A New Compartmentalized Scale (PN) for Measuring Polarity Applied to Novel Ether-Functionalized Amino Acid Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Density, Surface Tension, and Refractive Index of [CnOC2mim][Ala](n = 1, 2)
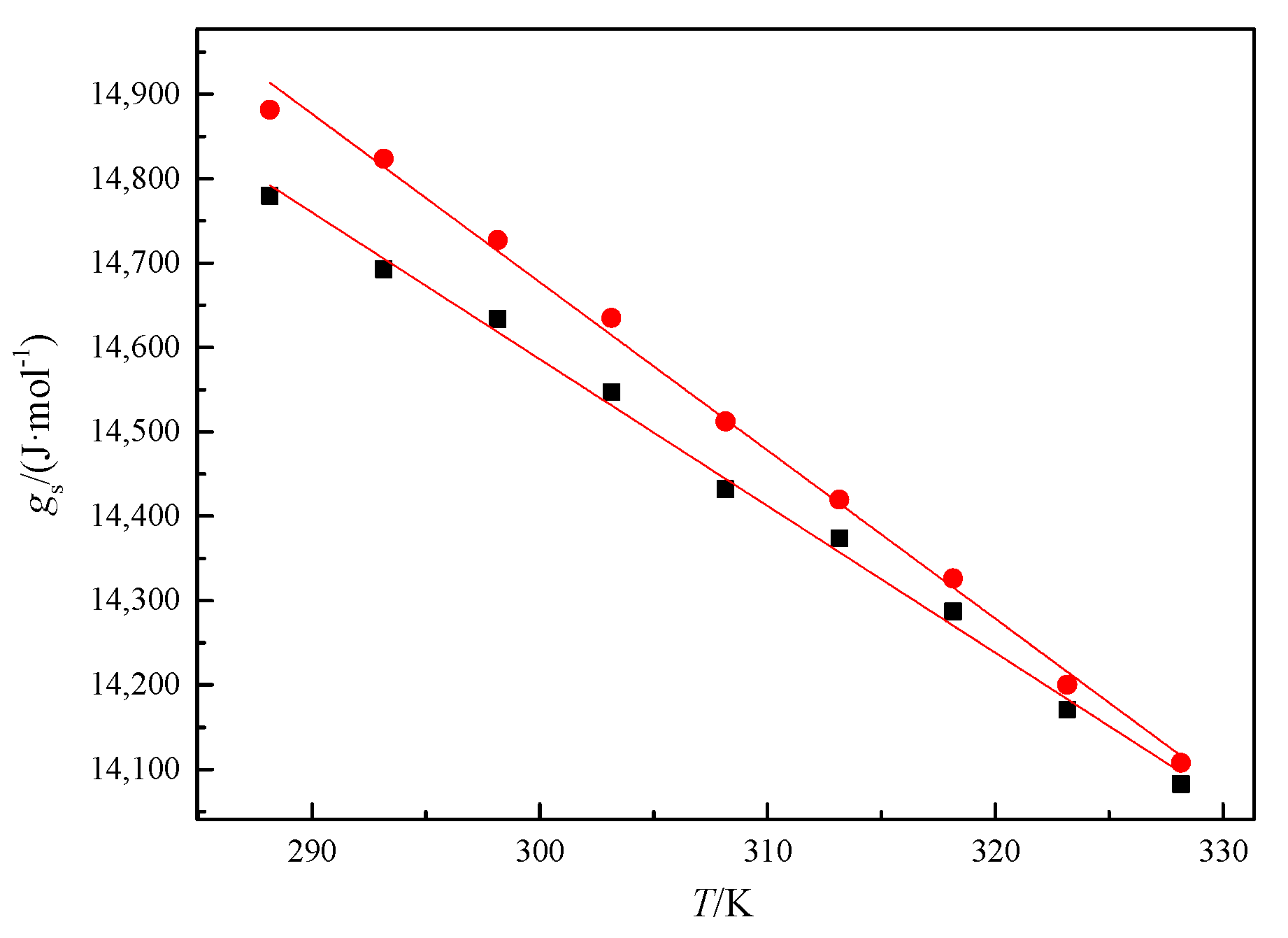
2.2. Strength of [CnOC2mim][Ala](n = 1, 2) Intermolecular Interactions
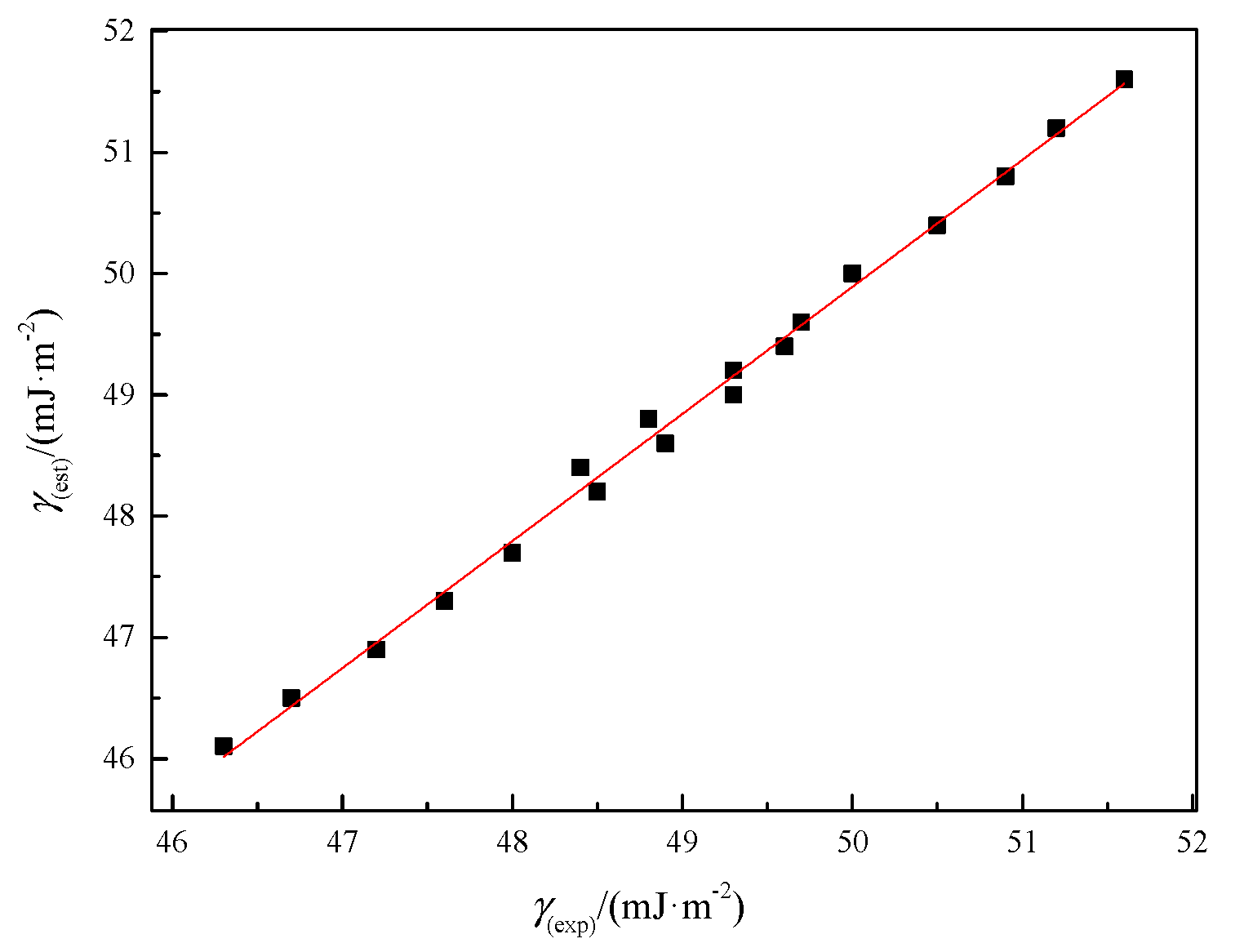
2.3. Prediction of Surface Tension Based on Molar Surface Gibbs Energy
2.4. Molar Surface Entropy: Polarity Contribution from Surface Liquid
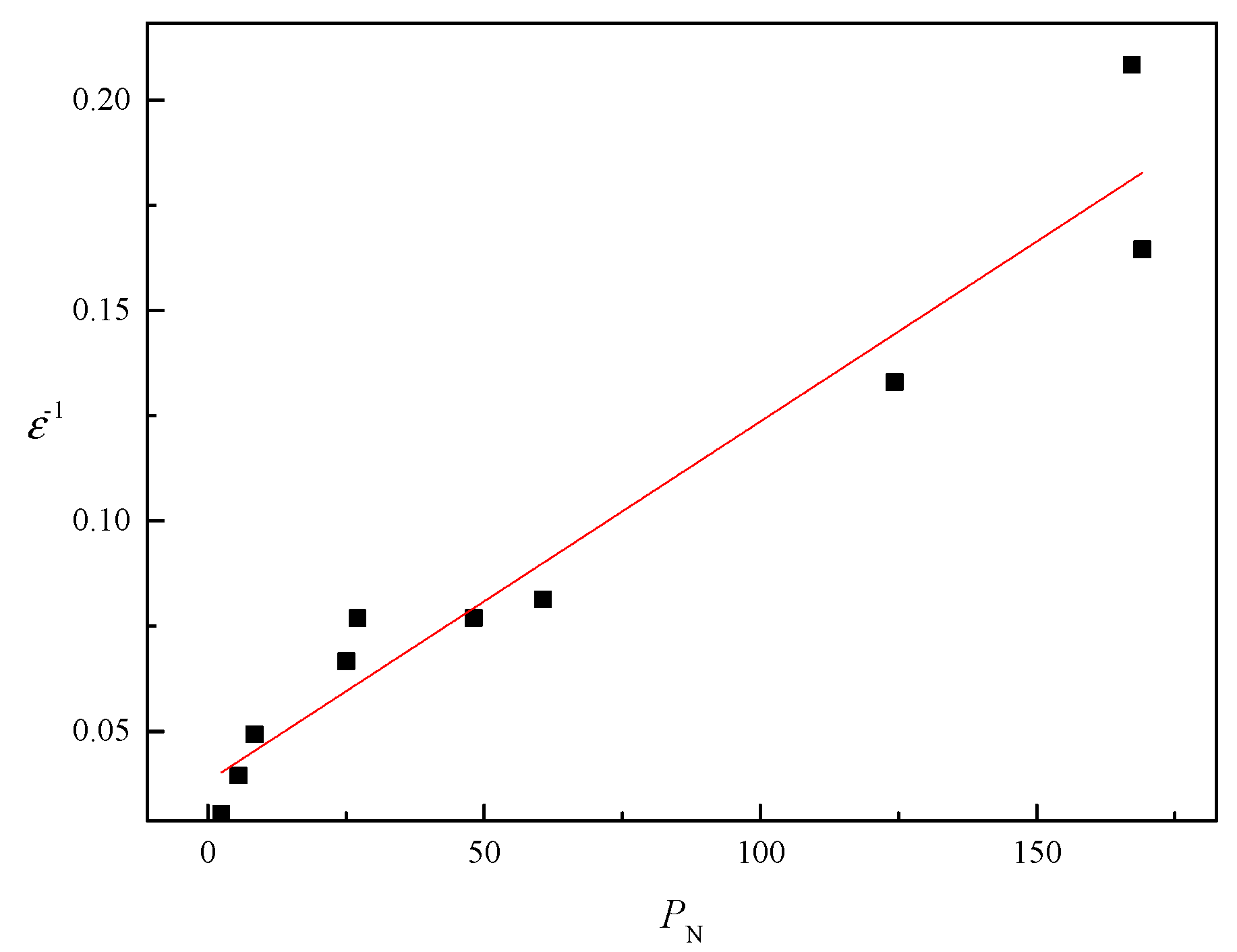
2.5. A New Model for Predicting Polarity
3. Materials and Methods
3.1. Materials
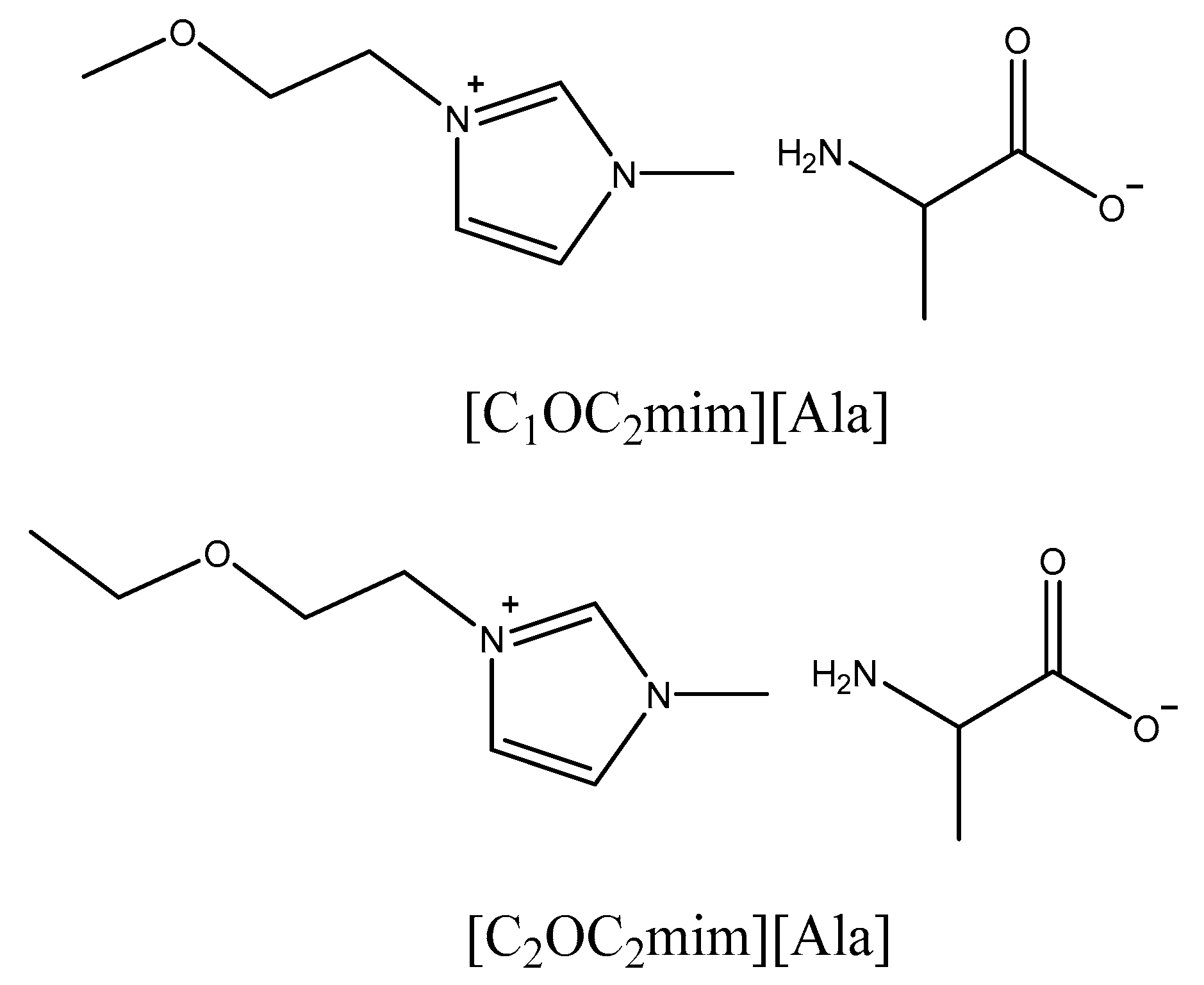
3.2. Preparation of ILs [CnOC2mim][Ala](n = 1, 2)
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Wei, G.-T.; Yang, Z.; Lee, C.-Y.; Yang, H.-Y.; Wang, C.C. Aqueous− organic phase transfer of gold nanoparticles and gold nanorods using an ionic liquid. J. Am. Chem. Soc. 2004, 126, 5036–5037. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-T.; Jeong, S.; Xue, M.-Z.; Balducci, A.; Winter, M.; Passerini, S.; Alessandrini, F.; Appetecchi, G. Development of ionic liquid-based lithium battery prototypes. J. Power Sources 2012, 199, 239–246. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Yao, H.; Zhou, Q.; Xin, J.; Lu, X.; Zhang, S. Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids. Green Chem. 2020, 22, 3122–3131. [Google Scholar] [CrossRef]
