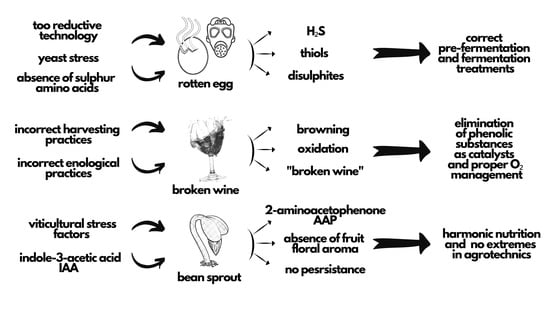
Wine Faults: State of Knowledge in Reductive Aromas, Oxidation and Atypical Aging, Prevention, and Correction Methods
Abstract
:1. Introduction
2. Reductive Faults
2.1. Sensory Attributes of Reductive Faults
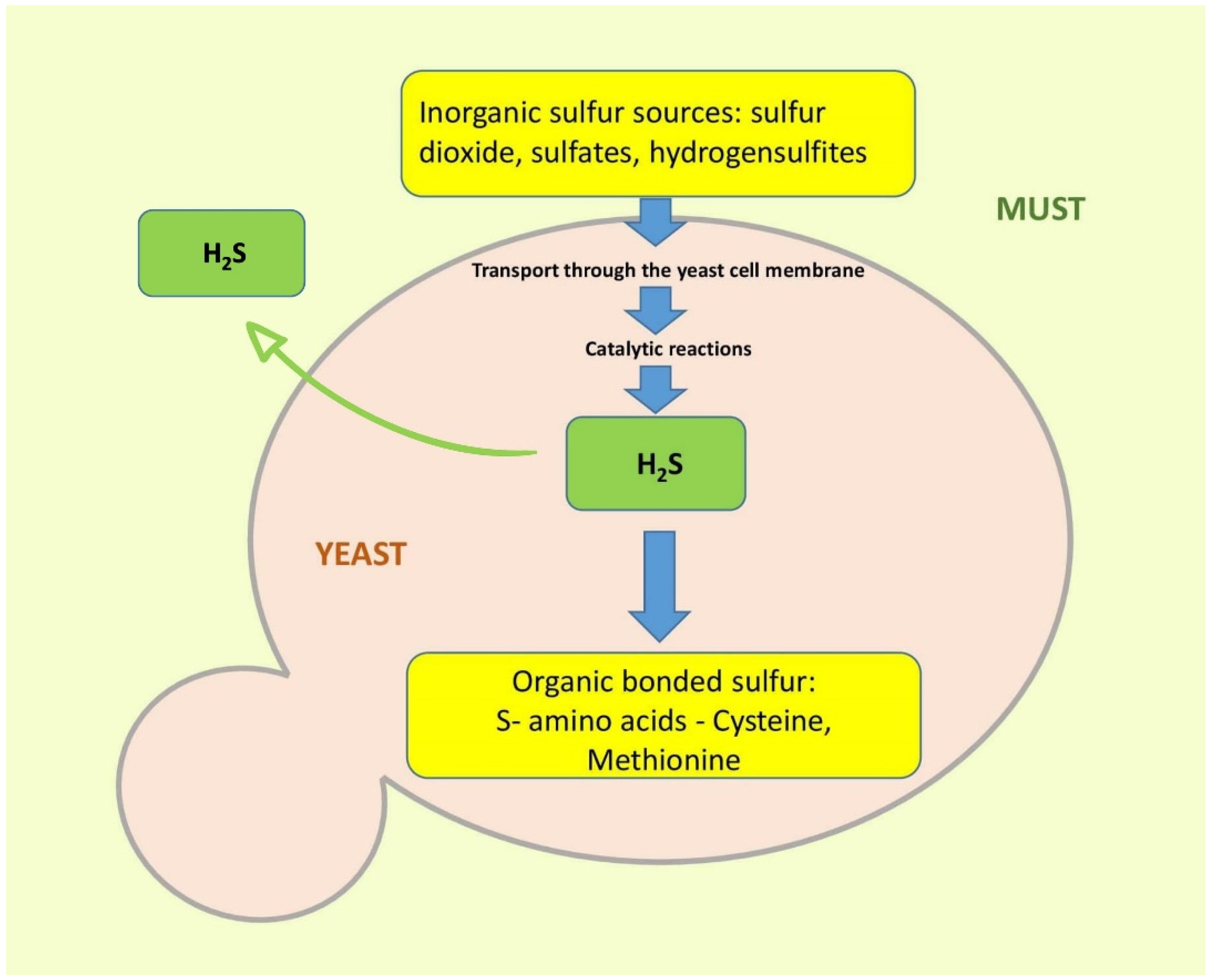
2.2. Biochemical Background of Volatile Sulfur Formation
2.2.1. Formation of Hydrogen Sulfide, Methanethiol, and Ethanethiol
2.2.2. Post-Fermentation Reductive Aromas—Thiols and Disulfides
2.3. Preventive Measures of Formation Reductive Aromas
2.4. Corrective Solutions in Reductive Aromas
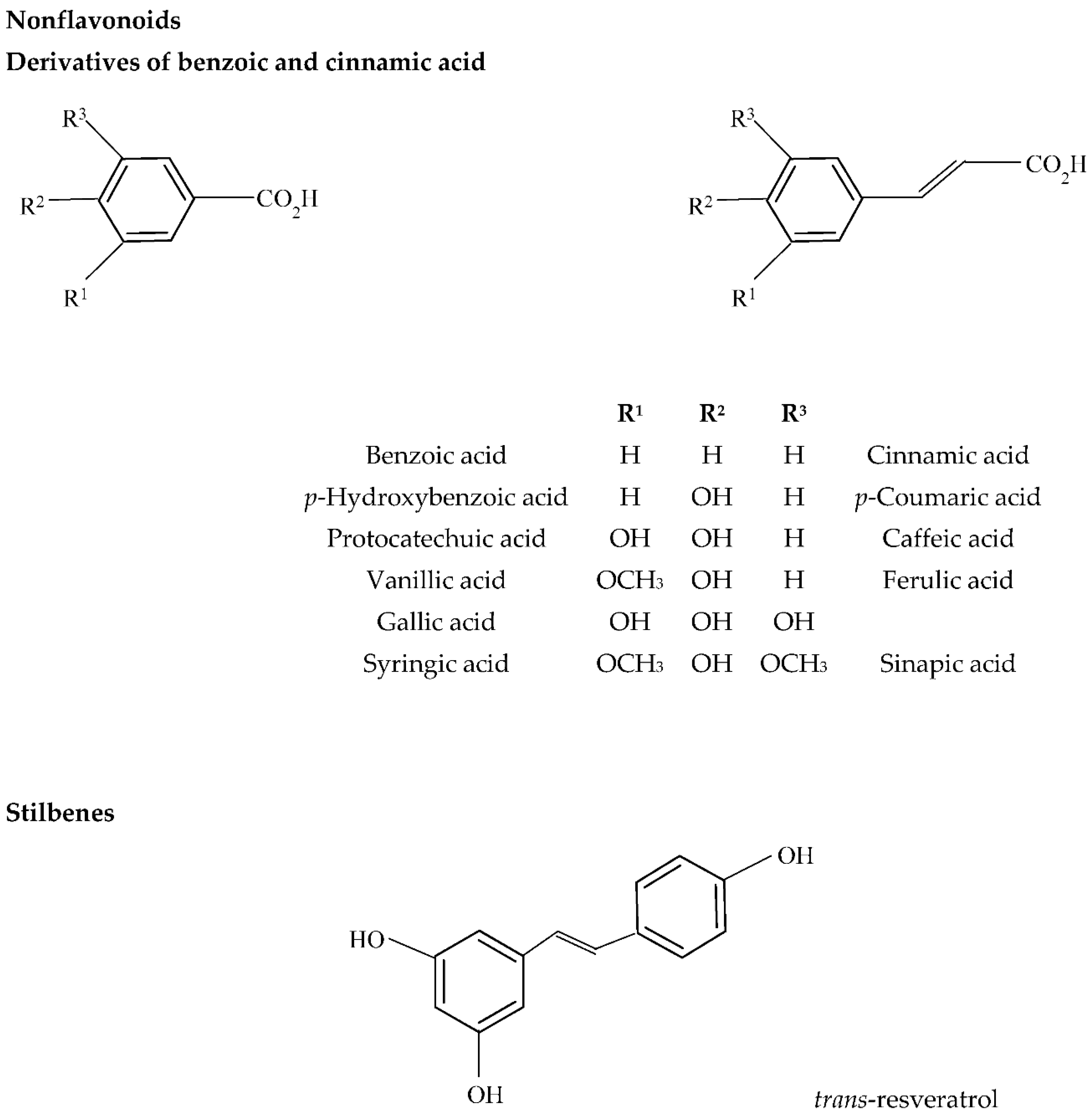
3. Wine Browning (Oxidation)
3.1. Sensory Attributes of Wine Browning
3.2. Biochemical Background of Wine Browning
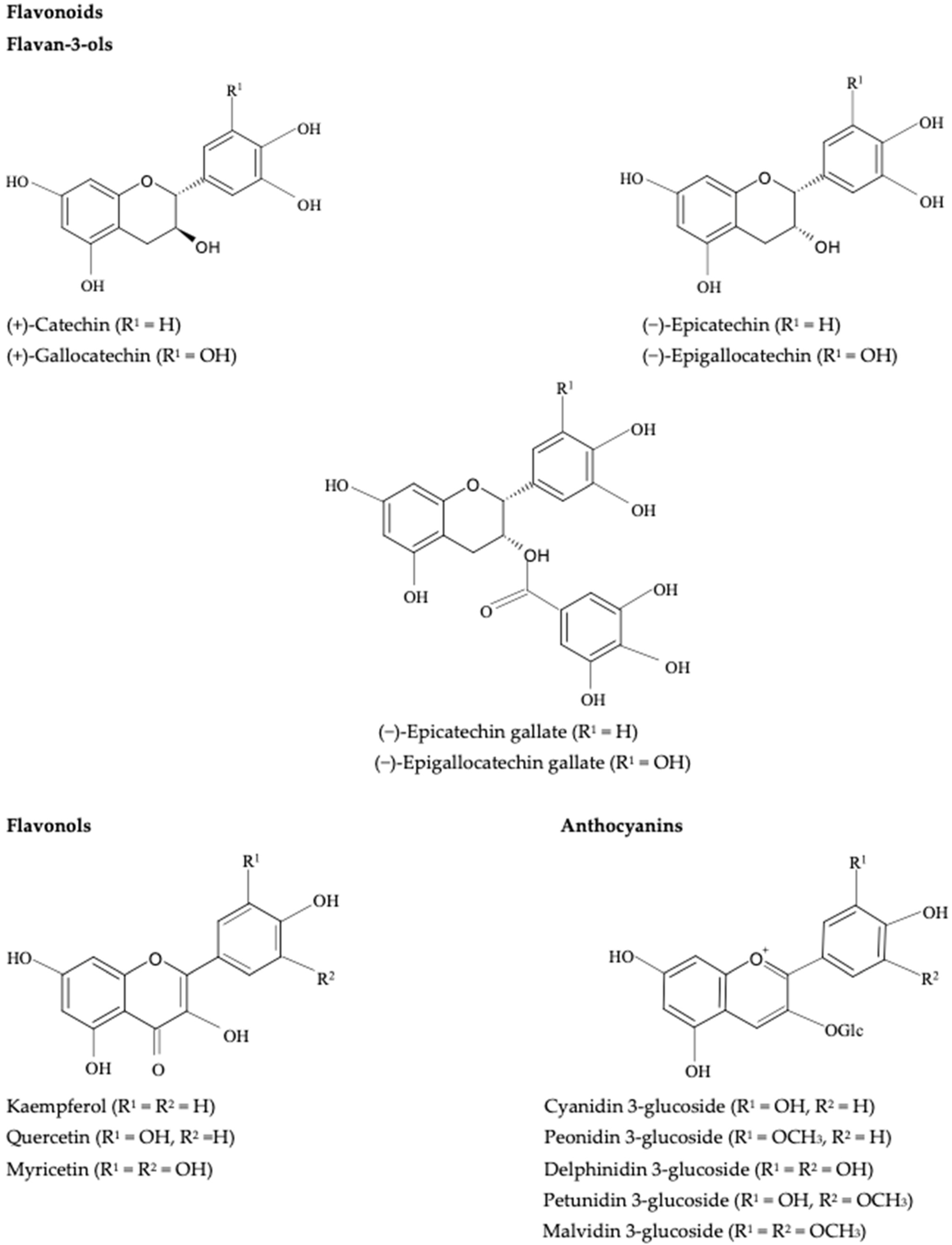
Enzymatic and Nonenzymatic Oxidation
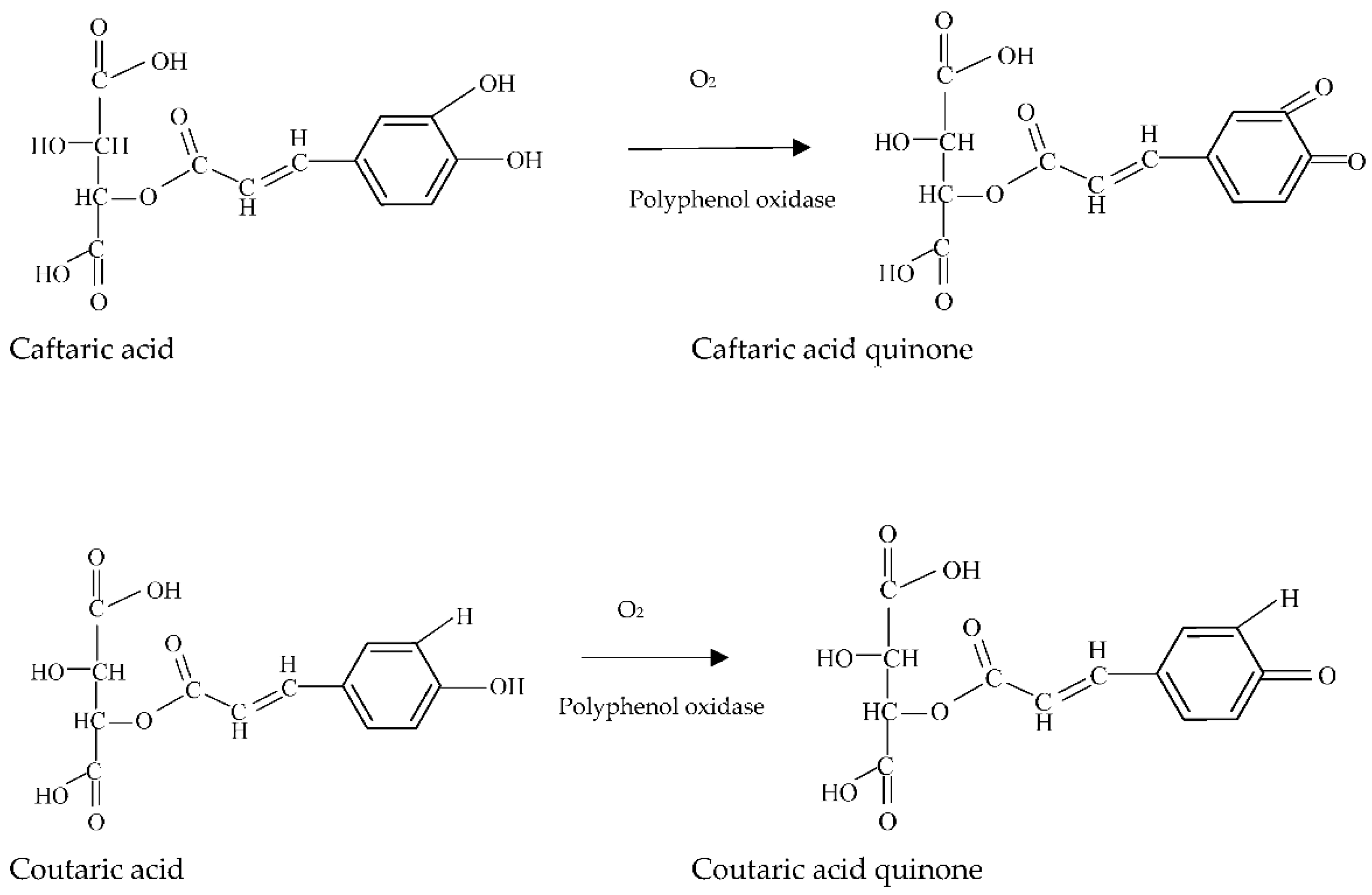
3.3. Preventive Measures of Wine Browning
3.4. Corrective Solutions in Browning
4. Atypical Aging
4.1. Sensory Attributes of ATA
4.1.1. Threshold Levels
4.1.2. Classification of the ATA Sensory Attributes
4.2. Biochemical Background of ATA Development
4.3. Preventive Measures of ATA
4.4. Corrective Solutions of ATA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ribeiro, T.; Fernandes, C.; Nunes, F.M.; Filipe-Ribeiro, L.; Cosme, F. Influence of the structural features of commercial mannoproteins in white wine protein stabilization and chemical and sensory properties. Food Chem. 2014, 159, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. The complexity of protein haze formation in wines. Food Chem. 2009, 112, 169–177. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Tasting: A Professional Handbook, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; p. 415. ISBN 978-0-12-801813-2. [Google Scholar]
- Ailer, Š. Vinárstvo & Somelierstvo (Winery & Sommelier Proficiency); Agriprint: Olomouc, Czech Republic, 2016; p. 197. ISBN 978-80-87091-63-0. [Google Scholar]
- Grainger, K. Faulty & undrinkable. IWFS 2021, 136, 6–9. [Google Scholar]
- Franco-Luesma, E.; Honoré-Chedozeau, C.; Ballester, J.; Valentin, D. Oxidation in wine: Does expertise influence the perception? LWT 2019, 116, 108511. [Google Scholar] [CrossRef]
- MacNeil, K. The Wine Bible, 2nd ed.; Workman Publishing: New York, NY, USA, 2015; p. 1009. ISBN 978-0-7611-8715-8. [Google Scholar]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef] [PubMed]
- Poláček, Š.; Tomáš, J.; Vietoris, V.; Lumnitzerová, M. Vinárstvo, Someliérstvo a Enogastronómia; Slovak University of Agriculture in Nitra: Nitra, Slovakia, 2018; p. 319. ISBN 978-80-552-1910-3. [Google Scholar]
