester isolated from Clinacanthus nutans leaves. Biomed. Pharmacother. 2019, 113, 108724. [Google Scholar] [CrossRef]
- Ismail, N.Z.; Md Toha, Z.; Muhamad, M.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Arsad, H. Antioxidant Effects, Antiproliferative Effects, and Molecular Docking of Clinacanthus nutans Leaf Extracts. Molecules 2020, 25, 2067. [Google Scholar] [CrossRef] [PubMed]
- De Melo, G.O.; Muzitano, M.F.; Legora-Machado, A.; Almeida, T.A.; De Oliveira, D.B.; Kaiser, C.R.; Koatz, V.L.; Costa, S.S. C-glycosylflavones from the aerial parts of Eleusine indica inhibit LPS-induced mouse lung inflammation. Planta Med. 2005, 4, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, G.; Song, M.; Wang, J.; Shen, C.; Chen, Z.; Huang, X.; Gao, Y.; Zhu, C.; Lin, C.; et al. Activation of Farnesoid X Receptor by Schaftoside Ameliorates Acetaminophen-Induced Hepatotoxicity by Modulating Oxidative Stress and Inflammation. Antioxid. Redox Signal. 2020, 33, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wu, J.; Chen, J.; Zhou, Y.; Chen, X.; Wu, Q.; Xu, Y.; Tu, W.; Lou, X.; Yang, G.; et al. Schaftoside ameliorates oxygen glucose deprivation-induced inflammation associated with the TLR4/Myd88/Drp1-related mitochondrial fission in BV2 microglia cells. J. Pharmacol. Sci. 2019, 139, 15–22. [Google Scholar] [CrossRef]
- Dang, J.; Paudel, Y.N.; Yang, X.; Ren, Q.; Zhang, S.; Ji, X.; Liu, K.; Jin, M. Schaftoside Suppresses Pentylenetetrazol-Induced Seizures in Zebrafish via Suppressing Apoptosis, Modulating Inflammation, and Oxidative Stress. ACS Chem. Neurosci. 2021, 12, 2542–2552. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef]
- Dong, L.Y.; Li, S.; Zhen, Y.L.; Wang, Y.N.; Shao, X.; Luo, Z.G. Cardioprotection of vitexin on myocardial ischemia/reperfusion injury in rat via regulating inflammatory cytokines and MAPK pathway. Am. J. Chin. Med. 2013, 41, 1251–1266. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, B.; Yu, W.Y.; Yao, X.; Ma, X.; Sheng, G.; Ma, Q. Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp. Ther. Med. 2016, 12, 1879–1884. [Google Scholar] [CrossRef]
- Wang, F.; Yin, J.; Ma, Y.; Jiang, H.; Li, Y. Vitexin alleviates lipopolysaccharide-induced islet cell injury by inhibiting HMGB1 release. Mol. Med. Rep. 2017, 15, 1079–1086. [Google Scholar] [CrossRef]
- Xie, C.L.; Li, J.L.; Xue, E.X.; Dou, H.C.; Lin, J.T.; Chen, K.; Wu, H.Q.; Wu, L.; Xuan, J.; Huang, Q.S. Vitexin alleviates ER-stress-activated apoptosis and the related inflammation in chondrocytes and inhibits the degeneration of cartilage in rats. Food Funct. 2018, 9, 5740–5749. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, T.; Liu, J.; Gu, L. Vitexin attenuates lipopolysaccharide-induced acute lung injury by controlling the Nrf2 pathway. PLoS ONE 2018, 13, e0196405. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1β-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1α pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef] [PubMed]
