Computer-Aided Detection of Quantitative Signatures for Breast Fibroepithelial Tumors Using Label-Free Multi-Photon Imaging
Abstract
:1. Introduction
2. Results
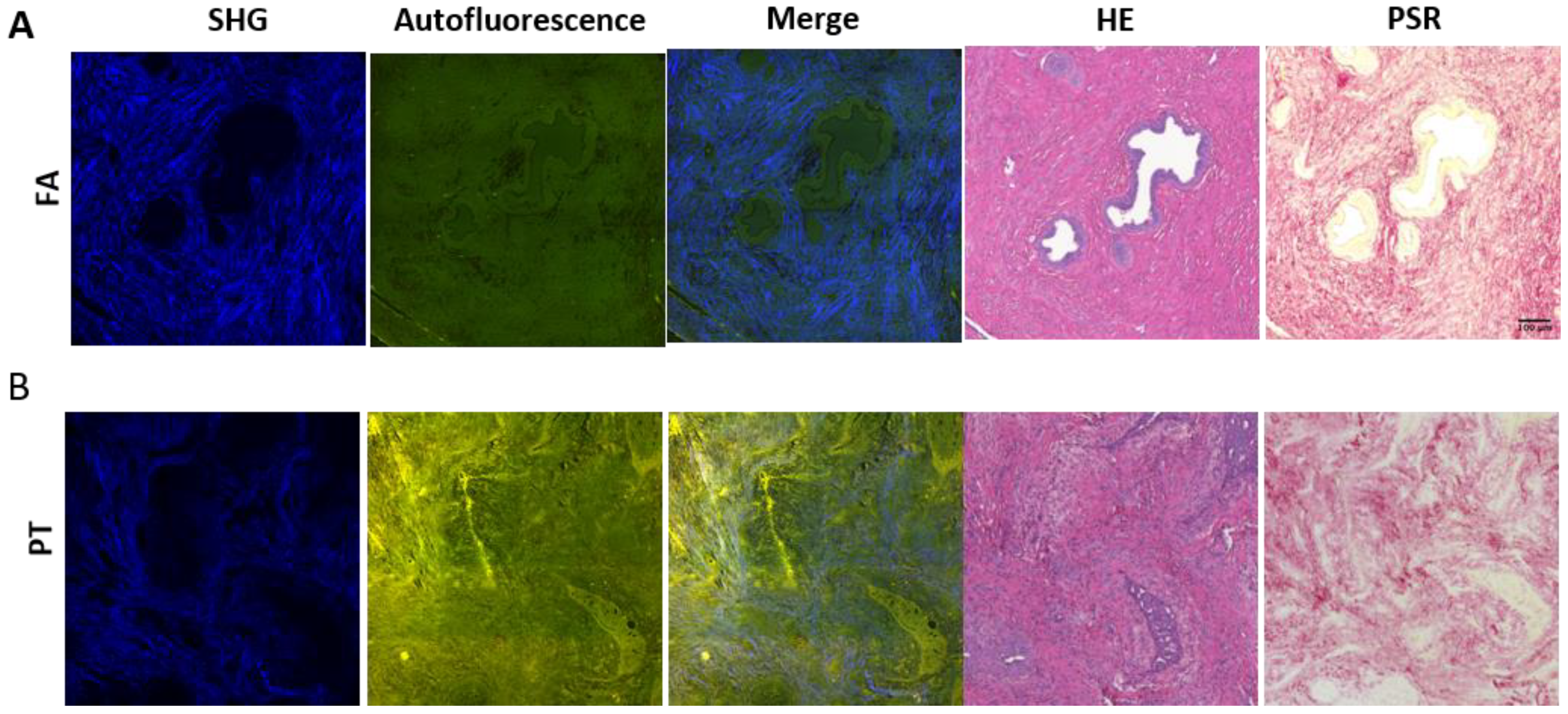
2.1. MPM Imaging Characterizes Morphological Distinctions between Epithelial and Stromal Regions for FA and PT Lesions
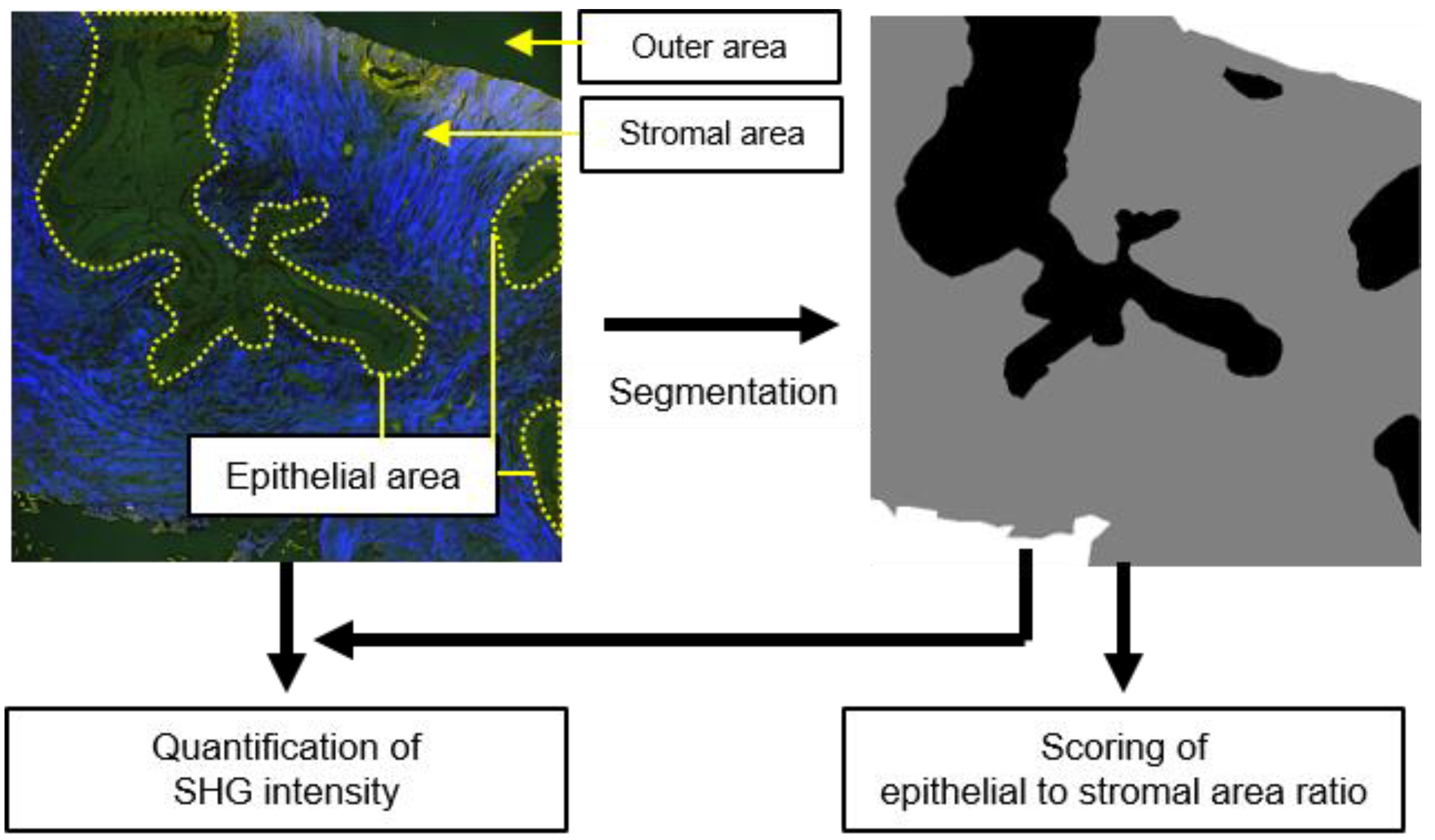
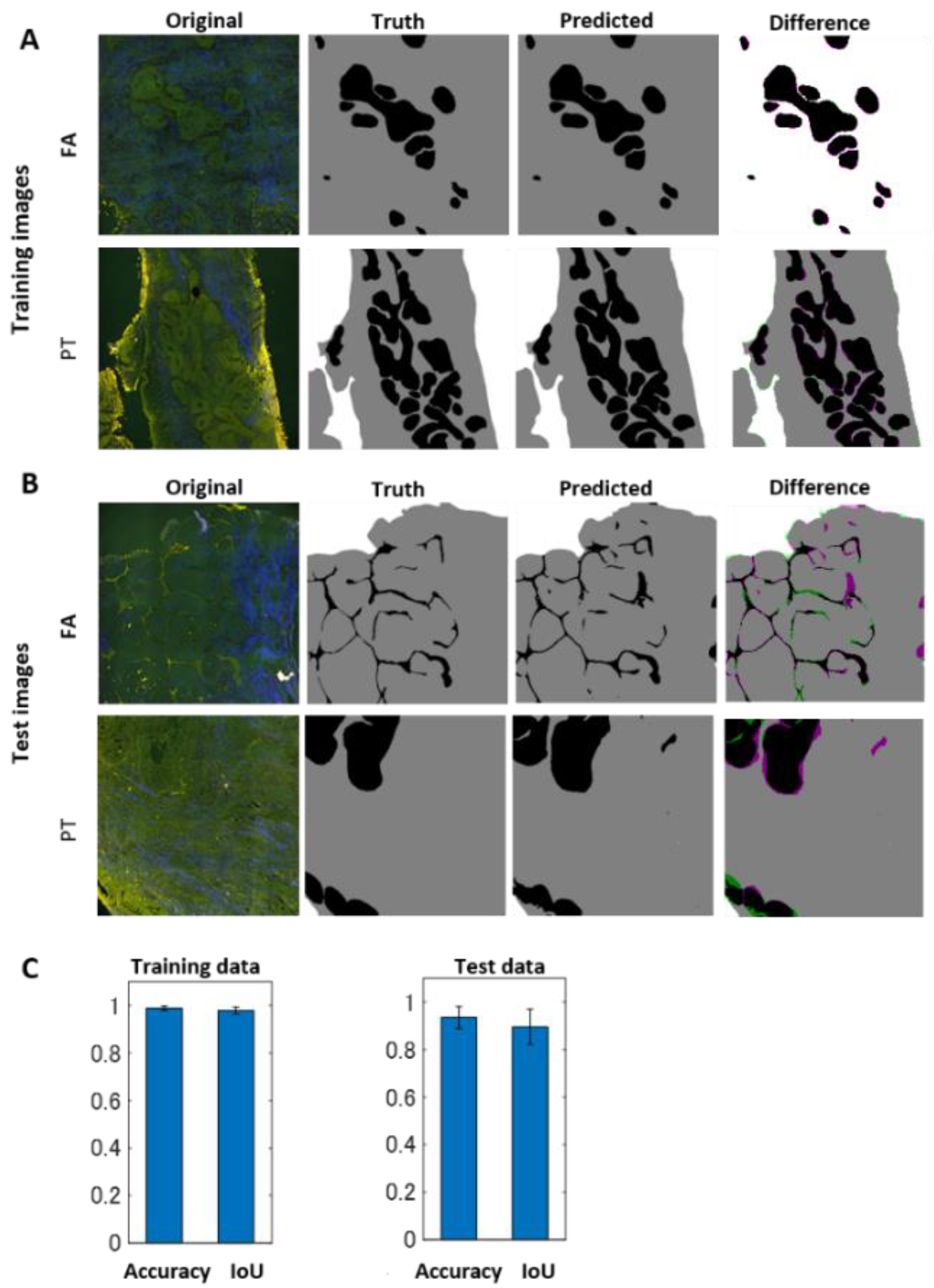
2.2. Deep-Learning-Based Image Segmentation Approach for Differentiating Epithelial and Stromal Morphologies
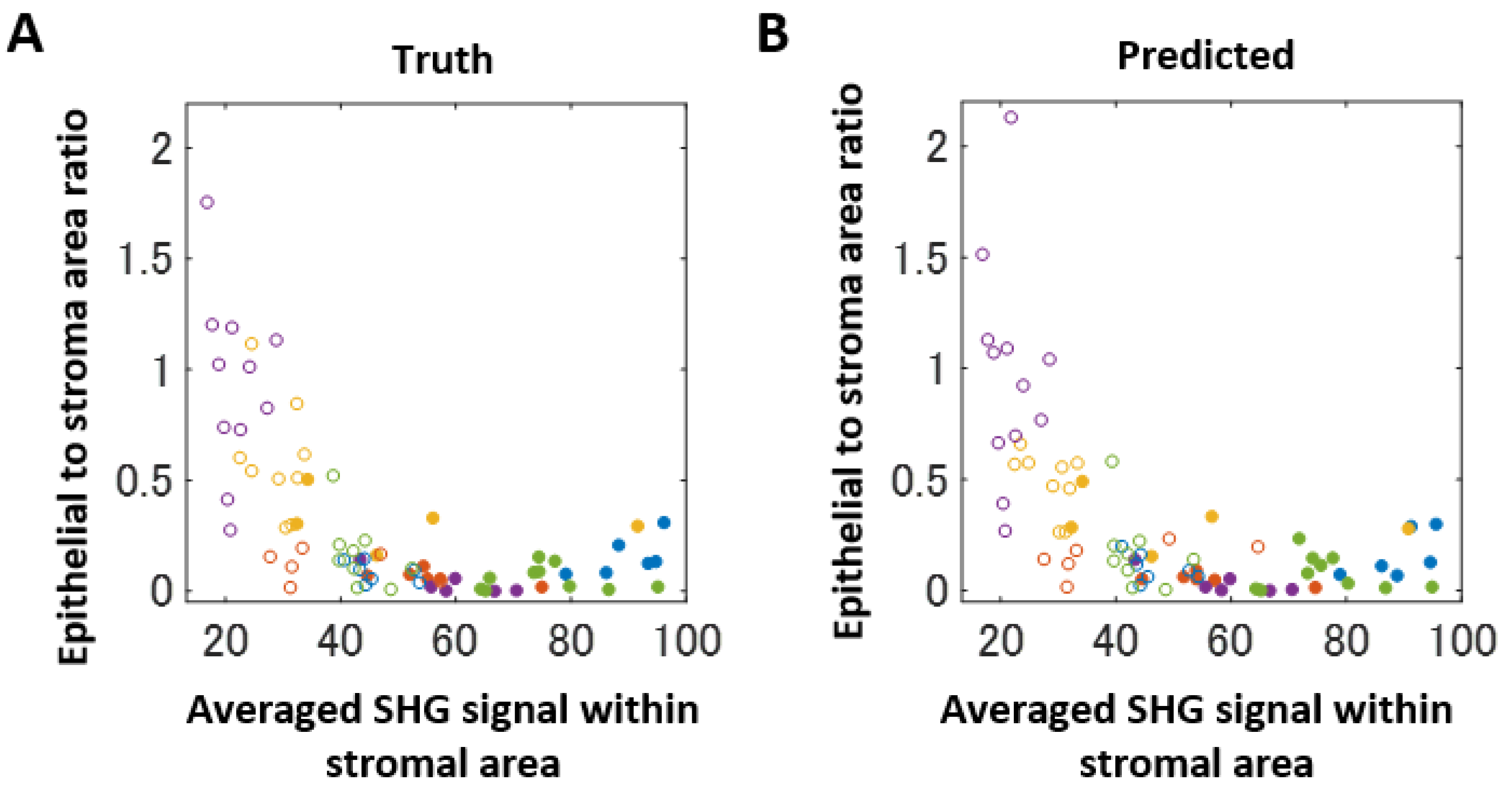
2.3. Computer-Assisted Scoring Helps to Diagnose FA and PT Lesions
3. Discussion
4. Materials and Methods
4.1. Patient-Derived Samples
4.2. Preparation of Tissue Sections
4.3. Image Acquisition by Multi-Photon Microscopy
4.4. Image Segmentation by SegNet
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
List of Abbreviations
| FA | Fibroadenoma |
| PT | Phyllodes tumor |
| CNB | Core needle biopsy |
| VAB | Vacuum-assisted biopsy |
| HE | Hematoxylin–eosin |
| PSR | Picro-sirius red |
| MPM | Multi-photon microscopy |
| DM | Dichroic mirror |
| FoV | Field of view |
| AF | Autofluorescence |
| SHG | Second harmonic generation |
| NADH | Nicotinamide adenine dinucleotide |
| FAD | Flavin adenine dinucleotide |
| AI | Artificial intelligence |
| IoU | Intersection of union |
References
