Elucidation of the Lipid Composition of Hemp (Cannabis sativa L.) Products by Means of Gas Chromatography and Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fatty Acid Methly Ester Analysis
2.1.1. Choice of the Chromatographic Method and Qualitative Results
2.1.2. Quantitative Results
2.2. Triacylglycerol Analysis
2.2.1. Choice of the Chromatographic Method
2.2.2. Qualitative Analysis: Application of a Novel LRI-Based Identification Strategy
2.2.3. Quantitative Results
3. Materials and Methods
3.1. Reagents and Materials
3.2. Samples
3.3. Sample Preparation
3.4. Instruments and Analytical Conditions
3.4.1. GC-MS/FID
+ (Ʃn3-PUFA/Ʃn6-PUFA)],
3.4.2. LC-MS
3.4.3. LC-MS Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica 2004, 140, 1–6. [Google Scholar] [CrossRef]
- Alcohol, Drugs and Addictive Behaviours Unit. Available online: www.who.int (accessed on 14 April 2022).
- European Monitoring Centre for Drugs and Drug Addiction (2017), Cannabis Legislation. Available online: https://www.emcdda.europa.eu/publications/adhoc/cannabis-legislation-europe_en (accessed on 30 April 2022).
- EU Regulation 1307/2013 Establishing Rules for Direct Payments to Farmers under Support Schemes within the Framework of the Common Agricultural Policy and Repealing Council Regulation (EC) No 637/2008 and Council Regulation (EC) No 73/2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32013R1307 (accessed on 30 April 2022).
- EU Plant Variety Database of the European Commission (v.3.4). Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchForm&ctl_type=A (accessed on 14 April 2022).
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a Nutritional Resource: An Overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.; Seguin, P.; Mustafa, A.F.; Charron, J. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Marzocchi, S.; Caboni, M.F. Effect of harvesting time on hemp (Cannabis sativa L.) seed oil lipid composition. Ital. J. Food Sci. 2020, 32, 1018–1029. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crop. Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M.; Boháč, M. Statistical Evaluation of Triacylglycerol Composition in Plant Oils Based on High-Performance Liquid Chromatography−Atmospheric Pressure Chemical Ionization Mass Spectrometry Data. J. Agric. Food Chem. 2009, 57, 6888–6898. [Google Scholar] [CrossRef]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152–182. [Google Scholar] [CrossRef]