- Grainger, K. Wine Faults and Flaws: A Practical Guide; John Wiley & Sons: Chichester, UK, 2021; p. 531. ISBN 978-1-118-97906-8. [Google Scholar]
- Hudelson, J. Wine Faults: Causes, Effects, Cures, 1st ed.; Board and Bench Publishing: San Francisco, CA, USA, 2010; p. 87. ISBN 978-1-934259-63-4. [Google Scholar]
- Ugliano, M.; Kolouchova, R.; Henschke, P.A. Occurrence of hydrogen sulfide in wine and in fermentation: Influence of yeast strain and supplementation of yeast available nitrogen. J. Ind. Microbiol. Biotechnol. 2011, 38, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ailer, Š.; Jedlička, J.; Paulen, O. Study of the extreme agro-technical and agro-climatic factors influence in vineyard for a qualitative wine potential. Eur. Int. J. Sci. Technol 2013, 2, 126–132. [Google Scholar]
- Winter, G.; Henschke, P.A.; Higgins, V.J.; Ugliano, M.; Curtin, C.D. Effects of rehydration nutrients on H2S metabolism and formation of volatile sulfur compounds by the wine yeast VL3. AMB Expr. 2011, 1, 36. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.E.; Bekker, M.Z.; Smith, P.A.; Wilkes, E.N. Sources of volatile sulfur compounds in wine. Aust. J. Grape Wine Res. 2015, 21, 705–712. [Google Scholar] [CrossRef]
- Müller, N.; Rauhut, D.; Tarasov, A. Sulfane sulfur compounds as source of reappearance of reductive off-odors in wine. Fermentation 2022, 8, 53. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Smith, M.E.; Smith, P.A.; Wilkes, E.N. Formation of hydrogen sulfide in wine: Interactions between copper and sulfur dioxide. Molecules 2016, 21, 1214. [Google Scholar] [CrossRef] [Green Version]
- Swiegers, J.H.; Pretorius, I.S. Modulation of volatile sulfur compounds by wine yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef]
- Giudici, P.; Kunkee, R.E. The effect of nitrogen deficiency and sulfur-containing amino acids on the reduction of sulfate to hydrogen sulfide by wine yeasts. Am. J. Enol. Vitic. 1994, 45, 107–112. [Google Scholar]
- Jordão, A.M.; Cosme, F. Grapes and Wines: Advances in Production, Processing, Analysis and Valorization, 1st ed.; InTech: Rijeka, Croatia, 2018; p. 386. ISBN 978-953-51-3833-4. [Google Scholar]
- Bekker, M.Z.; Espinase Nandorfy, D.; Kulcsar, A.C.; Faucon, A.; Bindon, K.; Smith, P.A. Comparison of remediation strategies for decreasing ‘Reductive’Characters in shiraz wines. Aust. J. Grape Wine Res. 2021, 27, 52–65. [Google Scholar] [CrossRef]
- Goode, J.; Harrop, S. Wine faults and their prevalence: Data from the world’s largest blind tasting. In Proceedings of the 20th Entretiens Scientifiques Lallemand, Horsens, Denmark, 15 May 2008. [Google Scholar]