- Fiks, A. Cystosarcoma phyllodes of the mammary gland—Müller’s tumor. For the 180th birthday of Johannes Müller. Virchows Arch. A Pathol. Anat. Histol. 1981, 392, 1–6. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Breast Tumours. WHO Classification of Breast Tumours: WHO Classification of Tumours; WHO Classification of Breast Tumours: Lyon, France, 2019; Volume 2, pp. 163–172. [Google Scholar]
- Zhou, Z.R.; Wang, C.C.; Yang, Z.Z.; Yu, X.L.; Guo, X.M. Phyllodes tumors of the breast: Diagnosis, treatment and prognostic factors related to recurrence. J. Thorac. Dis. 2016, 8, 3361–3368. [Google Scholar] [CrossRef] [Green Version]
- Ward, S.T.; Jewkes, A.J.; Jones, B.G.; Chaudhri, S.; Hejmadi, R.K.; Ismail, T.; Hallissey, M.T. The sensitivity of needle core biopsy in combination with other investigations for the diagnosis of phyllodes tumours of the breast. Int. J. Surg. 2012, 10, 527–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessauvagie, B.F.; Lee, A.H.S.; Meehan, K.; Nijhawan, A.; Tan, P.H.; Thomas, J.; Tie, B.; Treanor, D.; Umar, S.; Handy, A.M. Interobserver variation in the diagnosis of fibroepithelial lesions of the breast: A multicentre audit by digital pathology. J. Clin. Pathol. 2018, 71, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Marcil, G.; Wong, S.; Trabulsi, N.; Allard-Coutu, A.; Parsyan, A.; Omeroglu, A.; Atinel, G.; Mesurolle, B.; Meterissian, S. Fibroepithelial breast lesions diagnosed by core needle biopsy demonstrate a moderate rate of upstaging to phyllodes tumors. Am. J. Surg. 2017, 214, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Jara-Lazaro, A.R.; Akhilesh, M.; Thike, A.A.; Lui, P.C.-W.; Tse, G.M.-K.; Tan, P.H. Predictors of phyllodes tumours on core biopsy specimens of fibroepithelial neoplasms. Histopathology 2010, 57, 220–232. [Google Scholar] [CrossRef]