- Sae-Tan, S.; Kumrungsee, T.; Yanaka, N. Mungbean seed coat water extract inhibits inflammation in LPS-induced acute liver injury mice and LPS-stimulated RAW 246.7 macrophages via the inhibition of TAK1/IkappaBalpha/NF-kappaB. Journal Food Sci. Technol. 2020, 57, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Yuan, X.; He, L.; Li, X.; Huang, S.; Hou, S.; Liang, J. Vitexin ameliorates chronic stress plub high fat diet-induced nonalcoholic fatty liver disease by inhibiting inflammation. Eur. J. Pharmacol. 2020, 882, 173264. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Yuan, X.; Lu, Y.; Hu, J.; Gao, J.; Lin, J.; Liang, J.; Hou, S.; Chen, S. Vitexin prevents colitis-associated carcinogenesis in mice through regulating macrophage polarization. Phytomed. Int. J. Phytother. Phytopharm. 2021, 83, 153489. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, H.; Gu, X.; Yin, X. The natural flavonoid glycoside vitexin displays preclinical antitumor activity by suppressing NF-κB signaling in nasopharyngeal carcinoma. OncoTarget Ther. 2019, 12, 4461–4468. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Li, X.S.; Tang, X.Y.; Su, W.; Li, X. Vitexin protects melanocytes from oxidative stress via activating MAPK-Nrf2/ARE pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 594–603. [Google Scholar] [CrossRef]
- Min, J.W.; Kong, W.L.; Han, S.; Bsoul, N.; Liu, W.H.; He, X.H.; Sanchez, R.M.; Peng, B.W. Vitexin protects against hypoxic-ischemic injury via inhibiting Ca2+/Calmodulin-dependent protein kinase II and apoptosis signaling in the neonatal mouse brain. Oncotarget 2017, 8, 25513–25524. [Google Scholar] [CrossRef]
- Malar, D.S.; Prasanth, M.I.; Shafreen, R.B.; Balamurugan, K.; Devi, K.P. Grewia tiliaefolia and its active compound vitexin regulate the expression of glutamate transporters and protect Neuro-2a cells from glutamate toxicity. Life Sci. 2018, 203, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Suryanarayanan, V.; Prasanth, M.I.; Singh, S.K.; Balamurugan, K.; Devi, K.P. Vitexin inhibits Aβ25-35 induced toxicity in Neuro-2a cells by augmenting Nrf-2/HO-1 dependent antioxidant pathway and regulating lipid homeostasis by the activation of LXR-α. Toxicol. Vitr. 2018, 50, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.D.; Min, J.W.; Huang, W.X.; Wang, X.; Peng, Y.Y.; Han, S.; Yin, J.; Liu, W.H.; He, X.H.; Peng, B.W. Vitexin reduces epilepsy after hypoxic ischemia in the neonatal brain via inhibition of NKCC1. J. Neuroinflamm. 2018, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dai, J.; Cui, H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed. Pharmacother. 2018, 99, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Kang, S.C. Vitexin inhibits acrylamide-induced neuroinflammation and improves behavioral changes in zebrafish larvae. Neurotoxicol. Teratol. 2019, 74, 106811. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Zhang, B.; Ren, M. The protective effect of vitexinin septic encephalopathy by reducing leukocyte-endothelial adhesion and inflammatory response. Ann. Palliat. Med. 2020, 9, 2079–2089. [Google Scholar] [CrossRef]
- Zhao, C.R.; Yang, F.F.; Cui, Q.; Wang, D.; Zhou, Y.; Li, Y.S.; Zhang, Y.P.; Tang, R.Z.; Yao, W.J.; Wang, X.; et al. Vitexin inhibits APEX1 to counteract the flow-induced endothelial inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, 48. [Google Scholar] [CrossRef]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, Z.; Xue, W.; Sun, F.; Zhu, H.; Huang, D.; Wang, Z.; Dong, L. Vitexin regulates Epac and NLRP3 and ameliorates chronic cerebral hypoperfusion injury. Can. J. Physiol. Pharmacol. 2021, 99, 1079–1087. [Google Scholar] [CrossRef]