- Rigano, F.; Arena, P.; Mangraviti, D.; Donnarumma, D.; Dugo, P.; Donato, P.; Mondello, L.; Micalizzi, G. Identification of high-value generating molecules from the wastes of tuna fishery industry by liquid chromatography and gas chromatography hyphenated techniques with automated sample preparation. J. Sep. Sci. 2021, 44, 1571–1580. [Google Scholar] [CrossRef]
- Holčapek, M.; Jandera, P.; Zderadička, P.; Hrubá, L. Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 1010, 195–215. [Google Scholar] [CrossRef]
- Holčapek, M.; Lísa, M.; Jandera, P.; Kabátová, N. Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering and UV detection. J. Sep. Sci. 2005, 28, 1315–1333. [Google Scholar] [CrossRef]
- Rigano, F.; Oteri, M.; Micalizzi, G.; Mangraviti, D.; Dugo, P.; Mondello, L. Lipid profile of fish species by liquid chromatography coupled to mass spectrometry and a novel linear retention index database. J. Sep. Sci. 2020, 43, 1773–1780. [Google Scholar] [CrossRef]
- Rigano, F.; Oteri, M.; Russo, M.; Dugo, P.; Mondello, L. Proposal of a Linear Retention Index System for Improving Identification Reliability of Triacylglycerol Profiles in Lipid Samples by Liquid Chromatography Methods. Anal. Chem. 2018, 90, 3313–3320. [Google Scholar] [CrossRef]
- Ruiz-Gutidrrez, V.; Barron, L.J.R. Methods for the analysis of triacylglycerols. J. Chromatogr. B 1995, 671, 133–168. [Google Scholar] [CrossRef]
- Rigano, F.; Arigò, A.; Oteri, M.; La Tella, R.; Dugo, P.; Mondello, L. The retention index approach in liquid chromatography: An historical review and recent advances. J. Chromatogr. A 2021, 1640, 461963–461975. [Google Scholar] [CrossRef]
- Dimić, E.; Romanić, R.; Vujasinović, V. Essential Fatty Acids, Nutritive Value and Oxidative Stability of Cold Pressed Hempseed (Cannabis sativa L.) Oil from Different Varieties. Acta Aliment. 2009, 38, 229–236. [Google Scholar] [CrossRef]
- Petrović, M.; Debeljak, Ž.; Kezić, N.; Džidara, P. Relationship between cannabinoids content and composition of fatty acids in hempseed oils. Food Chem. 2015, 170, 218–225. [Google Scholar] [CrossRef]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in Seed Traits in a Collection of Cannabis sativa L. Genotypes. Front. Plant Sci. 2016, 7, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Kriese, U.; Schumann, E.; Weber, W.; Beyer, M.; Brühl & Matthäus, L. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Micalizzi, G.; Ragosta, E.; Farnetti, S.; Dugo, P.; Tranchida, P.Q.; Mondello, L.; Rigano, F. Rapid and miniaturized qualitative and quantitative gas chromatography profiling of human blood total fatty acids. Anal. Bioanal. Chem. 2020, 412, 2327–2337. [Google Scholar] [CrossRef]