- Rauhut, D. Volatile sulfur compounds: Impact on “Reduced Sulfur” flavor defects and “Atypical Aging” in wine. In Proceedings of the 31st New York Wine Industry Workshop, Geneva, NY, USA, 3–5 April 2002; New York State Agricultural Experiment Station, Cornell University: Geneva, NY, USA, 2002. [Google Scholar]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef]
- Müller, N.; Rauhut, D. Recent developments on the origin and nature of reductive sulfurous off-odours in wine. Fermentation 2018, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Siebert, T.E.; Bramley, B.; Solomon, M.R. Hydrogen sulfide: Aroma detection threshold study in red and white wine. AWRI Tech. 2009, 183, 14–16. [Google Scholar]
- Lu, Y.; Fong, A.S.Y.L.; Chua, J.-Y.; Huang, D.; Lee, P.-R.; Liu, S.-Q. The possible reduction mechanism of volatile sulfur compounds during durian wine fermentation verified in modified buffers. Molecules 2018, 23, 1456. [Google Scholar] [CrossRef] [Green Version]
- Goniak, O.J.; Noble, A.C. Sensory study of selected volatile sulfur compounds in white wine. Am. J. Enol. Vitic. 1987, 38, 223–227. [Google Scholar]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr.A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Validation of bismuth-containing indicator media for predicting H2S-producing potential of saccharomyces cerevisiae wine yeasts under enological conditions. Am. J. Enol. Vitic. 1995, 46, 269–273. [Google Scholar]
- Spiropoulos, A.; Tanaka, J.; Flerianos, I.; Bisson, L.F. Characterization of hydrogen sulfide formation in commercial and natural wine isolates of saccharomyces. Am. J. Enol. Vitic. 2000, 51, 233–248. [Google Scholar]
- Mendes-Ferreira, A.; Mendes-Faia, A.; Leao, C. Survey of hydrogen sulphide production by wine yeasts. J. Food Prot. 2002, 65, 1033–1037. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Rauhut, D. Yeasts−production of sulfur compounds. In Wine Microbiology and Biotechnology; CRC Press: Chur, Switzerland, 1993; pp. 183–223. ISBN 978-0-415-27850-8. [Google Scholar]
- Ugliano, M.; Henschke, P.A. Yeasts and wine flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. ISBN 978-0-387-74118-5. [Google Scholar]
- Ugliano, M.; Fedrizzi, B.; Siebert, T.; Travis, B.; Magno, F.; Versini, G.; Henschke, P.A. Effect of nitrogen supplementation and saccharomyces species on hydrogen sulfide and other volatile sulfur compounds in shiraz fermentation and wine. J. Agric. Food Chem. 2009, 57, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidi, I.D.; Farines, V.; Legras, J.-L.; Blondin, B. Development of a new assay for measuring H2S production during alcoholic fermentation: Application to the evaluation of the main factors impacting H2S production by three saccharomyces cerevisiae wine Strains. Fermentation 2021, 7, 213. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Puxeu, M.; Martín, L.; Nart, E.; Hidalgo, C.; Andorrà, I. Microbiological, physical, and chemical procedures to elaborate high-quality SO2-free wines. Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; InTech: Rijeka, Croatia, 2018; pp. 171–193. [Google Scholar]
- Yamagata, S. Roles of O-Acetyl-l-Homoserine sulfhydrylases in microorganisms. Biochimie 1989, 71, 1125–1143. [Google Scholar] [CrossRef]
- Henschke, P.A.; Jiranek, V. Yeast−Growth during Fermentation. In Wine Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1993; pp. 27–54. ISBN 978-0-415-27850-8. [Google Scholar]
- Park, S.K.; Boulton, R.B.; Noble, A.C. Formation of hydrogen sulfide and glutathione during fermentation of white grape musts. Am. J. Enol. Vitic. 2000, 51, 91–97. [Google Scholar]
- Moreira, N.; Mendes, F.; Pereira, O.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Volatile sulphur compounds in wines related to yeast metabolism and nitrogen composition of grape musts. Anal. Chim. Acta 2002, 458, 157–167. [Google Scholar] [CrossRef]
- Vos, P.J.A.; Gray, R.S. The origin and control of hydrogen sulfide during fermentation of grape must. Am. J. Enol. Vitic. 1979, 30, 187–197. [Google Scholar]
- Henschke, P.A.; Jiranek, V. Hydrogen sulfide formation during fermentation: Effect of nitrogen composition in model grape must. In Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, WA, USA, 18–19 June 1991; American Society for Enology and Viticulture: Davis, CA, USA, 1991; pp. 172–184, ISBN 0-9630711-0-6. [Google Scholar]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Determination of Sulphite reductase activity and its response to assimilable nitrogen status in a commercial saccharomyces cerevisiae wine yeast. J. Appl. Bacteriol. 1996, 81, 329–336. [Google Scholar] [CrossRef]
- Prokes, K.; Baron, M.; Mlcek, J.; Jurikova, T.; Adamkova, A.; Ercisli, S.; Sochor, J. The influence of traditional and immobilized yeast on the amino-acid content of sparkling wine. Fermentation 2022, 8, 36. [Google Scholar] [CrossRef]
- Hallinan, C.P.; Saul, D.J.; Jiranek, V. Differential utilisation of sulfur compounds for H2S liberation by nitrogen-starved wine yeasts. Aust. J. Grape Wine Res. 1999, 5, 82–90. [Google Scholar] [CrossRef]