- Jacobs, T.W.; Chen, Y.-Y.; Guinee, D.G., Jr.; Holden, J.A.; Cha, I.; Bauermeister, D.E.; Hashimoto, B.; Wolverton, D.; Hartzog, G. Fibroepithelial lesions with cellular stroma on breast core needle biopsy: Are there predictors of outcome on surgical excision? Am. J. Clin. Pathol. 2005, 124, 342–354. [Google Scholar] [CrossRef]
- Yasir, S.; Gamez, R.; Jenkins, S.; Visscher, D.W.; Nassar, A. Significant histologic features differentiating cellular fibroadenoma from phyllodes tumor on core needle biopsy specimens. Am. J. Clin. Pathol. 2014, 142, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Gould, D.J.; Salmans, J.A.; Lassinger, B.K.; Contreras, A.; Gutierrez, C.; Bonefas, E.; Liscum, K.R.; Silberfein, E.J. Factors associated with phyllodes tumor of the breast after core needle biopsy identifies fibroepithelial neoplasm. J. Surg. Res. 2012, 178, 299–303. [Google Scholar] [CrossRef]
- Wang, Y.; Resnick, M.B.; Lu, S.; Hui, Y.; Brodsky, A.S.; Yang, D.; Yakirevich, E.; Wang, L. Collagen type III alpha1 as a useful diagnostic immunohistochemical marker for fibroepithelial lesions of the breast. Hum. Pathol. 2016, 57, 176–181. [Google Scholar] [CrossRef]
- Perry, S.W.; Burke, R.M.; Brown, E.B. Two-photon and second harmonic microscopy in clinical and translational cancer research. Ann. Biomed. Eng. 2012, 40, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Mizuno, H.; Sudo, T.; Kikuta, J.; Haraguchi, N.; Ikeda, J.I.; Mizushima, T.; Yamamoto, H.; Morii, E.; Mori, M.; et al. Non-labeling multiphoton excitation microscopy as a novel diagnostic tool for discriminating normal tissue and colorectal cancer lesions. Sci. Rep. 2017, 7, 6959. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Oshima, Y.; Saitou, T.; Watanabe, T.; Miyake, T.; Yoshida, O.; Tokumoto, Y.; Abe, M.; Matsuura, B.; Hiasa, Y.; et al. Quantitative imaging of fibrotic and morphological changes in liver of non-alcoholic steatohepatitis (NASH) model mice by second harmonic generation (SHG) and auto-fluorescence (AF) imaging using two-photon excitation microscopy (TPEM). Biochem. Biophys. Rep. 2016, 8, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, T.; Takanezawa, S.; Ninomiya, H.; Watanabe, T.; Yamamoto, S.; Hiasa, Y.; Imamura, T. Tissue Intrinsic Fluorescence Spectra-Based Digital Pathology of Liver Fibrosis by Marker-Controlled Segmentation. Front. Med. 2018, 5, 350. [Google Scholar] [CrossRef] [PubMed]
- Kiyomatsu, H.; Oshima, Y.; Saitou, T.; Miyazaki, T.; Hikita, A.; Miura, H.; Iimura, T.; Imamura, T. Quantitative SHG imaging in osteoarthritis model mice, implying a diagnostic application. Biomed. Opt. Express 2015, 6, 405–420. [Google Scholar] [CrossRef] [Green Version]
- Saitou, T.; Kiyomatsu, H.; Imamura, T. Quantitative Morphometry for Osteochondral Tissues Using Second Harmonic Generation Microscopy and Image Texture Information. Sci. Rep. 2018, 8, 2826. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.L.; Hu, P.S.; Ghazaryan, A.; Chen, S.J.; Tsai, T.H.; Dong, C.Y. Quantitative analysis of multiphoton excitation autofluorescence and second harmonic generation imaging for medical diagnosis. Comput. Med. Imaging Graph. 2012, 36, 519–526. [Google Scholar] [CrossRef]
