Preclinical Pharmacokinetics and Bioavailability of Oxypeucedanin in Rats after Single Intravenous and Oral Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiments
2.3. Analytical Method for Oxypeucedanin and Method Validation
2.4. Pharmacokinetic Calculations
2.5. Statistical Analysis
3. Results and Discussion
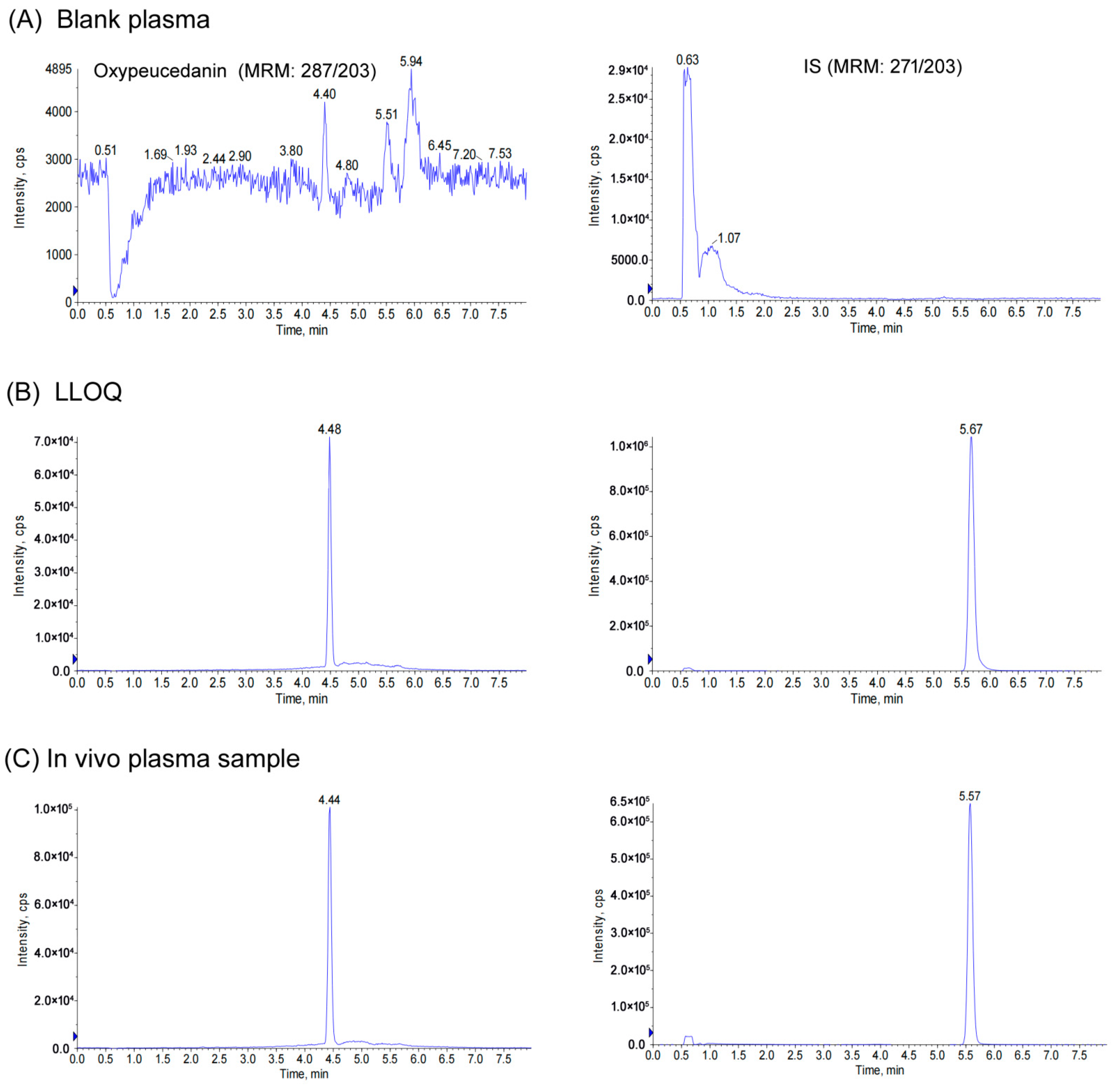
3.1. Method Development
3.2. Method Validation
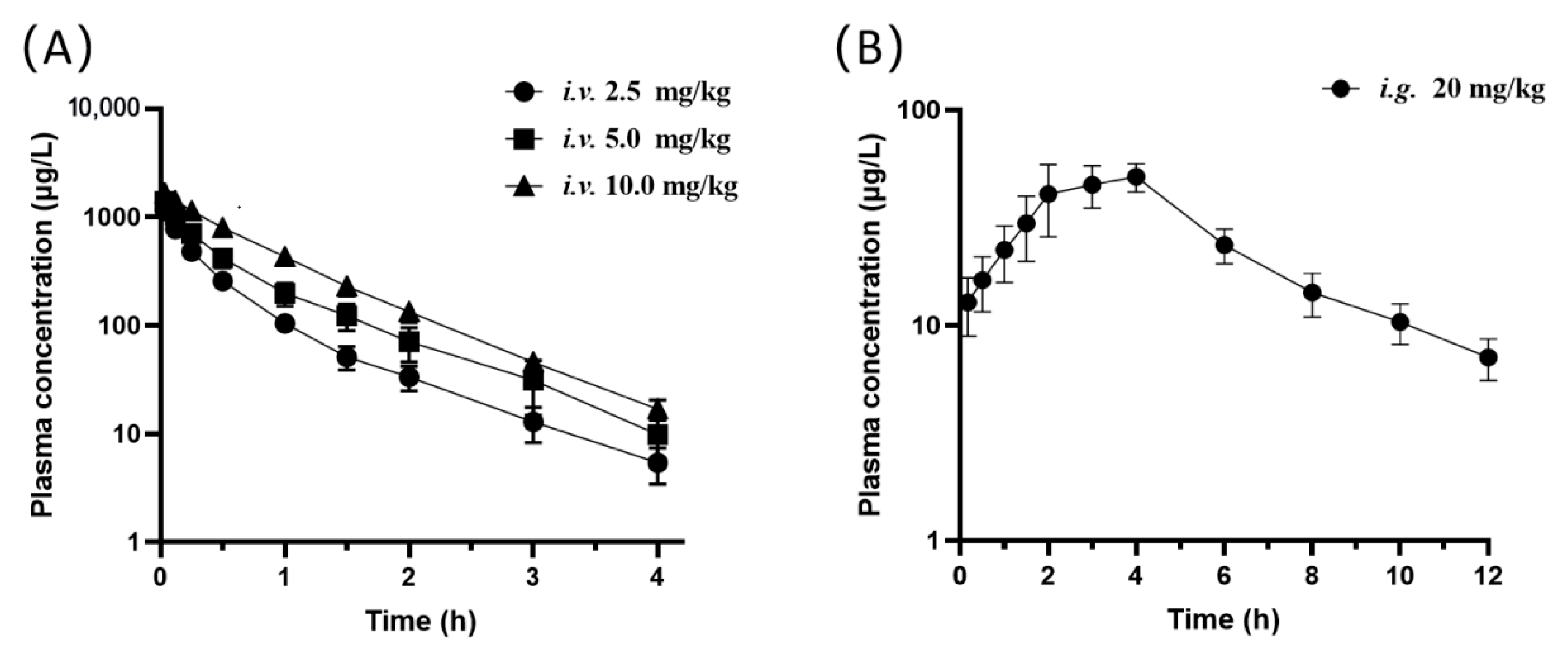
3.3. Pharmacokinetics and Bioavailability of Oxypeucedanin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UPLC/MS/MS | ultra-high performance liquid chromatography–tandem mass spectrometry |
| i.v. | intravenous administration |
| i.g. | intragastric administration |
| T1/2Z | elimination half-life |
| MRT | mean residence time |
| VZ | apparent volume of distribution |
| CLZ | systemic clearance |
| Tmax | the time to reach the peak concentration |
| AUC | the area under concentration-time curve |
| ADR | Angelicae Dahurica Radix |
| IS | internal standard |
| MRM | multiple-reaction monitoring |
| QC | quality control |
| LLOQ | lower limits of quantification |
| RSD | relative standard deviation |
References
- Wei, Y.; Ito, Y. Preparative isolation of imperatorin, oxypeucedanin and isoimperatorin from traditional Chinese herb bai zhi Angelica dahurica (Fisch. Ex Hoffm) Benth. Et Hook using multidimensional high-speed counter-current chromatography. J. Chromatogr. A 2006, 1115, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Q.; Zhang, X.M.; Wang, R.R.; Liu, Q.M.; Zheng, Y.T.; Zhou, J.; Chen, J.J. Anti-HIV Active Constituents from Angelica apaensis. Nat. Prod. Res. Dev. 2008, 20, 239–244. [Google Scholar]
- Xiao, W.L.; Li, S.H.; Shen, Y.H.; Niu, X.M.; Sun, H.D. Two new coumarin glucosides from the roots of Angelica apaensis and their anti-platelet aggregation activity. Arch. Pharm. Res. 2007, 30, 799–802. [Google Scholar] [CrossRef]
