Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System
Abstract
:1. Introduction
2. Results
2.1. Polyphenols with Antioxidant Activity within the Concentration Range of 5 µmol/L to 50 µmol/L
2.2. Polyphenols with Pro-Oxidant Activity within the Concentration Range of 5 µmol/L to 50 µmol/L
2.3. Polyphenols Which Altered Their Antioxidant Activity into Pro-Oxidant Activity (or Vice Versa) within the Concentration Range of 5 µmol/L to 50 µmol/L
3. Discussion
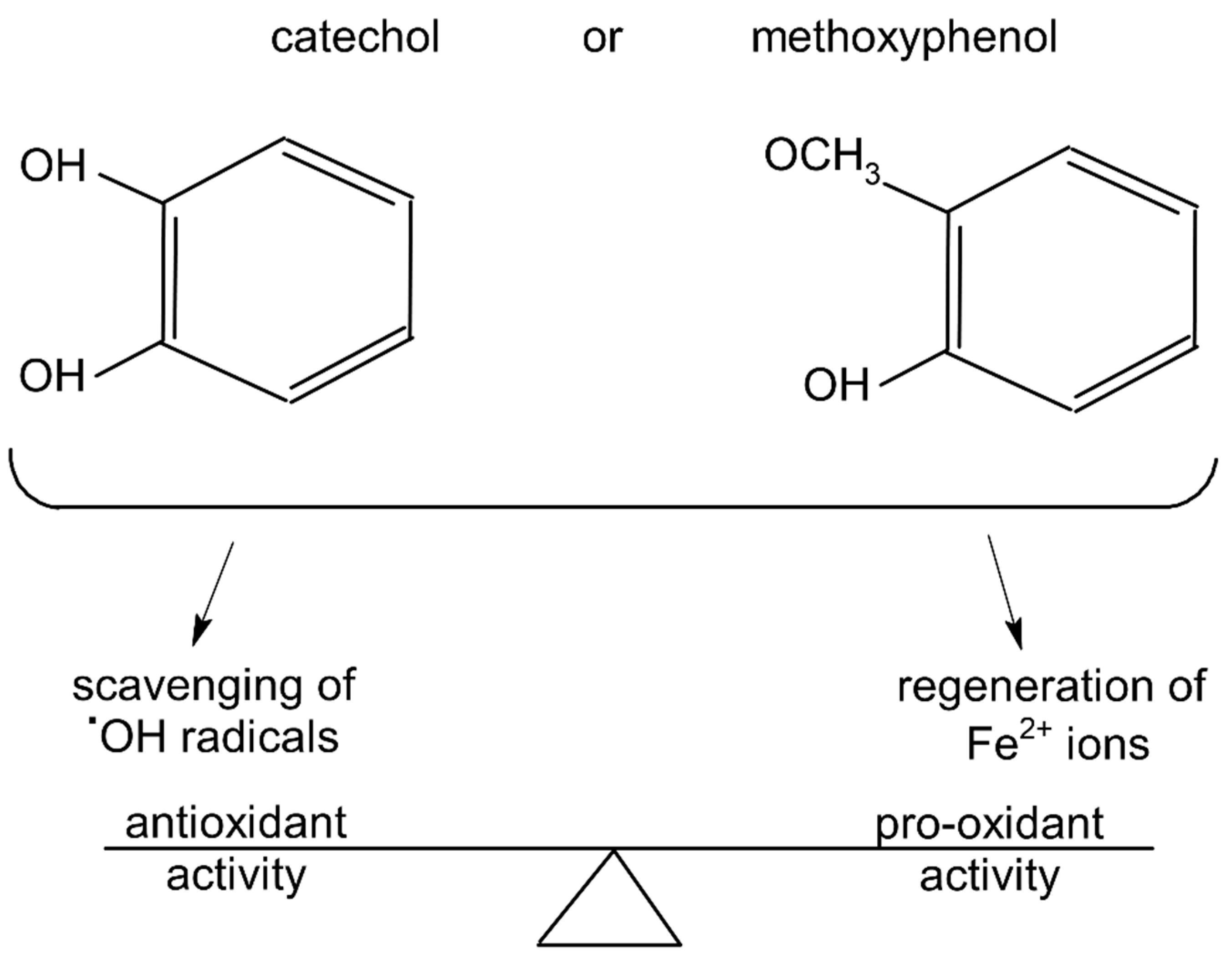
3.1. Plausible Mechanism by Which Polyphenols May Affect the UPE of the Fe2+-EGTA-H2O2 System
3.2. Phenolics That Suppressed Light Emission from the Fe2+-EGTA-H2O2 System within the Concentration Range of 5µmol/L to 50 µmol/L
3.3. Phenolics That Enhanced Light Emission from the Fe2+-EGTA-H2O2 System within the Concentration Range of 5 µmol/L to 50 µmol/L
3.4. Polyphenols Which Altered Their Antioxidant Activity into Pro-Oxidant Activity (or Vice Versa) within the Concentration Range of 5 µmol/L to 50 µmol/L
3.5. Strengths and Limitations of the Study
4. Materials and Methods
4.1. Reagents
4.2. Emitting Light System and Measurements of Total Light Emanation
4.3. Effect of Various Concentrations of Selected Plant Polyphenols on Light Emanation from the Fe2+-EGTA-H2O2 System
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Phan, H.T.; Chikae, M.; Takamura, Y.; Azo-Oussou, A.F.; Vestergaard, M.C. Black tea polyphenol theaflavin as promising antioxidant and potential copper chelator. J. Sci. Food Agric. 2020, 100, 3126–3135. [Google Scholar] [CrossRef]
- DeGraft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric-reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef]
- DeGraft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G. Plant polyphenols: How to translate their in vitro antioxidant actions to in vivo conditions. IUBMB Life 2007, 59, 308–315. [Google Scholar] [CrossRef]
- Giordano, M.E.; Caricato, R.; Lionetto, M.G. Concentration dependence of the antioxidant and prooxidant activity of Trolox in HeLa cells: Involvement in the induction of apoptotic volume decrease. Antioxidants 2020, 9, 1058. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Ferreira, H.; Lima, J.L.F.C.; Reis, S. Antioxidant activity of vitamin E and Trolox: Understanding of the factors that govern lipid peroxidation studies in vitro. Food Biophysics. 2009, 4, 312–320. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food. Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Winterton, P.; Britz, T.J.; Gelderblom, W.C. Antioxidant and pro-oxidant activities of aqueous extracts and crude polyphenolic fractions of rooibos (Aspalathus linearis). J. Agric. Food. Chem. 2005, 53, 10260–10267. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Light emission from the Fe2+-EGTA-H2O2 system: Possible application for the determination of antioxidant activity of plant phenolics. Molecules 2018, 23, 866. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Effect of physiological concentrations of vitamin C on the inhibitation of hydroxyl radical induced light emission from Fe2+-EGTA-H2O2 and Fe3+-EGTA-H2O2 systems in vitro. Molecules 2021, 26, 1993. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Kubicova, L. In vitro evaluation of pro- and antioxidant effects of flavonoid tricetin in comparison to myricetin. Molecules 2020, 25, 5850. [Google Scholar] [CrossRef]
- Dennis, K.K.; Go, Y.M.; Jones, D.P. Redox systems biology of nutrition and oxidative stress. J. Nutr. 2019, 149, 553–565. [Google Scholar] [CrossRef]
- Held, J.M. Redox systems biology: Harnessing the sentinels of the cysteine redoxome. Antioxid. Redox Signal. 2020, 32, 659–676. [Google Scholar] [CrossRef] [Green Version]
- Graf, E.; Mahoney, J.R.; Bryant, R.G.; Eaton, J.W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 1984, 259, 3620–3624. [Google Scholar] [CrossRef]
- Sánchez, M.; Sabio, L.; Gálvez, N.; Capdevila, M.; Dominguez-Vera, J.M. Iron chemistry at the service of life. IUBMB Life 2017, 69, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- René, A.; Abasq, M.L.; Hauchard, D.; Hapiot, P. How do phenolic compounds react toward superoxide ion? A simple electrochemical method for evaluating antioxidant capacity. Anal. Chem. 2010, 82, 8703–8710. [Google Scholar] [CrossRef]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food. Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Rana, M.S.; Guzman, M.I. Oxidation of phenolic aldehydes by ozone and hydroxyl radicals at the air-water interface. J. Phys. Chem. A 2020, 124, 8822–8833. [Google Scholar] [CrossRef]
- Kroflič, A.; Schaefer, T.; Huš, M.; Le, H.P.; Otto, T.; Herrmann, H. OH radicals reactivity towards phenol-related pollutants in water: Temperature dependence of the rate constants and novel insights into the [OH-phenol] adduct formation. Phys. Chem. Chem. Phys. 2020, 22, 1324–1332. [Google Scholar] [CrossRef]
- Roobinisha, S.; Elango, K.; Jothimani, S.; Subramanian, R. Isolation of hippuric acid from buffalo urine and its antioxidant activity. Buffalo Bull. 2017, 36, 530–536. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids-ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Ryan, P.; Hynes, M.J. The kinetics and mechanisms of the complex formation and antioxidant behavior of the polyphenols EGCg and ECG with iron (III). J. Inorg. Biochem. 2007, 101, 585–593. [Google Scholar] [CrossRef]