- Wang, B.G.; Konig, K.; Halbhuber, K.J. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J. Microsc. 2010, 238, 1–20. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef] [Green Version]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Williams, J.; Botvinick, E.L.; Potma, E.O.; Tromberg, B.J. Visualization of Breast Cancer Metabolism Using Multimodal Nonlinear Optical Microscopy of Cellular Lipids and Redox State. Cancer Res. 2018, 78, 2503–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Huang, Y.; Tang, Q.; Li, Z.; Horng, H.; Li, J.; Wu, Z.; Chen, Y.; Li, H. Quantitative evaluation of redox ratio and collagen characteristics during breast cancer chemotherapy using two-photon intrinsic imaging. Biomed. Opt. Express 2018, 9, 1375–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnola, P.J.; Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef]
- Burke, K.; Tang, P.; Brown, E. Second harmonic generation reveals matrix alterations during breast tumor progression. J. Biomed. Opt. 2013, 18, 31106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, A.J.; Cook, R.S.; Lee, J.H.; Arteaga, C.L.; Skala, M.C. Collagen density and alignment in responsive and resistant trastuzumab-treated breast cancer xenografts. J. Biomed. Opt. 2015, 20, 26004. [Google Scholar] [CrossRef] [Green Version]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Fried, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Kakkad, S.M.; Solaiyappan, M.; Argani, P.; Sukumar, S.; Jacobs, L.K.; Leibfritz, D.; Bhujwalla, Z.; Glunde, K. Collagen I fiber density increases in lymph node positive breast cancers: Pilot study. J. Biomed. Opt. 2012, 17, 116017. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.T.; Wu, Y.; Fu, F.M.; Lian, Y.E.; Zhuo, S.M.; Wang, C.; Chein, J.X. Differentiating the two main histologic categories of fibroadenoma tissue from normal breast tissue by using multiphoton microscopy. J. Microsc. 2015, 258, 79–85. [Google Scholar] [CrossRef]
- Tan, W.J.; Yan, J.; Xu, S.; Thike, A.A.; Bay, B.H.; Yu, H.; Tan, M.-H.; Tan, P.H. Second harmonic generation microscopy is a novel technique for differential diagnosis of breast fibroepithelial lesions. J. Clin. Pathol. 2015, 68, 1033–1035. [Google Scholar] [CrossRef] [Green Version]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef]

| Characteristic | Post-Operative Diagnosis | |
|---|---|---|
| Fibroadenoma | Phyllodes | |
| No. of patients | 5 | 5 |
| Age (median years) | 38 (IQR; 27–41) | 44 (IQR; 40–47) |
| Median size on Imaging (cm) | 3.0 (IQR; 3.0–3.1) | 2.9 (IQR; 1.4–3.5) |
| Number of biopsy (n) (min–max) | 3 (2–4) | 3 (3–6) |
| Type of biopsy (n) | ||
| Core needle biopsy (14 gauge) | 3 | 4 |
| Vacuum-assisted breast biopsy (10 gauge) | 2 | 1 |
| Pre-operative diagnosis (n) | ||
| Fibroadenoma | 3 | 2 |
| Phyllodes | 0 | 3 |
| Difficult to distinguish | 2 | 0 |