- Hwangbo, H.; Choi, E.O.; Kim, M.Y.; Kwon, D.H.; Ji, S.Y.; Lee, H.; Hong, S.H.; Kim, G.Y.; Hwang, H.J.; Hong, S.H.; et al. Suppression of tumor growth and metastasis by ethanol extract of Angelica dahurica Radix in murine melanoma B16F10 cells. Biosci. Trends 2020, 14, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Hu, Z.; Yan, F.; Lei, S.; Li, T.; Li, X.; Xu, C.; Sun, B.; Pan, C.; Chen, L. Angelica dahurica promoted angiogenesis and accelerated wound healing in db/db mice via the HIF-1alpha/PDGF-beta signaling pathway. Free Radic. Biol. Med. 2020, 160, 447–457. [Google Scholar] [CrossRef]
- Mottaghipisheh, J. Oxypeucedanin: Chemotaxonomy, isolation, and bioactivities. Plants 2021, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.J.; Lee, S.Y.; Singh, R.P.; Agarwal, R.; Yim, D.S. Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells. Acta Oncol. 2009, 48, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Shin, H.Y.; Kwon, K.S.; Shin, S.; Choung, S.Y.; Kwon, Y.S.; Lee, J.W.; Choi, B.H.; Lee, C.K. Effects of oxypeucedanin on global gene expression and MAPK signaling pathway in mouse neuroblastoma Neuro-2A cells. Planta Med. 2011, 77, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Hong, J.Y.; Park, H.J.; Lee, S.K. The antiproliferative activity of oxypeucedanin via induction of G2/M phase cell cycle arrest and p53-Dependent MDM2/p21 expression in human hepatoma cells. Molecules 2020, 25, 501. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Wang, J.; Yuan, L.; Hao, L.; Wang, D. Study on the mechanism and intervention strategy of sunitinib induced nephrotoxicity. Eur. J. Pharmacol. 2019, 864, 172709. [Google Scholar] [CrossRef]
- Singh, S.S. Preclinical pharmacokinetics: An approach towards safer and efficacious drugs. Curr. Drug Metab. 2006, 7, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, J.; Ma, G.; Zhang, J.; Zhu, Q.; Liu, H.; Zhang, P.; Zhu, Y.; Cai, W. Bioavailability and pharmacokinetics of S-propargyl-L-cysteine, a novel cardioprotective agent, after single and multiple doses in Beagle dogs. Xenobiotica 2012, 42, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jian, Y.; Wei, N.; Yuan, M.; Zhuang, X.; Li, H. Separation and simultaneous quantification of nine furanocoumarins from Radix Angelicae dahuricae using liquid chromatography with tandem mass spectrometry for bioavailability determination in rats. J. Sep. Sci. 2015, 38, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Peng, C.; Du, W.; Wang, S. Pharmacokinetic study of eight coumarins of Radix Angelicae dahuricae in rats by gas chromatography-mass spectrometry. Fitoterapia 2013, 89, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Yang, H.J.; Ma, J.Y. Simultaneous determination of three furanocoumarins by UPLC/MS/MS: Application to pharmacokinetic study of Angelica dahurica radix after oral administration to normal and experimental Colitis-Induced rats. Molecules 2017, 22, 416. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Yu, C.; Yuan, M.; Li, H. High hepatic exposure of furanocoumarins in Radix Angelica dahuricae is associated with transporter mediated active uptake. J. Ethnopharmacol. 2018, 212, 74–85. [Google Scholar] [CrossRef]
- Huo, H.L.; Yu, S.H.; Liu, X.Z.; Meng, Y.; Ren, Y.P.; Zhang, L.T. Simultaneous and sensitive determination of eight coumarins in rat bile and urine after oral administration of. Acta Chromatogr. 2013, 25, 201–219. [Google Scholar] [CrossRef]
- Liu, M.; Shi, X.; Yang, W.; Liu, S.; Wang, N.; Shi, R.; Qiao, S.; Wang, Q.; Wang, Y. Quantitative analysis of nine coumarins in rat urine and bile after oral administration of Radix Glehniae extract by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2011, 25, 783–793. [Google Scholar] [CrossRef]
- Dong, W.; Liao, Z.G.; Zhao, G.W.; Guan, X.J.; Zhang, J.; Liang, X.L.; Yang, M. Reversal Effect of Oxypeucedanin on P-glycoprotein-mediated Drug Transport. Molecules 2018, 23, 1841. [Google Scholar] [CrossRef] [Green Version]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef]
- Liu, Y.; Flynn, T.J. CYP3A4 inhibition by Psoralea corylifolia and its major components in human recombinant enzyme, differentiated human hepatoma HuH-7 and HepaRG cells. Toxicol. Rep. 2015, 2, 530–534. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Shi, X.; Zhang, G.; Guo, F.; Shan, L.; Cai, J. Metabolism and metabolic inhibition of xanthotoxol in human liver microsomes. Evid. Based Complement. Alternat. Med. 2016, 2016, 5416509. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, Y.; Fu, Y.; Cui, T.; Wang, Q.; Mao, X.; Peng, Y.; Zheng, J. Oxypeucedanin is a mechanism-based inactivator of CYP2B6 and CYP2D6. Curr. Drug Metab. 2021, 22, 882–892. [Google Scholar] [CrossRef]