- Ragonese, C.; Tranchida, P.Q.; Dugo, P.; Dugo, G.; Sidisky, L.M.; Robillard, M.V.; Mondello, L. Evaluation of use of a dicationic liquid stationary phase in the fast and conventional gas chromatographic analysis of health-hazardous C18 cis/trans fatty acids. Anal. Chem. 2009, 81, 5561–5568. [Google Scholar] [CrossRef]
- Fardin-Kia, A.R.; Delmonte, P.; Kramer, J.K.; Jahreis, G.; Kuhnt, K.; Santercole, V.; Rader, J.I. Separation of the fatty acids in menhaden oil as methyl esters with a highly polar ionic liquid gas chromatographic column and identification by time of flight mass spectrometry. Lipids 2013, 48, 1279–1295. [Google Scholar] [CrossRef]
- Delmonte, P.; Fardin-Kia, A.R.; Kramer, J.K.; Mossoba, M.M.; Sidisky, L.; Tyburczy, C.; Rader, J.I. Evaluation of highly polar ionic liquid gas chromatographic column for the determination of the fatty acids in milk fat. J. Chromatogr. A 2012, 1233, 137–146. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Wan, S.; Hua, K.; Jiang, C.; Wang, D.; He, C.; Guo, X. Enhanced yields and soil quality in a wheat–maize rotation using buried straw mulch. J. Sci. Food Agric. 2017, 97, 3333–3341. [Google Scholar] [CrossRef]
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary Supplementation with γ-Linolenic Acid Alters Fatty Acid Content and Eicosanoid Production in Healthy Humans. J. Nutr. 1997, 127, 1435–1444. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [Green Version]
- Grover, S.; Kumari, P.; Kumar, A.; Soni, A.; Sehgal, S.; Sharma, V. Preparation and Quality Evaluation of Different Oil Blends. Lett. Appl. NanoBioSci. 2021, 10, 2126–2137. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2021, 21, 5695. [Google Scholar] [CrossRef]
- Moussa, E.-R.W.H.; Shereen, A.N.; Manal, A.; Mehanni, A.-H.E.; Rasha, A.E. Nutritional value and fatty acid composition of household cooking on fish fatty acids profile using atherogenicity and thrombogenicity indices. J. Food Chem. Nutr. 2014, 2, 27–41. [Google Scholar]
- Guimarães, R.C.A.; Macedo, M.L.R.; Munhoz, C.L.; Filiu, W.; Viana, L.H.; Nozaki, V.T.; Hiane, P.A. Sesame and flaxseed oil: Nutritional quality and effects on serum lipids and glucose in rats. Food Sci. Technol. 2013, 33, 209–217. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.-Q.; Wu, Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS ONE 2020, 15, e0228276. [Google Scholar] [CrossRef] [Green Version]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in Edible Oils: Determination, Characterization, Quantitation, Chemometric Approach and Evaluation of Adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef]
- Tringaniello, C.; Cossignani, L.; Blasi, F. Characterization of the Triacylglycerol Fraction of Italian and Extra-European Hemp Seed Oil. Foods 2021, 10, 916. [Google Scholar] [CrossRef]