- Jiranek, V.; Langridge, P.; Henschke, P. Regulation of hydrogen sulfide liberation in wine-producing saccharomyces cerevisiae strains by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, s-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Ferreira, V. Reductive off-odors in wines: Formation and release of H2S and methanethiol during the accelerated anoxic storage of wines. Food Chem. 2016, 199, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Perpète, P.; Duthoit, O.; De Maeyer, S.; Imray, L.; Lawton, A.I.; Stavropoulos, K.E.; Gitonga, V.W.; Hewlins, M.J.E.; Richard Dickinson, J. Methionine catabolism in saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Gruenwedel, D.W.; Patnaik, R.K. Release of hydrogen sulfide and methyl mercaptan from sulfur-containing amino acids. J. Agric. Food Chem. 1971, 19, 775–779. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagaard, O.; Waters, E.J. Evolution of 3-mercaptohexanol, hydrogen sulfide, and methyl mercaptan during bottle storage of sauvignon blanc wines. Effect of Glutathione, copper, oxygen exposure, and closure-derived oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Day, M.P.; Holt, H.; Wilkes, E.; Smith, P.A. Effect of oxygen exposure during fermentation on volatile sulfur compounds in S Hiraz wine and a comparison of strategies for remediation of reductive character. Aust. J. Grape Wine Res. 2016, 22, 24–35. [Google Scholar] [CrossRef]
- Nguyen, D.-D.; Nicolau, L.; Dykes, S.I.; Kilmartin, P.A. Influence of microoxygenation on reductive sulfur off-odors and color development in a cabernet sauvignon wine. Am. J. Enol. Vitic. 2010, 61, 457–464. [Google Scholar] [CrossRef]
- Guitart, A.; Orte, P.H.; Ferreira, V.; Peña, C.; Cacho, J. Some observations about the correlation between the amino acid content of musts and wines of the chardonnay variety and their fermentation aromas. Am. J. Enol. Vitic. 1999, 50, 253–258. [Google Scholar]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Copper(II)-Mediated hydrogen sulfide and thiol oxidation to disulfides and organic polysulfanes and their reductive cleavage in wine: Mechanistic elucidation and potential applications. J. Agric. Food Chem. 2017, 65, 2564–2571. [Google Scholar] [CrossRef]
- Kreitman, G.Y.; Elias, R.J.; Jeffery, D.W.; Sacks, G.L. Loss and formation of malodorous volatile sulfhydryl compounds during wine storage. Crit. Rev. Food Sci. Nut. 2019, 59, 1728–1752. [Google Scholar] [CrossRef]
- Vela, E.; Hernández-Orte, P.; Franco-Luesma, E.; Ferreira, V. The effects of copper fining on the wine content in sulfur off-odors and on their evolution during accelerated anoxic storage. Food Chem. 2017, 231, 212–221. [Google Scholar] [CrossRef] [Green Version]
- International Code of Oenological Practices. Available online: https://www.oiv.int/public/medias/3570/e-code-ii-358.pdf (accessed on 10 March 2022).