- Huang, S.T.; Chen, C.T.; Chieng, K.T.; Huang, S.H.; Chiang, B.H.; Wang, L.F.; Kuo, H.S.; Lin, C.M. Inhibitory effects of a rice hull constituent on tumor necrosis factor alpha, prostaglandin E2, and cyclooxygenase-2 production in lipopolysaccharide-activated mouse macrophages. Ann. N. Y. Acad. Sci. 2005, 1042, 387–395. [Google Scholar] [CrossRef][Green Version]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin Exerts Anti-Inflammatory and Anti-Oxidant Activities on Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting MAPK and NF-kappaB and Activating HO-1/Nrf2 Pathways. Int. J. Biol. Sci. 2016, 12, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Huang, S.T.; Liang, Y.C.; Lin, M.S.; Shih, C.M.; Chang, Y.C.; Chen, T.Y.; Chen, C.T. Isovitexin suppresses lipopolysaccharide-mediated inducible nitric oxide synthase through inhibition of NF-kappa B in mouse macrophages. Planta Med. 2005, 71, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Wang, H.; Pan, C.W.; Lin, M.X. Isovitexin alleviates liver injury induced by lipopolysaccharide/d-galactosamine by activating Nrf2 and inhibiting NF-kappaB activation. Microb. Pathog. 2018, 119, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Wang, J. Isovitexin protects against cisplatin-induced kidney injury in mice through inhibiting inflammatory and oxidative responses. Int. Immunopharmacol. 2020, 83, 106437. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ma, Y.; He, Y.; Yang, H.; Chen, Y.; Wang, L.; Huang, D.; Qiu, S.; Tao, X.; Chen, W. A Network Pharmacology-Based Investigation to the Pharmacodynamic Material Basis and Mechanisms of the Anti-Inflammatory and Anti-Viral Effect of Isatis indigotica. Drug Des. Dev. Ther. 2021, 15, 3193–3206. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, R.; Chandel, S.; Upadhyay, S.; Bendre, R.; Ganugula, R.; Potunuru, U.R.; Giri, H.; Sahu, G.; Kumar, P.U.; Reddy, G.B.; et al. Gentiana lutea exerts anti-atherosclerotic effects by preventing endothelial inflammation and smooth muscle cell migration. Nutr. Metab. Cardiovasc. Dis. NMCD 2016, 26, 293–301. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Hu, G.; He, D.; Li, Y.; Ran, X.; Du, J.; Fu, S.; Liu, D. Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKβ/AMPK-PGC-1α Signaling Axis. Front. Immunol. 2019, 10, 2650. [Google Scholar] [CrossRef]
- Xiao, Q.; Qu, Z.; Zhao, Y.; Yang, L.; Gao, P. Orientin Ameliorates LPS-Induced Inflammatory Responses through the Inhibitory of the NF-κB Pathway and NLRP3 Inflammasome. Evid. Based Complementary Altern. Med. eCAM 2017, 2017, 2495496. [Google Scholar] [CrossRef]
- Xiao, Q.; Piao, R.; Wang, H.; Li, C.; Song, L. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int. J. Biol. Macromol. 2018, 118, 747–755. [Google Scholar] [CrossRef]
- Dhakal, H.; Lee, S.; Choi, J.K.; Kwon, T.K.; Khang, D.; Kim, S.H. Inhibitory effects of orientin in mast cell-mediated allergic inflammation. Pharmacol. Rep. PR 2020, 72, 1002–1010. [Google Scholar] [CrossRef]
- Xiao, Q.; Cui, Y.; Zhao, Y.; Liu, L.; Wang, H.; Yang, L. Orientin relieves lipopolysaccharide-induced acute lung injury in mice: The involvement of its anti-inflammatory and anti-oxidant properties. Int. Immunopharmacol. 2021, 90, 107189. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Vaiyapuri, M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1,2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017, 96, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Bae, J.S. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vasc. Pharmacol. 2014, 62, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Kwak, S.; Bae, J.S. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo. Inflammation 2014, 37, 2164–2173. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, P.; Hao, L.; Tao, L.; Jia, J.; Gao, B.; Liu, J.Y.; Zheng, L.T.; Zhen, X. Antiinflammatory effects of orientin-2′′-O-galactopyranoside on lipopolysaccharide-stimulated microglia. Biol. Pharm. Bull. 2014, 37, 1282–1294. [Google Scholar] [CrossRef]