- Mungure, T.E.; Birch, E.J. Analysis of Intact Triacylglycerols in Cold Pressed Canola, Flax and Hemp Seed Oils by HPLC and ESI-MS. SOP Trans. Anal. Chem. 2014, 1, 48–61. [Google Scholar] [CrossRef]
- Taaifi, Y.; Benmoumen, A.; Belhaj, K.; Aazza, S.; Abid, M.; Azeroual, E.; Elamrani, A.; Mansouri, F.; Serghini Caid, H. Seed Composition of Non-Industrial Hemp (Cannabis sativa L.) Varieties from Four Regions in Northern Morocco. Int. J. Food Sci. Technol. 2021, 56, 5931–5947. [Google Scholar] [CrossRef]
- Oteri, M.; Rigano, F.; Micalizzi, G.; Casale, M.; Malegori, C.; Dugo, P.; Mondello, L. Comparison of lipid profile of Italian Extra Virgin Olive Oils by using rapid chromatographic approaches. J. Food Compos. Anal. 2022, 110, 104531–104544. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M. Triacylglycerols in Nut and Seed Oils: Detailed Characterization Using High-Performance Liquid Chromatography/Mass Spectrometry. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 43–54. [Google Scholar] [CrossRef]
- Beccaria, M.; Inferrera, V.; Rigano, F.; Gorynski, K.; Purcaro, G.; Pawliszyn, J.; Dugo, P.; Mondello, L. Highly informative multiclass profiling of lipids by ultra-high performance liquid chromatography—Low resolution (quadrupole) mass spectrometry by using electrospray ionization and atmospheric pressure chemical ionization interfaces. J. Chromatogr. A 2017, 1509, 69–82. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
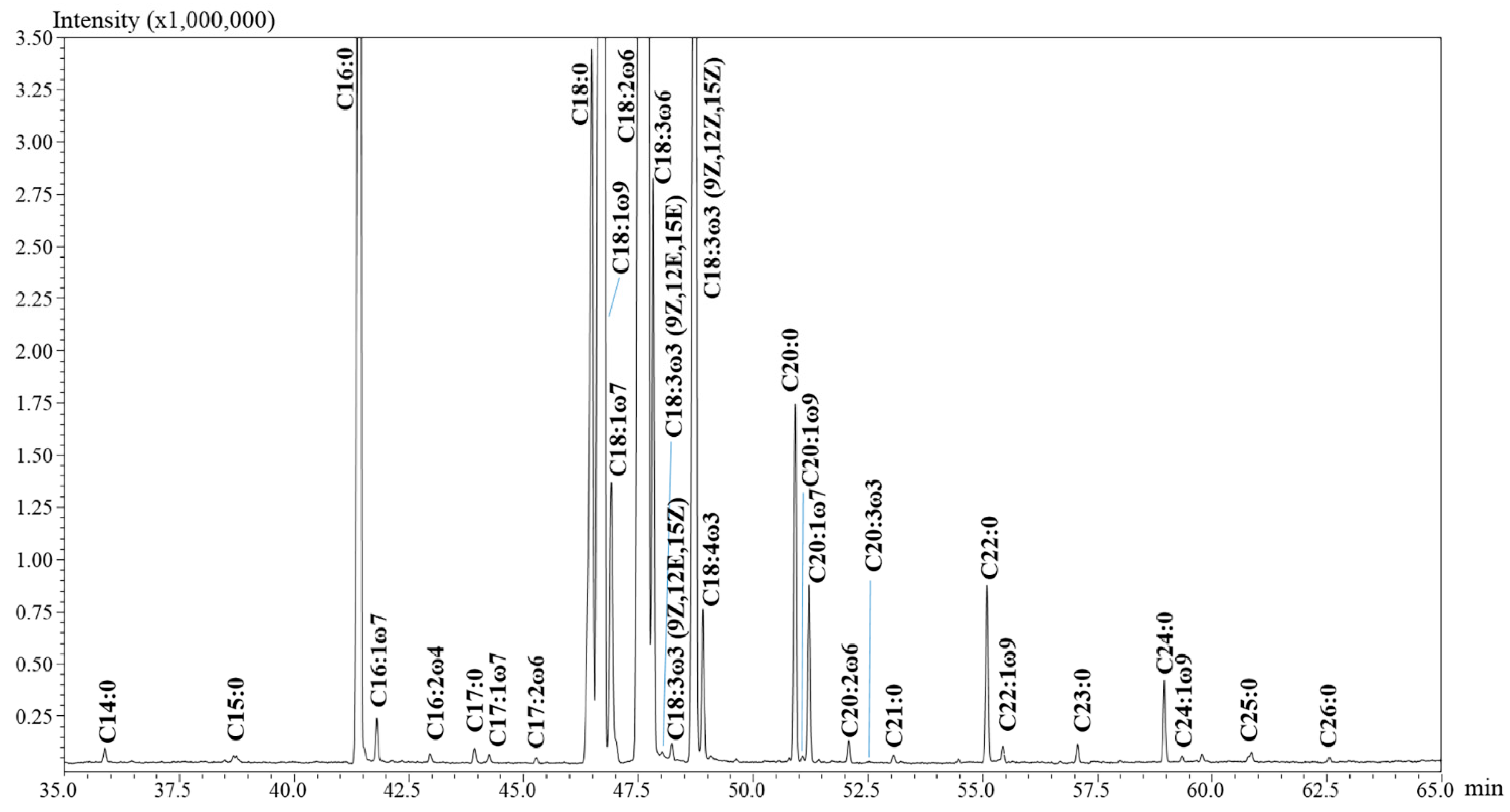
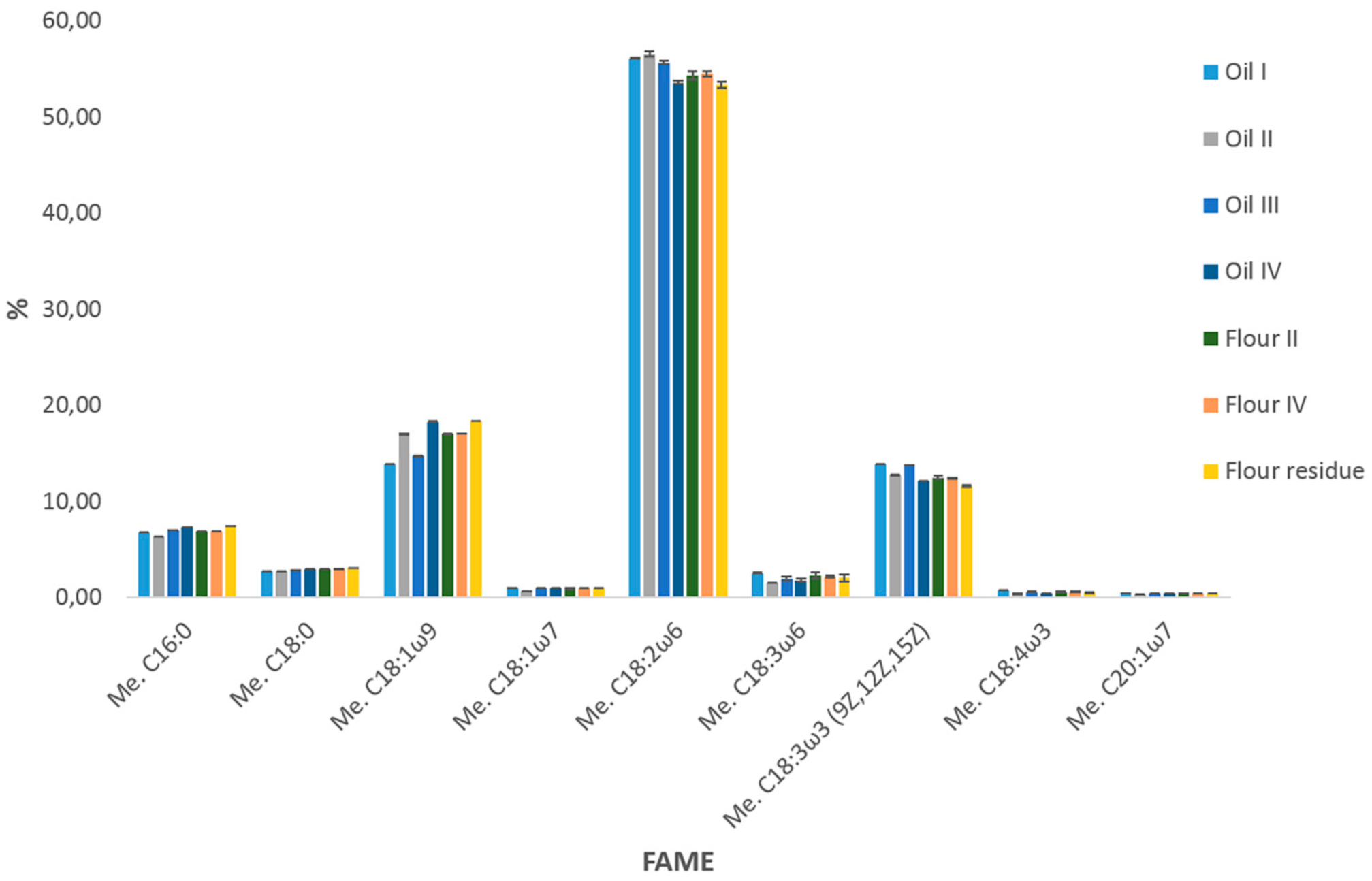
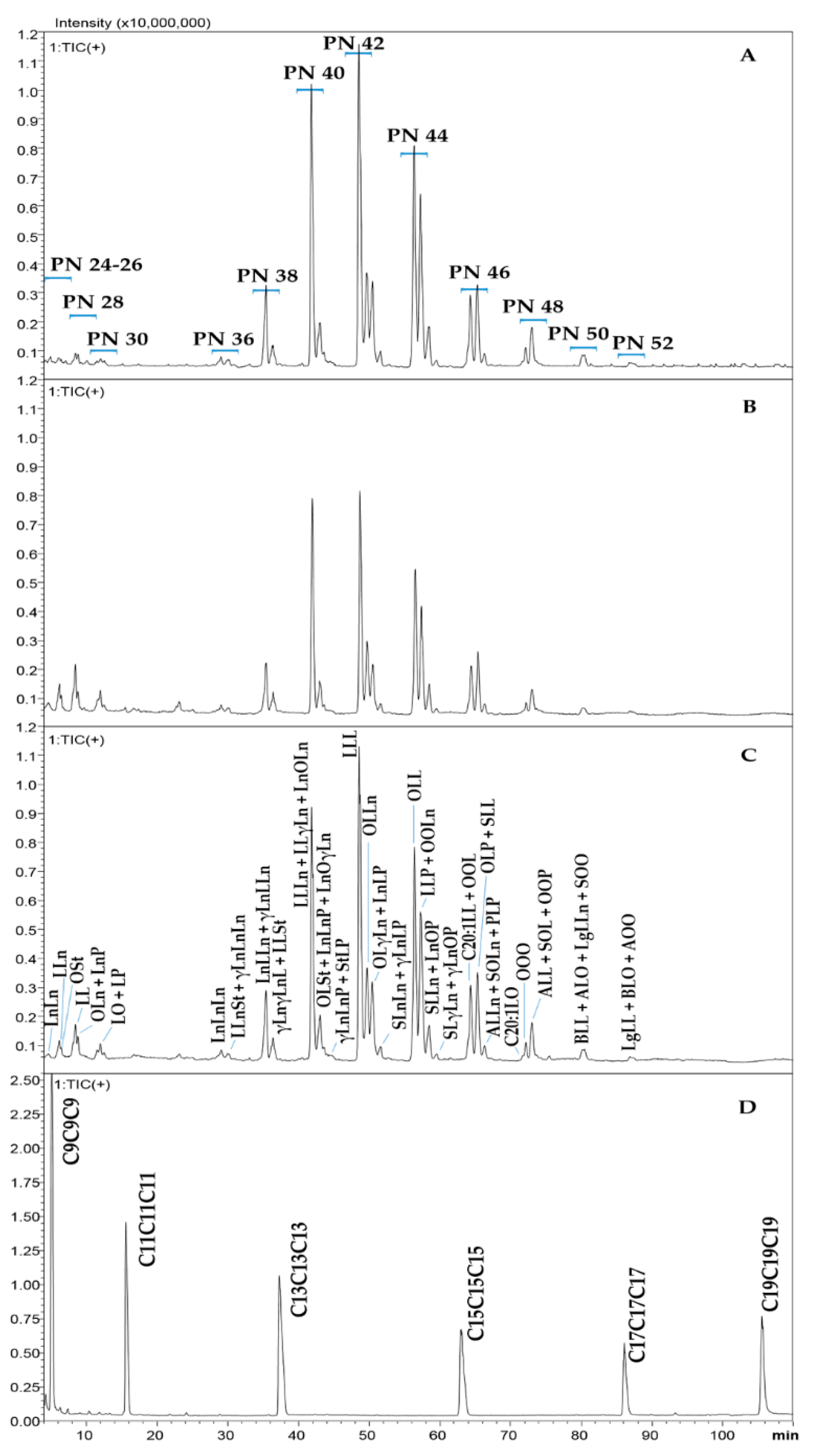


| n. | Compound | LRIexp | LRIlib | MS % Similarity | Oil I | Oil II | Oil III | Oil IV | Flour II | Flour IV | Residue IV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Me. C14:0 | 1401 | 1400 | 96 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.00 |
| 2 | Me. C15:0 | 1503 | 1500 | 95 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.01 |
| 3 | Me. C16:0 | 1603 | 1600 | 97 | 6.76 ± 0.01 | 6.36 ± 0.03 | 7.01 ± 0.01 | 7.31 ± 0.00 | 6.94 ± 0.01 | 6.87 ± 0.00 | 7.46 ± 0.01 |
| 4 | Me. C16:1n7 | 1618 | 1609 | 97 | 0.11 ± 0.00 | 0.03 ± 0.00 | 0.11 ± 0.00 | 0.12 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.12 ± 0.00 |
| 5 | Me. C16:2n4 (9Z.12Z) | 1665 | 1663 | 90 | 0.02 ± 0.00 | 0.09 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 |
| 6 | Me. C17:0 | 1703 | 1700 | 95 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| 7 | Me. C17:1n7 | 1716 | 1713 | 94 | 0.02± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 |