- Hynes, M.J.; Coinceanainn, M.Ó. The kinetics and mechanisms of the reaction of iron (III) with gallic acid, gallic acid methyl ester and catechin. J. Inorg. Biochem. 2001, 85, 131–142. [Google Scholar] [CrossRef]
- Moalin, M.; Strijdonck, G.P.; Beckers, M.; Hagemen, G.; Borm, P.; Bast, A.; Haenen, G.R. A planar conformation and the hydroxyl groups in the B and C rings play a pivotal role in the antioxidant capacity of quercetin and quercetin derivatives. Molecules 2011, 16, 9636–9650. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Sochor, J.; Ryvolova, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully automated spectrometric protocols for determination of antioxidant activity: Advantages and disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar] [CrossRef]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.; Stefano, R.D.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A.; et al. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef]
- Burkitt, M.J.; Milne, L. Hydroxyl radical formation from Cu(II)-trolox mixtures: Insights into the pro-oxidant properties of alpha-tocopherol. FEBS Lett. 1996, 379, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dziuba, N.; Hardy, J.; Lindahl, P.A. Low-molecular-mass iron complexes in blood plasma of iron-deficient pigs do not originate directly from nutrient iron. Metallomics 2019, 11, 1900–1911. [Google Scholar] [CrossRef] [Green Version]
- Steensma, A.; Faassen-Peters, M.A.; Noteborn, H.P.; Rietjens, I.M. Bioavailability of genistein and its glycoside genistin as measured in the portal vein of freely moving unanesthetized rats. J. Agric. Food Chem. 2006, 54, 8006–8012. [Google Scholar] [CrossRef]
- Hicks, M.; Gebicki, J.M. Rate constants for reaction of hydroxyl radicals with Tris, Tricine and Hepes buffers. FEBS J. 1986, 199, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Matsuzaki, Y.; Watanabe, T.; Uchiyama, K.; Ohsawa, K.; Imaeda, K. Effects of buffer solutions and chelators on the generation of hydroxyl radical and the lipid peroxidation in the Fenton reaction system. J. Clin. Biochem. Nutr. 1992, 13, 147–154. [Google Scholar] [CrossRef]
- Chen, H.Y. Why the reactive oxygen species of the Fenton reaction switches from oxoiron (IV) species to hydroxyl radical in phosphate buffer solutions? A computational rationale. ACS Omega 2019, 4, 14105–14113. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.W.; Gibson, Q.H. The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J. Biol. Chem. 1970, 245, 2409–2413. [Google Scholar] [CrossRef]
- Pospíšil, P.; Prasad, A.; Rác, M. Role of reactive oxygen species in ultra-weak photon emission in biological systems. J. Photochem. Photobiol. B 2014, 139, 11–23. [Google Scholar] [CrossRef]