| Nominal Concentration (ng/mL) | Determined Concentration (ng/mL) | Accuracy (%) | Precision (%) |
|---|---|---|---|
| 2(LLOQ) | 1.84 ± 0.29 | 87.05 | 16.19 |
| 5 | 5.4092 ± 0.59 | 106.55 | 10.98 |
| 10 | 9.7690 ± 0.86 | 94.91 | 8.77 |
| 50 | 53.5744 ± 5.88 | 95.71 | 10.97 |
| 100 | 114.2086 ± 10.72 | 104.98 | 9.39 |
| 500 | 513.9346 ± 17.32 | 101.61 | 3.37 |
| 1000 | 968.5228 ± 31.61 | 98.98 | 3.26 |
| Nominal Concentration (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 15) | ||||
|---|---|---|---|---|---|---|
| Determined Concentration (ng/mL) | Accuracy (%) | Precision (%) | Determined Concentration (ng/mL) | Accuracy (%) | Precision (%) | |
| 2(LLOQ) | 2.00 ± 0.24 | 100.87 | 12.19 | 1.92 ± 0.27 | 96.25 | 13.86 |
| 6 | 6.02 ± 0.37 | 106.98 | 6.19 | 6.36 ± 0.49 | 106.48 | 7.71 |
| 60 | 57.05 ± 2.62 | 98.75 | 4.61 | 59.09 ± 4.86 | 98.49 | 8.22 |
| 900 | 861.82 ± 22.42 | 97.24 | 2.60 | 832.55 ± 39.68 | 92.51 | 4.77 |
| Conditions | Determined Concentration (ng/mL) | Accuracy (%) | Precision (%) |
|---|---|---|---|
| 6 ng/mL | |||
| Autosampler (room temperature, 12 h) | 5.72 ± 0.45 | 95.39 | 7.91 |
| Bench-top (room temperature, 12 h) | 5.98 ± 0.68 | 99.67 | 11.40 |
| Freeze–thaw (three cycles) | 6.14 ± 0.22 | 102.36 | 3.52 |
| Long-term (1 month at −80 °C) | 5.73 ± 0.32 | 95.50 | 5.65 |
| 60 ng/mL | |||
| Autosampler (room temperature, 12 h) | 66.76 ± 1.33 | 111.26 | 1.99 |
| Bench-top (room temperature, 12 h) | 62.56 ± 1.66 | 104.26 | 2.66 |
| Freeze–thaw (three cycles) | 59.87 ± 2.19 | 99.79 | 3.66 |
| Long-term (1 month at −80 °C) | 63.12 ± 2.33 | 105.20 | 3.69 |
| 900 ng/mL | |||
| Autosampler (room temperature, 12 h) | 923.85 ± 38.89 | 102.65 | 4.21 |
| Bench-top (room temperature, 12 h) | 943.02 ± 52.15 | 104.78 | 5.53 |
| Freeze–thaw (three cycles) | 873.81 ± 16.52 | 97.09 | 1.89 |
| Long-term (1 month at −80 °C) | 870.48 ± 68.68 | 96.72 | 7.89 |
| QC Samples | Recovery(%) | Precision for Recovery (RSD %) | Matrix Effect (%) | Precision for Matrix Effect (RSD %) |
|---|---|---|---|---|
| 6 ng/mL | 103.68 | 6.02 | 109.03 | 3.92 |