| 8 | Me. C17:2n6 (9Z.12Z) a | 1757 | - | - | 0.03± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 9 | Me. C18:0 | 1806 | 1800 | 97 | 2.77 ± 0.01 | 2.78 ± 0.01 | 2.83 ± 0.01 | 2.97 ± 0.00 | 2.96 ± 0.01 | 2.97 ± 0.01 | 3.04 ± 0.01 |
| 10 | Me. C18:1n9 | 1817 | 1810 | 94 | 13.86 ± 0.00 | 17.01 ± 0.04 | 14.75 ± 0.05 | 18.31 ± 0.02 | 17.01 ± 0.01 | 17.09 ± 0.01 | 18.40 ± 0.00 |
| 11 | Me. C18:1n7 | 1824 | 1826 | 96 | 0.98 ± 0.01 | 0.69 ± 0.01 | 0.98 ± 0.00 | 1.01 ± 0.01 | 0.94 ± 0.02 | 0.96 ± 0.01 | 1.01 ± 0.00 |
| 12 | Me. C18:2n6 (9Z.12Z) | 1860 | 1851 | 98 | 56.11 ± 0.07 | 56.52 ± 0.28 | 55.66 ± 0.17 | 53.56 ± 0.17 | 54.28 ± 0.42 | 54.48 ± 0.24 | 53.30 ± 0.32 |
| 13 | Me. C18:3n6 (6Z.9Z.12Z) | 1865 | 1858 | 96 | 2.58 ± 0.03 | 1.49 ± 0.00 | 1.98 ± 0.17 | 1.78 ± 0.18 | 2.28 ± 0.35 | 2.21 ± 0.15 | 2.08 ± 0.17 |
| 14 | Me. C18:3n3 (9Z.12E.15E) | 1873 | 1875 | 92 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.01 ± 0.00 |
| 15 | Me. C18:3n3 (9Z.12E.15Z) | 1883 | 1880 | 96 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.02 | 0.04 ± 0.00 |
| 16 | Me. C18:3n3 (9Z.12Z.15Z) | 1906 | 1902 | 98 | 13.85 ± 0.00 | 12.78 ± 0.06 | 13.77 ± 0.04 | 12.13 ± 0.01 | 12.52 ± 0.15 | 12.38 ± 0.07 | 11.56 ± 0.09 |
| 17 | Me. C18:4n3 (6Z.9Z.12Z.15Z) | 1913 | 1909 | 97 | 0.75 ± 0.00 | 0.40 ± 0.03 | 0.61 ± 0.01 | 0.44 ± 0.00 | 0.61 ± 0.08 | 0.58 ± 0.06 | 0.52 ± 0.08 |
| 18 | Me. C20:0 | 2002 | 2000 | 96 | 0.89 ± 0.00 | 0.72 ± 0.00 | 0.90 ± 0.01 | 0.90± 0.00 | 0.85 ± 0.01 | 0.87 ± 0.01 | 0.89 ± 0.01 |
| 19 | Me. C20:1n9 | 2009 | 2008 | 92 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 |
| 20 | Me. C20:1n7 | 2016 | 2015 | 98 | 0.40 ± 0.00 | 0.33 ± 0.01 | 0.43 ± 0.00 | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.47 ± 0.00 |
| 21 | Me. C20:2n6 (11Z.14Z) | 2057 | 2055 | 96 | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.06 ± 0.00 |
| 22 | Me. C21:0 | 2103 | 2102 | 90 | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 |
| 23 | Me. C20:3n3 (11Z.14Z.17Z) | 2110 | 2109 | 89 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 24 | Me. C22:0 | 2201 | 2200 | 91 | 0.37 ± 0.00 | 0.31 ± 0.00 | 0.40 ± 0.00 | 0.42 ± 0.00 | 0.40 ± 0.00 | 0.41 ± 0.00 | 0.45 ± 0.01 |
| 25 | Me. C22:1n9 | 2219 | 2217 | 91 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.07 ± 0.00 |
| 26 | Me. C23:0 | 2303 | 2301 | 95 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 |
| 27 | Me. C24:0 | 2401 | 2400 | 93 | 0.16 ± 0.00 | 0.13 ± 0.01 | 0.18 ± 0.00 | 0.19 ± 0.00 | 0.18 ± 0.00 | 0.19 ± 0.00 | 0.22 ± 0.00 |
| 28 | Me. C24:1n9 | 2421 | 2420 | 90 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.00 |