- Cadenas, E.; Varsavsky, A.I.; Boveris, A.; Chance, B. Oxygen-or organic hydroperoxide-induced chemiluminescence of brain and liver homogenates. Biochem. J. 1981, 198, 645–654. [Google Scholar] [CrossRef] [Green Version]
| Polyphenol | Chemical Structure | % Inhibition at Concentrations of | Graph # | ||
|---|---|---|---|---|---|
| 5 µmol/L | 25 µmol/L | 50 µmol/L | |||
| 3,4-dihydroxyphenyl-acetic acid | 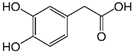 | 97 ± 17 (92; 18) *† | 103 ± 21 (99; 19) *† | 101 ± 16 (101; 14) *† | 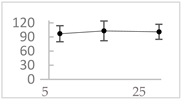 |
| Orthocresol |  | 86 ± 4 (86; 3) *† | 94 ± 1 (94; 1) *† | 94 ± 6 (93; 6) *† | 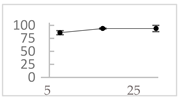 |
| Homovanillic acid | 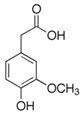 | 46 ± 14 (37; 15) *‡ | 85 ± 11 (86; 11) *‡ | 81 ± 13 (82; 15) *‡ | 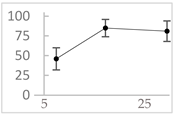 |
| Vanillic acid | 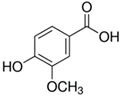 | 39 ± 23 (45; 24) *‡ | 70 ± 16 (73; 10) *‡ | 69 ± 3 (70; 2) *‡ | 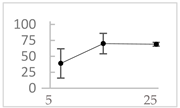 |
| Caffeic acid | 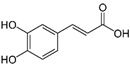 | 30 ± 12 (25; 11) * | 66 ± 15 (63; 20) * | 84 ± 20 (79;17) * |  |
| 4-hydroxyphenyl acetic acid |  | 24 ± 7 (26; 5) | 40 ± 7 (40; 10) * | 50 ± 1 (50; 2) * |  |
| 3-hydroxybenzoic acid | 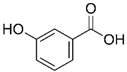 | 12 ± 12 (12; 15) | 10 ± 13 (8; 20) | 29 ± 17 (34; 10) * | 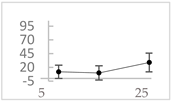 |
| hippuric acid | 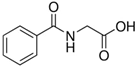 | 6 ± 12 (20; 19) | 29 ± 14 (30; 17) | 32 ± 12 (29; 14) * | 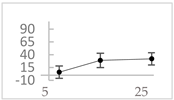 |
| Polyphenol | Chemical Structure | % Enhancement at Concentrations of | Graph # | ||
|---|---|---|---|---|---|
| 5 µmol/L | 25 µmol/L | 50 µmol/L | |||
| Gallic acid | 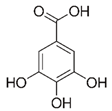 | 1689 ± 358 (1701; 567) *† | 3594 ± 912 (3350; 1534) *† | 2069 ± 484 (2128; 645) *† | 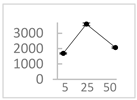 |
| Phloroglucinol |  | 349 ± 30 (346; 46) * | 1634 ± 132 (1630; 92) *† | 2730 ± 127 (2720; 171) *† |  |
| Pelargonidin | 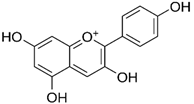 | 194 ± 109 (228; 92) * | 187 ± 92 (227; 65) * | 195 ± 98 (216; 60) * | 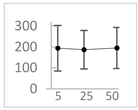 |
| Ellagic acid |  | 146 ± 51 (141; 85) * | 398 ± 114 (366; 110) * | 893 ± 180 (855; 245) * | 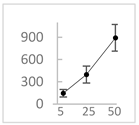 |
| pelargonidin-3-O-rutinoside | 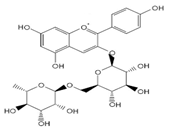 | 75 ± 7 (73; 9) * | 264 ± 50 (265; 86) * | 258 ± 28 (260; 38) * | 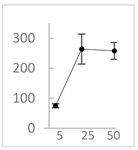 |
| Polyphenol | Chemical Structure | % Inhibition (↓) or Enhancement (↑) at Concentrations of | Graph # | ||