- Williams, H.D.; Sahbaz, Y.; Ford, L.; Nguyen, T.-H.; Scammells, P.J.; Porter, C.J. Ionic liquids provide unique opportunities for oral drug delivery: Structure optimization and in vivo evidence of utility. Chem. Commun. 2014, 50, 1688–1690. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nat. News 2015, 528, 188. [Google Scholar] [CrossRef] [Green Version]
- Rahim, A.H.A.; Yunus, N.M.; Hamzah, W.S.W.; Sarwono, A.; Muhammad, N. Low-Viscosity Ether-Functionalized Ionic Liquids as Solvents for the Enhancement of Lignocellulosic Biomass Dissolution. Processes 2021, 9, 261. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Jiang, N.; Li, X.; Pasupath, S.; Fang, Y.; Liu, Q.; Dang, D. Rational Design of an Ionic Liquid-Based Electrolyte with High Ionic Conductivity Towards Safe Lithium/Lithium-Ion Batteries. Chem.—Asian J. 2019, 14, 2810–2814. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, J.; Bai, L.; Wang, B.; Gao, H.; Shang, D.; Zhang, X.; Zhang, S. Highly selective capture of CO2 by ether-functionalized pyridinium ionic liquids with low viscosity. Energy Fuels 2015, 29, 6039–6048. [Google Scholar] [CrossRef]