- Franco-Luesma, E.; Ferreira, V. Formation and release of H2S, methanethiol, and dimethylsulfide during the anoxic storage of wines at room temperature. J. Agric. Food Chem. 2016, 64, 6317–6326. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, Q.; Hao, H.; Wei, Q.; Shi, B.; Yu, J.; Wang, C.; Wang, Y. Evaluation of the treatability of various odor compounds by powdered activated carbon. Water Res. 2019, 156, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Ailer, Š. Vplyv Aplikácie Kontaktnỳch Listovỳch Hnojív Na Obsah hygienicky vỳznamnỳch chemickỳch prvkov v hroznovom mušte a víne. Bull. Food Res. 1999, 38, 183–193. [Google Scholar]
- Dias, D.; Guarda, A.; Wiethan, B.A.; Claussen, L.E.; Bohrer, D.; De Carvalho, L.M.; Do Nascimento, P.C. Influence of ethanethiol in antioxidant activity and in total phenolics concentration of wines. Comparative study against control samples. J. Food Qual. 2013, 36, 432–440. [Google Scholar] [CrossRef]
- Allison, R.B. Hydrogen Sulfide Development In Wine During Anoxic Storage. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2021. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 512. ISBN 978-0-470-01035-8. [Google Scholar]
- Kraft, D.N. Impact of Lees Content, Nitrogen, and Elemental Sulfur on Volatile Sulfur Compound Formation in Vitis Vinifera L. cv. “Pinot noir” Wine. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2015. [Google Scholar]
- Goode, J. Flawless: Understanding Faults in Wine; University of California Press: Berkely, CA, USA, 2018; p. 237. ISBN 978-0-520-97131-8. [Google Scholar]
- Viviers, M.Z.; Smith, M.E.; Wilkes, E.; Smith, P. Effects of five metals on the evolution of hydrogen sulfide, methanethiol, and dimethyl sulfide during anaerobic storage of chardonnay and shiraz wines. J. Agric. Food Chem. 2013, 61, 12385–12396. [Google Scholar] [CrossRef]
- Clark, A.C.; Grant-Preece, P.; Cleghorn, N.; Scollary, G.R. Copper(II) addition to white wines containing hydrogen sulfide: Residual copper concentration and activity. Aust. J. Grape Wine Res. 2015, 21, 30–39. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Nandorfy, D.E.; Kulcsar, A.C.; Faucon, A.; Smith, P.A. Remediating ‘Reductive’Characters in wine. In Proceedings of the 17th Australian Wine Industry Technical Conference, Adelaide, Australia, 21–24 July 2019; 2020; p. 97. [Google Scholar]
- Pokrỳvková, J.; Ailer, Š.; Jedlička, J.; Chlebo, P.; Jurík, L. The use of a targeted must oxygenation method in the process of developing the archival potential of natural wine. Appl. Sci. 2020, 10, 4810. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2019/934—of 12 March 2019—Supplementing Regulation (EU) No 1308 /2013 of the European Parliament and of the Council as Regards Wine-Growing Areas Where the Alcoholic Strength May Be Increased, Authorised Oenological Practices and Restrictions Applicable to the Production and Conservation of Grapevine Products, the Minimum Percentage of Alcohol for by-Products and Their Disposal, and Publication of OIV Files. 52. Off. J. Eur. Union L. 2019, 149, 1–52. [Google Scholar]
- Singleton, V.L.; Kramlinga, T.E. Browning of white wines and an accelerated test for browning capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar]
- Salacha, M.-I.; Kallithraka, S.; Tzourou, I. Browning of white wines: Correlation with antioxidant characteristics, total polyphenolic composition and flavanol content. IJFST 2008, 43, 1073–1077. [Google Scholar] [CrossRef]
- Karbowiak, T.; Mansfield, A.K.; Barrera-García, V.D.; Chassagne, D. Sorption and diffusion properties of volatile phenols into cork. Food Chem. 2010, 122, 1089–1094. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Siuta, M. The impact of oxygen at various stages of vinification on the chemical composition and the antioxidant and sensory properties of white and red wines. Int. J. Food Sci. 2020, 2020, 7902974. [Google Scholar] [CrossRef] [Green Version]
- Makhotkina, O.; Kilmartin, P.A. Uncovering the influence of antioxidants on polyphenol oxidation in wines using an electrochemical method: Cyclic voltammetry. J. Electroanal. Chem. 2009, 633, 165–174. [Google Scholar] [CrossRef]
- Kilmartin, P.A. The oxidation of red and white wines and its impact on wine aroma. Chem. New Zealand 2009, 73, 79–83. [Google Scholar]
- Michlovský, M. Bobule; Vinselekt Michlovský a.s.: Luční, Rakvice, Czech, 2014. [Google Scholar]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.-L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of winemaking treatment and wine aging on phenolic content in vranec wines. J. Food Sci. Technol. 2012, 49, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, V.; Dörnyei, Á.; Márk, L.; Vojnoski, B.; Stafilov, T.; Stefova, M.; Kilár, F. Polyphenolic content of vranec wines produced by different vinification conditions. Food Chem. 2011, 124, 316–325. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for dealcoholization of wines: Their impact on wine phenolic composition, volatile composition and sensory characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Deshaies, S.; Cazals, G.; Enjalbal, C.; Constantin, T.; Garcia, F.; Mouls, L.; Saucier, C. Red wine oxidation: Accelerated ageing tests, possible reaction mechanisms and application to syrah red wines. Antioxidants 2020, 9, 663. [Google Scholar] [CrossRef]