- Yoo, H.; Ku, S.K.; Lee, T.; Bae, J.S. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation 2014, 37, 1705–1717. [Google Scholar] [CrossRef]
- Bae, J.S. Inhibitory Effect of Orientin on Secretory Group IIA Phospholipase A2. Inflammation 2015, 38, 1631–1638. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Chen, X.; Yang, H.; Li, X.; Xu, Y.; Zhu, X. Orientin alleviates cognitive deficits and oxidative stress in Aβ1-42-induced mouse model of Alzheimer’s disease. Life Sci. 2015, 121, 104–109. [Google Scholar] [CrossRef]
- Wang, X.; An, F.; Wang, S.; An, Z.; Wang, S. Orientin Attenuates Cerebral Ischemia/Reperfusion Injury in Rat Model through the AQP-4 and TLR4/NF-κB/TNF-α Signaling Pathway. J. Stroke Cerebrovasc. Dis. 2017, 26, 2199–2214. [Google Scholar] [CrossRef]
- Guo, D.; Hu, X.; Zhang, H.; Lu, C.; Cui, G.; Luo, X. Orientin and neuropathic pain in rats with spinal nerve ligation. Int. Immunopharmacol. 2018, 58, 72–79. [Google Scholar] [CrossRef]
- Li, C.; Cai, C.; Zheng, X.; Sun, J.; Ye, L. Orientin suppresses oxidized low-density lipoproteins induced inflammation and oxidative stress of macrophages in atherosclerosis. Biosci. Biotechnol. Biochem. 2020, 84, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Jalil, J.; Husain, K.; Mohamad, H.F.; Ahmad, A. Luteolin and apigenin derived glycosides from Alphonsea elliptica abrogate LPS-induced inflammatory responses in human plasma. J. Ethnopharmacol. 2021, 275, 114120. [Google Scholar] [CrossRef] [PubMed]
- Küpeli, E.; Aslan, M.; Gürbüz, I.; Yesilada, E. Evaluation of in vivo biological activity profile of isoorientin. Z. Naturforschung. C J. Biosci. 2004, 59, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, H.S.; Choi, J.K.; Lee, I.S.; Choi, H.J. Isoorientin induces Nrf2 pathway-driven antioxidant response through phosphatidylinositol 3-kinase signaling. Arch. Pharm. Res. 2007, 30, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Han, X.; Li, W.; Ren, D.; Yang, X. Isoorientin Prevents Hyperlipidemia and Liver Injury by Regulating Lipid Metabolism, Antioxidant Capability, and Inflammatory Cytokine Release in High-Fructose-Fed Mice. J. Agric. Food Chem. 2016, 64, 2682–2689. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Y.; Lv, S.; Tan, S.; Zhang, S.; Huang, R.; Zhuo, L.; Liang, S.; Lu, Z.; Huang, Q. Gypsophila elegans isoorientin attenuates CCl(4)-induced hepatic fibrosis in rats via modulation of NF-kappaB and TGF-β1/Smad signaling pathways. Int. Immunopharmacol. 2015, 28, 305–312. [Google Scholar] [CrossRef]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of Anti-Inflammatory Properties of Isoorientin Isolated from Tubers of Pueraria tuberosa. Oxidative Med. Cell. Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef]
- Fan, X.; Wei, W.; Huang, J.; Liu, X.; Ci, X. Isoorientin Attenuates Cisplatin-Induced Nephrotoxicity Through the Inhibition of Oxidative Stress and Apoptosis via Activating the SIRT1/SIRT6/Nrf-2 Pathway. Front. Pharmacol. 2020, 11, 264. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Xu, Y. Isoorientin suppresses sepsis-induced acute lung injury in mice by activating an EPCR-dependent JAK2/STAT3 pathway. J. Mol. Histol. 2021, 53, 97–109. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Ren, X.; Liu, Q.; Wang, J.; Liu, X. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-kappaB signaling pathway in BV-2 microglia. Mol. Cell. Biochem. 2014, 386, 153–165. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kwon, S.H.; Lee, S.Y.; Jang, C.G. Isoorientin improves scopolamine-induced cognitive impairments by restoring the cholinergic system, antioxidant defense, and p-CREB/BDNF signaling in the hippocampus and frontal cortex. Arch. Pharm. Res. 2019, 42, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Tan, X.; Liu, J.; Zhi, Y.; Yi, L.; Bai, S.; Du, Q.; Li, Q.X.; Dong, Y. Isoorientin Inhibits Inflammation in Macrophages and Endotoxemia Mice by Regulating Glycogen Synthase Kinase 3β. Mediat. Inflamm. 2020, 2020, 8704146. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, K.Y.; Park, B. Isoorientin Inhibits Amyloid β(25-35)-Induced Neuronal Inflammation in BV2 Cells by Blocking the NF-κB Signaling Pathway. Molecules 2021, 26, 7056. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Geng, X.; Teng, T.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; Lee, J.C. Dynamic Role of Phospholipases A2 in Health and Diseases in the Central Nervous System. Cells 2021, 10, 2693. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Ho, C.F.Y.; Ng, Y.K.; Farooqui, A.A. Use of Phytochemicals against Neuroinflammation. In Neuroprotective Effects of Phytochemicals in Neurological Disorders; Wiley: Hoboken, NJ, USA, 2017; p. 4. [Google Scholar]
- Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Gu, Z.; Lee, J.C.; Folk, W.R.; Greenlief, C.M.; Sun, G.Y. Yin-Yang Mechanisms Regulating Lipid Peroxidation of Docosahexaenoic Acid and Arachidonic Acid in the Central Nervous System. Front. Neurol. 2019, 10, 642. [Google Scholar] [CrossRef]
- Tan, C.S.; Ho, C.F.; Heng, S.S.; Wu, J.S.; Tan, B.K.; Ng, Y.K.; Sun, G.Y.; Lin, T.N.; Ong, W.Y. Clinacanthus nutans Extracts Modulate Epigenetic Link to Cytosolic Phospholipase A2 Expression in SH-SY5Y Cells and Primary Cortical Neurons. Neuromol. Med. 2016, 18, 441–452. [Google Scholar] [CrossRef]
- Tsai, H.D.; Wu, J.S.; Kao, M.H.; Chen, J.J.; Sun, G.Y.; Ong, W.Y.; Lin, T.N. Clinacanthus nutans Protects Cortical Neurons Against Hypoxia-Induced Toxicity by Downregulating HDAC1/6. Neuromol. Med. 2016, 18, 274–282. [Google Scholar] [CrossRef]
- Wu, J.S.; Kao, M.H.; Tsai, H.D.; Cheung, W.M.; Chen, J.J.; Ong, W.Y.; Sun, G.Y.; Lin, T.N. Clinacanthus nutans Mitigates Neuronal Apoptosis and Ischemic Brain Damage Through Augmenting the C/EBPβ-Driven PPAR-gamma Transcription. Mol. Neurobiol. 2018, 55, 5425–5438. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wu, J.S.; Tsai, H.D.; Huang, C.Y.; Chen, J.J.; Sun, G.Y.; Lin, T.N. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) and neurodegenerative disorders. Mol. Neurobiol. 2012, 46, 114–124. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.J.; Gao, Y.; Bennett, M.V.L.; et al. Peroxisome proliferator-activated receptor gamma (PPARgamma): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018, 163, 27–58. [Google Scholar] [CrossRef]
- Kao, M.-H.; Wu, J.-S.; Cheung, W.-M.; Chen, J.-J.; Sun, G.Y.; Ong, W.-Y.; Herr, D.R.; Lin, T.-N. Clinacanthus nutans mitigates neuronal death and reduces ischemic brain injury: Role of NF-κB-driven IL-1β transcription. Neuromolecular Med. 2020, 23, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Azam, A.; Ismail, I.S.; Shaikh, M.F.; Shaari, K.; Abas, F. Effects of Clinacanthus nutans leaf extract on lipopolysaccharide-induced neuroinflammation in rats: A behavioral and (1)H NMR-based metabolomics study. Avicenna J. Phytomed. 2019, 9, 164–186. [Google Scholar]