- Trush, M.; Metelytsia, L.; Semenyuta, I.; Kalashnikova, L.; Papeykin, O.; Venger, I.; Tarasyuk, O.; Bodachivska, L.; Blagodatnyi, V.; Rogalsky, S. Reduced ecotoxicity and improved biodegradability of cationic biocides based on ester-functionalized pyridinium ionic liquids. Environ. Sci. Pollut. Res. 2019, 26, 4878–4889. [Google Scholar] [CrossRef]
- Qu, Y.; Lan, J.; Chen, Y.; Sun, J. Amino acid ionic liquids as efficient catalysts for CO2 capture and chemical conversion with epoxides under metal/halogen/cocatalyst/solvent-free conditions. Sustain. Energy Fuels 2021, 5, 2494–2503. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, C.; Zhang, C.; Zhao, S.; Cai, M.; Liu, Z.; Yu, Q. Amino acid ionic liquids as anticorrosive and lubricating additives for water and their environmental impact. Tribol. Int. 2021, 153, 106663. [Google Scholar] [CrossRef]
- Jiang, J.; Mu, X.; Qiao, J.; Su, Y.; Qi, L. New chiral ligand exchange capillary electrophoresis system with chiral amino amide ionic liquids as ligands. Talanta 2017, 175, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhang, Q.; Ren, D.; Nie, Z.; Liu, Q.; Yao, S. Functional amino acid ionic liquids as solvent and selector in chiral extraction. J. Chromatogr. A 2010, 