| 29 | Me. C25:0 | 2496 | 2494 | 89 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 30 | Me. C26:0 | 2598 | 2600 | 91 | 0.03 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| SFA | 11.15 ± 0.04 | 10.51 ± 0.18 | 11.53 ± 0.05 | 12.02 ± 0.04 | 11.59 ± 0.08 | 11.54 ± 0.05 | 12.30 ± 0.06 | ||||
| MUFA | 15.43 ± 0.02 | 18.13 ± 0.06 | 16.33 ± 0.06 | 19.96 ± 0.03 | 18.61 ± 0.04 | 18.70 ± 0.04 | 20.13 ± 0.02 | ||||
| PUFA | 73.49 ± 0.11 | 71.41 ± 0.38 | 72.21 ± 0.40 | 68.07 ± 0.37 | 69.85 ± 1.00 | 69.81 ± 0.56 | 67.63 ± 0.87 | ||||
| ω6 | 58.78 ± 0.10 | 58.07 ± 0.28 | 57.74 ± 0.34 | 55.42 ± 0.35 | 56.63 ± 0.78 | 56.76 ± 0.39 | 55.46 ± 0.71 | ||||
| ω3 | 14.69 ± 0.01 | 13.25 ± 0.09 | 14.45 ± 0.05 | 12.64 ± 0.01 | 13.21 ± 0.22 | 13.04 ± 0.16 | 12.14 ± 0.17 | ||||
| ω6/ω3 | 4.00 ± 0.01 | 4.38 ± 0.05 | 4.00 ± 0.04 | 4.39 ± 0.03 | 4.29 ± 0.13 | 4.35 ± 0.09 | 4.57 ± 0.12 | ||||
| SFA/MUFA | 0.72 ± 0.01 | 0.58 ± 0.01 | 0.71 ± 0.01 | 0.60 ± 0.01 | 0.62 ± 0.01 | 0.62 ± 0.01 | 0.61 ± 0.01 | ||||
| PUFA/SFA | 6.59 ± 0.04 | 6.79 ± 0.16 | 6.26 ± 0.06 | 5.66 ± 0.05 | 6.03 ± 0.13 | 6.05 ± 0.08 | 5.49 ± 0.10 | ||||
| AI | 0.08 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | ||||
| TI | 0.12 ± 0.00 | 0.11 ± 0.01 | 0.12 ± 0.00 | 0.13 ± 0.02 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.14 ± 0.00 | ||||
| h/H | 12.86 ± 0.03 | 13.81 ± 0.16 | 12.33 ± 0.09 | 11.73 ± 0.08 | 12.42 ± 0.16 | 12.61 ± 0.19 | 11.45 ± 0.14 |
| PN | Compound | CN | DB | LRIexp | LRItab a | Δ | Oil I | Oil II | Oil III | Oil IV | Flour II | Flour IV | Residue IV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | LnLn | 36 | 6 | 2608 # | - | - | 0.67 ± 0.04 | 0.27 ± 0.01 | 0.88 ± 0.01 | 0.68 ± 0.04 | 1.16 ± 0.04 | 1.37 ± 0.09 | 0.54 ± 0.01 |
| 26 | LLn | 36 | 5 | 2764 | - | - | 1.00 ± 0.06 | 1.04 ± 0.04 | 1.6 ± 0.02 | 0.57 ± 0.03 | 2.83 ± 0.08 | 3.59 ± 0.23 | 1.71 ± 0.00 |
| 26 | OSt | 36 | 5 | 2781 | - | - | 0.15 ± 0.01 | 0.18 ± 0.02 | 0.26 ± 0.01 | 0.14 ± 0 | 0.66 ± 0.02 | 0.97 ± 0.11 | 0.46 ± 0.04 |
| 28 | LL | 36 | 4 | 2895 | - | - | 1.53 ± 0.15 | 1.92 ± 0.05 | 2.02 ± 0.13 | 0.79 ± 0.02 | 4.23 ± 0.1 | 5.29 ± 0.07 | 2.79 ± 0.04 |
| 28 | OLn + LnP | 36/34 | 4/3 | 2917 | - | - | 0.3 ± 0.02 | 0.35 ± 0.01 | 0.46 ± 0.05 | 0.37 ± 0.01 | 0.91 ± 0.09 | 1.11 ± 0.1 | 0.82 ± 0.02 |
| 30 | LO + LP | 36/34 | 3/2 | 3100 | - | - | 0.54 ± 0.10 | 0.34 ± 0.06 | 0.51 ± 0.02 | 0.32 ± 0.01 | 1.13 ± 0.08 | 1.29 ± 0.15 | 0.94 ± 0.12 |
| 36 | LnLnLn | 54 | 9 | 3675 * | 3668 | 7 | 0.87 ± 0.02 | 1.14 ± 0.05 | 1.08 ± 0.04 | 0.89 ± 0.01 | 0.86 ± 0.06 | 1.37 ± 0.16 | 0.83 ± 0.04 |