- Ahmad Azam, A.; Ismail, I.S.; Kumari, Y.; Shaikh, M.F.; Abas, F.; Shaari, K. The anti-neuroinflammatory effects of Clinacanthus nutans leaf extract on metabolism elucidated through 1H NMR in correlation with cytokines microarray. PLoS ONE 2020, 15, e0238503. [Google Scholar] [CrossRef] [PubMed]
- Kuo, X.; Herr, D.R.; Ong, W.-Y. Anti-inflammatory and Cytoprotective Effect of Clinacanthus nutans Leaf but not Stem Extracts on 7-Ketocholesterol Induced Brain Endothelial Cell Injury. Neuromol. Med. 2021, 23, 176–183. [Google Scholar] [CrossRef] [PubMed]

| Solvent | Phytochemical Components | Potential Health Effects | References |
|---|---|---|---|
| Distilled water | Glucoside Sulfur-containing compounds Phytosterols Triterpenoids Flavones Amino acids | Anti-inflammatory | [27] |
| Methanol | Schaftoside Isoorientin Orientin Isovitexin Vitexin | Anti-inflammatory | [6] |
| Methanol | Entadamide C Clinamide D | Anticancer | [22] |
| Methanol | Palmitic acid Phytol 1-Monopalmitin Stigmast-5-ene Pentadecanoic acid Heptadecanoic acid 1-linolenoylglycerol Glycerol monostearate Alpha-tocospiro B Stigmasterol | Anti-diabetic | [28] |
| Methanol | Betulin Stigmasterol Sitosterol β-Amyrin Vitamin E Campesterol | Anticancer | [29] |
| Methanol | Schaftoside Isomollupentin 7-O-β-glucopyranoside Orientin Isoorientin Vitexin Isovitexin | [14] | |
| Ethanol | Glyceryl 1,3-disterate ester (C39H76O5), Kaempferol 3-O-feruloyl-sophoroside 7-O-glucoside (C43H48O24) Hydroxypthioceranic acid (C46H92O3) | Antiapoptotic | [30] |
| Ethanol | Myricetin Orientin Isoorientin Vitexin Isovitexin Apigenin Ferulic acid | [10] | |
| Ethanol | Clinamides 2-cis-entadamide | [16] | |
| Ethanol | Schaftoside Orientin Isovitexin Vitexin | Anti-inflammatory | [15] |
| Chloroform | Purpurin-18 phytyl ester | Anti-inflammatory | [31] |
| Dichloromethane | Palmitic acid Linolenyl alcohol | Anticancer | [32] |
| Hexane | Palmitic acid Phytol Hexadecanoic acid 1-Monopalmitin Stigmast-5-ene Pentadecanoic acid Heptadecanoic acid 1-Linolenoylglycerol Stigmasterol | [23] | |
| Hexane | Schaftoside Stigmasterol β-sitosterol Triterpenoid lupeol | Immuno-modulatory | [25] |
| Hexane | 13(2)-hydroxy-(13(2)-R)-phaeophytin b, 13(2)-hydroxy-(13(2)-S)-phaeophytin a 13(2)-hydroxy-(13(2)-R)-phaeophytin a. | Antiviral | [24] |
| Ethyl acetate | Lupeol Lup-20(29)-en-3-one Lup-20(29)-en-ol acetate Stigmasterol Sitosterol Betulin Campesterol Squalene Vitamin E Oleic acid | Anticancer | [29] |
| Microwave Assisted Extraction | Polyphenols Flavonoids | [26] | |
| Supercritical carbon dioxide | Phytosterols β-Sitosterol | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, W.-Y.; Herr, D.R.; Sun, G.Y.; Lin, T.-N. Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans. Molecules 2022, 27, 3607. https://doi.org/10.3390/molecules27113607
Ong W-Y, Herr DR, Sun GY, Lin T-N. Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans. Molecules. 2022; 27(11):3607. https://doi.org/10.3390/molecules27113607
Chicago/Turabian StyleOng, Wei-Yi, Deron R. Herr, Grace Y. Sun, and Teng-Nan Lin. 2022. "Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans" Molecules 27, no. 11: 3607. https://doi.org/10.3390/molecules27113607
APA StyleOng, W.-Y., Herr, D. R., Sun, G. Y., & Lin, T.-N. (2022). Anti-Inflammatory Effects of Phytochemical Components of Clinacanthus nutans. Molecules, 27(11), 3607. https://doi.org/10.3390/molecules27113607