- Vlahou, E.; Christofi, S.; Roussis, I.G.; Kallithraka, S. Browning development and antioxidant compounds in white wines after selenium, iron, and peroxide addition. Appl. Sci. 2022, 12, 3834. [Google Scholar] [CrossRef]
- Cacho, J.; Castells, J.E.; Esteban, A.; Laguna, B.; Sagristá, N. Iron, copper, and manganese influence on wine oxidation. Am. J. Enol. Vitic. 1995, 46, 380–384. [Google Scholar]
- Moreno, J.; Peinado, R. Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012; p. 443. ISBN 978-0-12-388439-8. [Google Scholar]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Cheynier, V.F.; Van Hulst, M.W. Oxidation of trans-caftaric acid and 2-S-glutathionylcaftaric acid in model solutions. J. Agric. Food Chem. 1988, 36, 10–15. [Google Scholar] [CrossRef]
- Ünal, M.Ü.; Şener, A. Correlation between browning degree and composition of importantturkish white wine grape varieties. Turk. J. Agric. For. 2016, 40, 62–67. [Google Scholar] [CrossRef]
- Sartor, S.; Burin, V.M.; Ferreira-Lima, N.E.; Caliari, V.; Bordignon-Luiz, M.T. Polyphenolic profiling, browning, and glutathione content of sparkling wines produced with nontraditional grape varieties: Indicator of quality during the biological aging. J. Food Sci. 2019, 84, 3546–3554. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid-induced browning of (+)-catechin in a model wine system. J. Agric. Food Chem. 2001, 49, 934–939. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Jourdes, M.; Riu-Aumatell, M.; Buxaderas, S.; Teissedre, P.-L.; López-Tamames, E. Kinetics of browning, phenolics, and 5-hydroxymethylfurfural in commercial sparkling wines. J. Agric. Food Chem. 2014, 62, 1159–1166. [Google Scholar] [CrossRef]
- Ailer, Š.; Serenčéš, R.; Kozelová, D.; Poláková, Z.; Jakabová, S. Possibilities for depleting the content of undesirable volatile phenolic compounds in white wine with the use of low-intervention and economically efficient grape processing technology. Appl. Sci. 2021, 11, 6735. [Google Scholar] [CrossRef]
- Baroň, M. Možnosti snížení obsahu oxidu siřičitého ve víne. Část 1. Vinařský Obzor. 2013, 7–8, 406–409. [Google Scholar]
- Baroň, M. Možnosti snížení obsahu oxidu siřičitého ve víne. Část 2. Vinařský Obzor. 2013, 9, 456–458. [Google Scholar]
- Baroň, M. Možnosti snížení obsahu oxidu siřičitého ve víne. Část 3. Vinařský Obzor. 2013, 10, 508–511. [Google Scholar]
- Day, M.P.; Schmidt, S.A.; Pearson, W.; Kolouchova, R.; Smith, P.A. Effect of passive oxygen exposure during pressing and handling on the chemical and sensory attributes of chardonnay wine. Aust. J. Grape Wine Res. 2019, 25, 185–200. [Google Scholar] [CrossRef]
- Roure, F.; Anderson, G. Characteristics of oenotannins. In The Australian & New Zealand Grapegrower and Winemaker; Winetitles Media: Broadview, Australia, 2006; p. 48. [Google Scholar]
- Nunes, P.; Muxagata, S.; Correia, A.C.; Nunes, F.M.; Cosme, F.; Jordão, A.M. Effect of oak wood barrel capacity and utilization time on phenolic and sensorial profile evolution of an encruzado white wine. J. Sci. Food Agric. 2017, 97, 4847–4856. [Google Scholar] [CrossRef]
- Renaud, S.; Gueguen, R. The french paradox and wine drinking. Novartis Found. Symp. 1998, 216, 208–217. [Google Scholar]
- Vidavalur, R.; Otani, H.; Singal, P.K.; Maulik, N. Significance of wine and resveratrol in cardiovascular disease: French Paradox revisited. Exp. Clin. Cardiol. 2006, 11, 217. [Google Scholar]
- Razmkhab, S.; Lopez-Toledano, A.; Ortega, J.M.; Mayen, M.; Merida, J.; Medina, M. Adsorption of phenolic compounds and browning products in white wines by yeasts and their cell walls. J. Agric. Food Chem. 2002, 50, 7432–7437. [Google Scholar] [CrossRef]
- Gil, M.; Avila-Salas, F.; Santos, L.S.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Rosé wine fining using polyvinylpolypyrrolidone: Colorimetry, targeted polyphenomics, and molecular dynamics simulations. J. Agric. Food Chem. 2017, 65, 10591–10597. [Google Scholar] [CrossRef]
- Gil, M.; Louazil, P.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Effect of polyvinylpolypyrrolidone treatment on rosés wines during fermentation: Impact on color, polyphenols and thiol aromas. Food Chem. 2019, 295, 493–498. [Google Scholar] [CrossRef]
- Bonilla, F.; Mayen, M.; Merida, J.; Medina, M. Yeasts used as fining treatment to correct browning in white wines. J. Agric. Food Chem. 2001, 49, 1928–1933. [Google Scholar] [CrossRef]
- Alpeza, I.; Linke, I.; Maslov, L.; Jeromel, A. Atypical aging off-flavour and relation between sensory recognition and 2-aminoacetophenone in croatian wines. J. Cent. Eur. Agric. 2021, 22, 408–419. [Google Scholar] [CrossRef]
- Christoph, N.; Bauer-Christoph, C.; Gessner, M.; Köhler, H.J. Die “Untypische Alterungsnote Im Wein”, Teil I: Untersuchungen zum auftreten und zur sensorischen charakterisierung der “untypischen alterungsnote”. Rebe Wein 1995, 48, 350–356. [Google Scholar]
- Rapp, A.; Versini, G.; Ullemeyer, H. 2-Aminoacetophenone-causal component of untypical aging flavor (Naphthalene Note, Hybrid Note) of wine. Vitis 1993, 32, 61–62. [Google Scholar]
- Schneider, V. UTA-Ein weltweites problem. Der Winzer 2013, 7, 24–28. [Google Scholar]
- Linsenmeier, A.; Pfliehinger, M. Wirkungskette stress–Das Ungeklärte UTA-Rätsel. Deut. Weinbau 2009, 24, 18–21. [Google Scholar]