| 36 | LLnSt + γLnLnLn | 54 | 9 | 3703 | - | - | 1.14 ± 0.05 | 1.08 ± 0.03 | 1.14 ± 0.05 | 0.66 ± 0.08 | 0.82 ± 0.07 | 0.82 ± 0.06 | 0.55 ± 0.01 |
| 36 | γLnγLnLn | 54 | 9 | 3720 | - | - | 0.13 ± 0.01 | 0.26 ± 0.02 | 0.22 ± 0.01 | 0.34 ± 0.04 | - | - | - |
| 38 | LnLLn + γLnLLn | 54 | 8 | 3845 * | 3830/- | 15/- | 6.95 ± 0.27 | 6.94 ± 0.20 | 7.42 ± 0.16 | 5.84 ± 0.22 | 6.27 ± 0.26 | 5.97 ± 0.28 | 6.29 ± 0.00 |
| 38 | γLnγLnL + LLSt | 54 | 8 | 3876 * | 3867/3890 b | 9/14 | 3.89 ± 0.03 | 2.61 ± 0.00 | 3.42 ± 0.05 | 2.29 ± 0.01 | 2.62 ± 0.05 | 2.69 ± 0.25 | 2.54 ± 0.04 |
| 40 | LLLn + LLγLn + LnOLn | 54 | 7 | 4008 | 3993/3999/4011 | 15/9/3 | 17.53 ± 0.49 | 15.66 ± 0.49 | 16.96 ± 0.53 | 16.60 ± 0.33 | 14.57 ± 0.76 | 15.65 ± 1.06 | 14.90 ± 0.11 |
| 40 | OLSt + LnLnP + LnOγLn | 54/52/54 | 7/6/7 | 4036 * | -/4023/- | -/13/- | 4.38 ± 0.13 | 2.98 ± 0.05 | 4.21 ± 0.3 | 2.93 ± 0.05 | 2.62 ± 0.32 | 2.97 ± 0.04 | 2.67 ± 0.01 |
| 40 | γLnLnP + StLP | 52 | 6 | 4048 * | -/4049 b | - | 0.46 ± 0.06 | 0.50 ± 0.05 | 0.68 ± 0.14 | 0.42 ± 0.07 | 0.34 ± 0.02 | 0.42 ± 0.03 | 0.36 ± 0.07 |
| 42 | LLL | 54 | 6 | 4165 * | 4160 | 5 | 20.31 ± 0.3 | 19.35 ± 0.21 | 19.86 ± 0.5 | 20.11 ± 0.13 | 18.31 ± 0.22 | 17.12 ± 0.37 | 19.18 ± 0.01 |
| 42 | OLLn | 54 | 6 | 4191 | 4192 | −1 | 5.56 ± 0.14 | 5.97 ± 0.04 | 5.04 ± 0.04 | 5.98 ± 0.35 | 5.56 ± 0.19 | 5.74 ± 0.20 | 5.79 ± 0.09 |
| 42 | OLγLn+ LnLP | 54/52 | 6/5 | 4209 | 4196/4217 | 13/−8 | 4.95 ± 0.15 | 4.54 ± 0.38 | 4.41 ± 0.34 | 4.88 ± 0.21 | 4.37 ± 0.18 | 3.90 ± 0.08 | 4.63 ± 0.13 |
| 42 | SLnLn + γLnLP | 54/52 | 6/5 | 4235 | 4216/-/4221/4221 | 19/-/14/14 | 1.19 ± 0.03 | 0.82 ± 0.01 | 0.94 ± 0.18 | 0.96 ± 0.14 | 0.91 ± 0.06 | 0.93 ± 0.08 | 1.02 ± 0.02 |
| 44 | OLL | 54 | 5 | 4348 | 4342 | 6 | 8.57 ± 0.16 | 9.76 ± 0.08 | 8.52 ± 0.43 | 11.13 ± 0.32 | 9.72 ± 0.42 | 9.55 ± 0.20 | 10.18 ± 0.14 |
| 44 | LLP + OOLn | 52/54 | 4/5 | 4369 | 4358/4360 | 11/9 | 6.84 ± 0.18 | 7.15 ± 0.03 | 6.41 ± 0.60 | 7.97 ± 0.12 | 6.86 ± 0.05 | 6.18 ± 0.21 | 7.69 ± 0.12 |
| 44 | SLLn + LnOP | 54/52 | 5/4 | 4396 | 4378/4383 | 18/13 | 2.15 ± 0.12 | 2.34 ± 0.07 | 1.99 ± 0.29 | 2.36 ± 0.13 | 2.36 ± 0.08 | 2.04 ± 0.08 | 2.05 ± 0.07 |
| 44 | SLγLn + γLnOP | 54/52 | 5/4 | 4420 | -/4403 | -/17 | 0.48 ± 0.07 | 0.40 ± 0.01 | 0.42 ± 0.07 | 0.38 ± 0.05 | 0.57 ± 0.12 | 0.40 ± 0.02 | 0.31 ± 0.02 |
| 46 | C20:1LL | 56 | 5 | 4502 | 4512 | −10 | 2.93 ± 0.23 | 4.24 ± 0.07 | 3.16 ± 0.19 | 4.14 ± 0.23 | 3.71 ± 0.28 | 3.66 ± 0.19 | 4.1 ± 0.05 |
| 46 | OOL | 54 | 4 | 4537 | 4522 | 15 | |||||||
| 46 | OLP + SLL | 52/54 | 3/4 | 4562 | 4548/4548 | 14/14 | 3.63 ± 0.07 | 4.64 ± 0.1 | 3.7 ± 0.04 | 4.26 ± 0.44 | 4.09 ± 0.37 | 3.8 ± 0.23 | 4.19 ± 0.03 |