|---|---|---|---|---|---|
| 5 µmol/L | 25 µmol/L | 50 µmol/L | |||
| Ferulic acid | 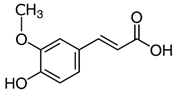 | ↑63 ± 22 (65; 35) * | ↑14 ± 13 (13; 18) | ↓28 ± 10 (28; 14) * | 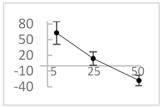 |
| Chlorogenic acid |  | ↑5 ± 22 (4; 22) | ↓78 ± 5 (78; 8) * | ↓94 ± 3 (94; 4) * | 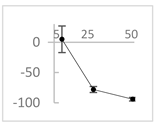 |
| cyanidin3-O-glucoside |  | ↑445 ± 65 (322; 97) *† | ↑80 ± 35 (64; 47) *‡ | ↓24 ± 14 (23; 21) | 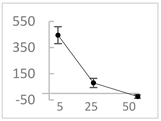 |
| Trolox | 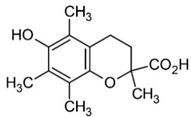 | ↑479 ± 51 (505; 45) *† | ↓104 ± 7 (100; 6) * | ↓105 ± 10 (99; 6) * | 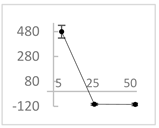 |
| Resorcinol | 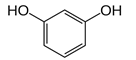 | ↓ 22 ± 17 (29; 24) | ↑10 ± 19 (6; 16) | ↑53 ± 23 (47; 19) * | 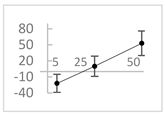 |
| Proposed Mechanism of Action | Effect on UPE |
|---|---|
| 1. Reaction with H2O2 | Suppression |
| 2. Reaction with •OH radicals | Suppression |
| 3. Chelation of Fe2+ ions to form less reactive complexes | Suppression |
| 4. Regeneration of Fe2+ ions by the reduction of Fe3+ into Fe2+ | Enhancement |
| 5. Reaction with H2O2 or •OH radicals to form products that emit light | Enhancement |
| 6. Reaction with O2− radicals | Suppression |
| No. | Sample | Volumes of Working Solutions Added to Luminometer Tube (µL) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| PBS | Polyphenol * | EGTA | FeSO4 | H2O2 | H2O | ||
| 1 | Complete system | 940 | - | 20 | 20 | 100 | - |
| 2 | Complete system + polyphenol | - | 940 | 20 | 20 | 100 | - |
| 3 | Incomplete system | 960 | - | - | 20 | 100 | - |
| 4 | Incomplete system + polyphenol | 20 | 940 | - | 20 | 100 | - |
| Additional controls | |||||||
| 5 | Fe2+-EGTA without H2O2 | 940 | - | 20 | 20 | - | 100 |
| 6 | Fe2+-EGTA without H2O2 + Polyphenol | - | 940 | 20 | 20 | - | 100 |
| 7 | Polyphenol alone | 40 | 940 | - | - | - | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. https://doi.org/10.3390/molecules27113453
Nowak M, Tryniszewski W, Sarniak A, Wlodarczyk A, Nowak PJ, Nowak D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules. 2022; 27(11):3453. https://doi.org/10.3390/molecules27113453
Chicago/Turabian StyleNowak, Michal, Wieslaw Tryniszewski, Agata Sarniak, Anna Wlodarczyk, Piotr J. Nowak, and Dariusz Nowak. 2022. "Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System" Molecules 27, no. 11: 3453. https://doi.org/10.3390/molecules27113453
APA StyleNowak, M., Tryniszewski, W., Sarniak, A., Wlodarczyk, A., Nowak, P. J., & Nowak, D. (2022). Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules, 27(11), 3453. https://doi.org/10.3390/molecules27113453