- Linsenmeier, A.W.; Rauhut, D.; Sponholz, W.R. Aging and flavor deterioration in wine. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2022; pp. 559–594. [Google Scholar]
- Ailer, Š.; Valšíková, M.; Jedlička, J.; Mankoveckỳ, J.; Baroň, M. Influence of sugar and ethanol content and color of wines on the sensory evaluation: From wine competition “Nemčiňany Wine Days” in Slovak Republic (2013–2016). Erwerbs-Obstbau 2020, 62, 9–16. [Google Scholar] [CrossRef]
- Schneider, V. Atypical aging defect: Sensory discrimination, viticultural causes, and enological consequences. A review. Am. J. Enol. Vitic. 2014, 65, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Fischer, U.; Sponholz, R. Die sensorische beschreibung der untypischen alterungsnote. Der Dtsch. Weinbau 2000, 3, 16–21. [Google Scholar]
- Henick-Kling, T.; Gerling, C.; Martinson, T.; Acree, T.; Lakso, A. Studies on the origin and sensory aspects of atypical aging in white wines. In Proceedings of the 15th International Enology Sympopsium; International Association of Enology, Management and Wine Marketing, Trier, Germany, 16 April 2008; p. 10. [Google Scholar]
- Gessner, M.; Köhler, H.J.; Christoph, N. Die “Untypische Alterungsnote” in Wein, Teil VIII: Auswirkungen von Inhaltsstoffen Und Antioxidantien Auf Die Bildung von o-Aminoacetophenon. Rebe Wein 1999, 51, 264–267. [Google Scholar]
- Christoph, N.; Bauer-Christoph, C.; Geßner, M.; Köhler, H.J.; Simat, T.; Hoenicke, K. Formation of 2-aminoacetophenone and formylaminoacetophenone in wine by reaction of sulfurous acid with indole-3-acetic acid. Vitic. Enol. Sci. 1998, 53, 79–86. [Google Scholar]
- Linsenmeier, A.; Rauhut, D.; Kurbel, H.; Lohnertz, O.; Schubert, S. Untypical ageing off-flavour and masking effects due to long-term nitrogen fertilization. Vitis-Geilweilerhof 2007, 46, 33. [Google Scholar]
- Maslov, L.; Jeromel, A.; Herjavec, S.; Korenika, A.-M.J.; Mihaljević, Ž.; Plavša, T. Indole-3-acetic acid and tryptophan in istrian malvasia grapes and wine. J. Food Agric. Environ. 2011, 9, 132–136. [Google Scholar]
- Horlacher, N.; Link, K.; Schwack, W. Bacillus thuringiensis insecticides: Source of 2-aminoacetophenone formation in wine. J. Exp. Food Chem. 2016, 2, 113. [Google Scholar] [CrossRef] [Green Version]
- Nardin, T.; Roman, T.; Dekker, S.; Nicolini, G.; Thei, F.; Masina, B.; Larcher, R. Evaluation of antioxidant supplementation in must on the development and potential reduction of different compounds involved in atypical ageing of wine using HPLC-HRMS. LWT 2022, 154, 112639. [Google Scholar] [CrossRef]
- Facchini, P.J.; Huber-Allanach, K.L.; Tari, L.W. Plant Aromatic L-Amino acid decarboxylases: Evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry 2000, 54, 121–138. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Hoenicke, K.; Simat, T.J.; Steinhart, H.; Christoph, N.; Geßner, M.; Köhler, H.-J. ‘Untypical Aging off-Flavor’ in Wine: Formation of 2-aminoacetophenone and evaluation of its influencing factors. Anal. Chim. Acta 2002, 458, 29–37. [Google Scholar] [CrossRef]
- Linsenmeier, A.; Rauhut, D.; Kürbel, H.; Schubert, S.; Löhnertz, O. Ambivalence of the influence of nitrogen supply on O-aminoacetophenone in riesling wine. Vitis 2007, 46, 91–97. [Google Scholar]
- Gessner, M.; Köhler, H.J.; Christoph, N.; Nagel-Derr, A.; Krell, U. Die “Untypische Alterungsnote” in Wein, Teil IX: Würzburger UTAFIX-Test: Ein einfaches diagnoseverfahren zur früherkennung von weinen Mit UTA-Neigung. Rebe Wein 1999, 52, 296–303. [Google Scholar]


| Compound | Aroma Description | Odor Detection Threshold (µg L−1) | References |
|---|---|---|---|
| Hydrogen sulfide | Rotten egg, sewage-like, vegetal | 1.1–1.6 | [24,26] |
| Methanethiol | Cooked cabbage, onion, putrefaction, rubber | 1.8–3.1 | [24,27] |
| Ethanethiol | Onion, rubber, natural gas, faecal, earthy | 1.1 | [27,28] |
| Dimethylsulfide | Asparagus, corn, molasses, boiled cabbage, canned corn, blackcurrant, truffle | 25 | [28] |
| Diethylsulfide | Cooked vegetables, onion, garlic, rubber | 0.90 | [27,28] |
| Dimethyl disulfide | Cooked cabbage, intense onion | 29 | [27,28] |
| Diethyl disulfide | Onion, garlic, burnt rubber | 4.3 | [27,28] |
| 3-(methylthio)-1-propanol (methionol) | Cauliflower, cabbage, potato | 500 | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailer, Š.; Jakabová, S.; Benešová, L.; Ivanova-Petropulos, V. Wine Faults: State of Knowledge in Reductive Aromas, Oxidation and Atypical Aging, Prevention, and Correction Methods. Molecules 2022, 27, 3535. https://doi.org/10.3390/molecules27113535
Ailer Š, Jakabová S, Benešová L, Ivanova-Petropulos V. Wine Faults: State of Knowledge in Reductive Aromas, Oxidation and Atypical Aging, Prevention, and Correction Methods. Molecules. 2022; 27(11):3535. https://doi.org/10.3390/molecules27113535
Chicago/Turabian StyleAiler, Štefan, Silvia Jakabová, Lucia Benešová, and Violeta Ivanova-Petropulos. 2022. "Wine Faults: State of Knowledge in Reductive Aromas, Oxidation and Atypical Aging, Prevention, and Correction Methods" Molecules 27, no. 11: 3535. https://doi.org/10.3390/molecules27113535
APA StyleAiler, Š., Jakabová, S., Benešová, L., & Ivanova-Petropulos, V. (2022). Wine Faults: State of Knowledge in Reductive Aromas, Oxidation and Atypical Aging, Prevention, and Correction Methods. Molecules, 27(11), 3535. https://doi.org/10.3390/molecules27113535