| Histological type (n) | ||
| Benign | 5 | |
| Borderline/malignant | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi-Taguchi, K.; Saitou, T.; Kamei, Y.; Murakami, A.; Nishiyama, K.; Aoki, R.; Kusakabe, E.; Noda, H.; Yamashita, M.; Kitazawa, R.; et al. Computer-Aided Detection of Quantitative Signatures for Breast Fibroepithelial Tumors Using Label-Free Multi-Photon Imaging. Molecules 2022, 27, 3340. https://doi.org/10.3390/molecules27103340
Kobayashi-Taguchi K, Saitou T, Kamei Y, Murakami A, Nishiyama K, Aoki R, Kusakabe E, Noda H, Yamashita M, Kitazawa R, et al. Computer-Aided Detection of Quantitative Signatures for Breast Fibroepithelial Tumors Using Label-Free Multi-Photon Imaging. Molecules. 2022; 27(10):3340. https://doi.org/10.3390/molecules27103340
Chicago/Turabian StyleKobayashi-Taguchi, Kana, Takashi Saitou, Yoshiaki Kamei, Akari Murakami, Kanako Nishiyama, Reina Aoki, Erina Kusakabe, Haruna Noda, Michiko Yamashita, Riko Kitazawa, and et al. 2022. "Computer-Aided Detection of Quantitative Signatures for Breast Fibroepithelial Tumors Using Label-Free Multi-Photon Imaging" Molecules 27, no. 10: 3340. https://doi.org/10.3390/molecules27103340
APA StyleKobayashi-Taguchi, K., Saitou, T., Kamei, Y., Murakami, A., Nishiyama, K., Aoki, R., Kusakabe, E., Noda, H., Yamashita, M., Kitazawa, R., Imamura, T., & Takada, Y. (2022). Computer-Aided Detection of Quantitative Signatures for Breast Fibroepithelial Tumors Using Label-Free Multi-Photon Imaging. Molecules, 27(10), 3340. https://doi.org/10.3390/molecules27103340