| 60 ng/mL | 92.52 | 4.79 | 99.86 | 10.13 |
| 900 ng/mL | 99.76 | 3.13 | 98.02 | 11.52 |
| Parameters | i.v. | i.g. | ||
|---|---|---|---|---|
| 2.5 mg/kg | 5 mg/kg | 10 mg/kg | 20 mg/kg | |
| Cmax (μg/L) | - | - | - | 64.64 ± 34.79 |
| C2min (μg/L) | 1140.35 ± 477.81 | 1393.22 ± 800.06 | 1662.94 ± 229.57 | - |
| Tmax (h) | - | - | - | 3.38 ± 0.74 |
| T1/2Z (h) | 0.61 ± 0.18 | 0.66 ± 0.13 | 0.61 ± 0.18 | 2.94 ± 1.59 |
| AUC(0–t) (μg/L∙h) | 479.75 ± 142.84 | 760.67 ± 414.15 | 1282.04 ± 328.82 | 284.91 ± 87.29 |
| AUC(0–∞) (μg/L∙h) | 484.47 ± 143.86 | 771.31 ± 425.43 | 1298.92 ± 332.04 | 318.25 ± 98.27 |
| AUC(0–t)/AUC(0–∞) | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.90 ± 0.09 |
| MRT (h) | 0.62 ± 0.27 | 0.72 ± 0.25 | 0.80 ± 0.19 | 5.86 ± 2.24 |
| VZ (L/kg) | 4.98 ± 2.33 | 7.50 ± 3.32 | 7.04 ± 2.50 | - |
| CLZ (L/kg/h) | 5.64 ± 1.90 | 8.55 ± 4.95 | 8.18 ± 2.22 | - |
| F (%) | - | - | - | 10.26 ± 2.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.-C.; Tang, W.-T.; Yu, L.-L.; Qian, X.-J.; Ren, J.; Li, J.-J.; Rong, W.-W.; Li, J.-X.; Zhu, Q. Preclinical Pharmacokinetics and Bioavailability of Oxypeucedanin in Rats after Single Intravenous and Oral Administration. Molecules 2022, 27, 3570. https://doi.org/10.3390/molecules27113570
Zheng M-C, Tang W-T, Yu L-L, Qian X-J, Ren J, Li J-J, Rong W-W, Li J-X, Zhu Q. Preclinical Pharmacokinetics and Bioavailability of Oxypeucedanin in Rats after Single Intravenous and Oral Administration. Molecules. 2022; 27(11):3570. https://doi.org/10.3390/molecules27113570
Chicago/Turabian StyleZheng, Ming-Cong, Wen-Ting Tang, Lu-Lu Yu, Xun-Jia Qian, Jie Ren, Jie-Jia Li, Wei-Wei Rong, Jun-Xu Li, and Qing Zhu. 2022. "Preclinical Pharmacokinetics and Bioavailability of Oxypeucedanin in Rats after Single Intravenous and Oral Administration" Molecules 27, no. 11: 3570. https://doi.org/10.3390/molecules27113570
APA StyleZheng, M.-C., Tang, W.-T., Yu, L.-L., Qian, X.-J., Ren, J., Li, J.-J., Rong, W.-W., Li, J.-X., & Zhu, Q. (2022). Preclinical Pharmacokinetics and Bioavailability of Oxypeucedanin in Rats after Single Intravenous and Oral Administration. Molecules, 27(11), 3570. https://doi.org/10.3390/molecules27113570