| 46 | ALLn + SOLn + PLP | 56/54/50 | 5/4/2 | 4586 | -/4575/4571 | /11/15 | 0.69 ± 0.09 | 0.84 ± 0.02 | 0.61 ± 0.04 | 0.72 ± 0.08 | 0.78 ± 0.03 | 0.73 ± 0.06 | 0.74 ± 0.07 |
| 48 | C20:1LO | 56 | 4 | 4728 | 4708 | 20 | 0.11 ± 0.01 | 0.16 ± 0 | 0.22 ± 0.03 | 0.13 ± 0 | 0.18 ± 0.02 | 0.12 ± 0.01 | 0.24 ± 0.02 |
| 48 | OOO | 54 | 3 | 4740 | 4729 | 11 | 0.24 ± 0.05 | 0.49 ± 0.04 | 0.39 ± 0 | 0.51 ± 0.02 | 0.4 ± 0.03 | 0.35 ± 0.07 | 0.56 ± 0.04 |
| 48 | ALL + SOL + OOP | 56/54/52 | 4/3/2 | 4761 | -/4746/4756 | -/15/5 | 1.41 ± 0.29 | 2.03 ± 0.13 | 1.63 ± 0.07 | 1.95 ± 0.07 | 1.81 ± 0.07 | 1.66 ± 0.36 | 2.26 ± 0.28 |
| 48 | BLLn + AOLn | 58/56 | 5/4 | 4777 | - | - | 0.19 ± 0.01 | 0.23 ± 0.02 | 0.25 ± 0 | ||||
| 48 | SLP + POP | 52/50 | 2/1 | 4789 | -/4776 | -/13 | 0.04 ± 0.01 | 0.06 ± 0 | 0.06 ± 0.01 | ||||
| 50 | BLL+ ALO +LgLLn+ SOO | 58/56/60/54 | 4/3/5/2 | 4948 | -/-/-/4948 | -/-/-/0 | 0.91 ± 0.03 | 1.24 ± 0.05 | 1.09 ± 0.02 | 1.23 ± 0.03 | 1 ± 0.16 | 1.08 ± 0.1 | 1.23 ± 0.01 |
| 52 | LgLL + BLO + AOO | 60/58/56 | 4/3/2 | 5126 | -/-/5138 c | -/-/-12 | 0.24 ± 0 | 0.38 ± 0 | 0.36 ± 0.02 | 0.43 ± 0.05 | 0.35 ± 0.06 | 0.34 ± 0.03 | 0.44 ± 0 |
| 54 | LgLO + BOO | 60/58 | 3/2 | 5336 | - | - | 0.05 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, P.; Rigano, F.; Guarnaccia, P.; Dugo, P.; Mondello, L.; Trovato, E. Elucidation of the Lipid Composition of Hemp (Cannabis sativa L.) Products by Means of Gas Chromatography and Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Detection. Molecules 2022, 27, 3358. https://doi.org/10.3390/molecules27103358
Arena P, Rigano F, Guarnaccia P, Dugo P, Mondello L, Trovato E. Elucidation of the Lipid Composition of Hemp (Cannabis sativa L.) Products by Means of Gas Chromatography and Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Detection. Molecules. 2022; 27(10):3358. https://doi.org/10.3390/molecules27103358
Chicago/Turabian StyleArena, Paola, Francesca Rigano, Paolo Guarnaccia, Paola Dugo, Luigi Mondello, and Emanuela Trovato. 2022. "Elucidation of the Lipid Composition of Hemp (Cannabis sativa L.) Products by Means of Gas Chromatography and Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Detection" Molecules 27, no. 10: 3358. https://doi.org/10.3390/molecules27103358
APA StyleArena, P., Rigano, F., Guarnaccia, P., Dugo, P., Mondello, L., & Trovato, E. (2022). Elucidation of the Lipid Composition of Hemp (Cannabis sativa L.) Products by Means of Gas Chromatography and Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Detection. Molecules, 27(10), 3358. https://doi.org/10.3390/molecules27103358