1217, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Ghavre, M.; Byrne, O.; Altes, L.; Surolia, P.K.; Spulak, M.; Quilty, B.; Thampi, K.R.; Gathergood, N. Low toxicity functionalised imidazolium salts for task specific ionic liquid electrolytes in dye-sensitised solar cells: A step towards less hazardous energy production. Green Chem. 2014, 16, 2252–2265. [Google Scholar] [CrossRef]
- Kebaili, H.; Pérez de los Ríos, A.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Kameche, M.; Hernández-Fernández, J.; Hernández-Fernández, F.J. Evaluating the toxicity of ionic liquids on Shewanella sp. for designing sustainable bioprocesses. Front. Mater. 2020, 7, 387. [Google Scholar] [CrossRef]
- Jouyban, A.; Mirheydari, S.N.; Barzegar-Jalali, M.; Shekaari, H.; Acree, W.E. Comprehensive models for density prediction of ionic liquid+ molecular solvent mixtures at different temperatures. Phys. Chem. Liq. 2020, 58, 309–324. [Google Scholar] [CrossRef]
- Chen, Z.; Huo, Y.; Cao, J.; Xu, L.; Zhang, S. Physicochemical Properties of Ether-Functionalized Ionic Liquids: Understanding Their Irregular Variations with the Ether Chain Length. Ind. Eng. Chem. Res. 2016, 55, 11589–11596. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Atashrouz, S.; Amini, E.; Pazuki, G. Modeling of surface tension for ionic liquids using group method of data handling. Ionics 2015, 21, 1595–1603. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Rogers, R.D.; Mantz, R.A.; Trulove, P.C.; Cocalia, V.A.; Visser, A.E.; Anderson, J.L.; Anthony, J.L.; Brennecke, J.F.; Maginn, E.J.; et al. Physicochemical Properties. In Ionic Liquids in Synthesis; 2007; pp. 57–174. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527621194 (accessed on 22 April 2022).
- Nemec, T. Prediction of surface tension of binary mixtures with the parachor method. In EPJ Web of Conferences; EDP Sciences: Les Ulis, France, 2015; Volume 92, p. 02054. [Google Scholar]
- Paduszynski, K. Extensive Databases and Group Contribution QSPRs of Ionic Liquid Properties. 3: Surface Tension. Ind. Eng. Chem. Res. 2021, 60, 5705–5720. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kumar, A. Polarity issues in room temperature ionic liquids. Clean Technol. Environ. Policy 2015, 17, 1111–1116. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Swift, L.; Hallett, J.P.; Welton, T.; Coutinho, J.A.P.; Freire, M.G. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 6593–6601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurnia, K.A.; Lima, F.; Cláudio, A.F.M.; Coutinho, J.A.P.; Freire, M.G. Hydrogen-bond acidity of ionic liquids: An extended scale. Phys. Chem. Chem. Phys. 2015, 17, 18980–18990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, M.; Liu, R.J.; Yang, H.X.; Guan, W.; Tong, J.; Yang, J.Z. Determination of the vaporisation enthalpies and estimation of the polarity for 1-alkyl-3-methylimidazolium propionate {C(n)mim Pro (n = 2, 3)} ionic liquids. J. Chem. Thermodyn. 2014, 70, 214–218. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, Y.; Jiang, W.; Yu, K.; Tong, J.; Yang, J. The application of molar surface Gibbs energy to predicting ILs’ properties. J. Mol. Liq. 2019, 288, 111004. [Google Scholar] [CrossRef]
- Tong, J.; Hong, M.; Chen, Y.; Wang, H.; Guan, W.; Yang, J.-Z. The surface tension, density and refractive index of amino acid ionic liquids: C(3)mim Gly and C(4)mim Gly. J. Chem. Thermodyn. 2012, 54, 352–357. [Google Scholar] [CrossRef]
- Singh, D.; Gardas, R.L. Influence of Cation Size on the Ionicity, Fluidity, and Physiochemical Properties of 1,2,4-Triazolium Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 4834–4842. [Google Scholar] [CrossRef]
- Glasser, L. Lattice and phase transition thermodynamics of ionic liquids. Thermochim. Acta 2004, 421, 87–93. [Google Scholar] [CrossRef]
- Gusain, R.; Panda, S.; Bakshi, P.S.; Gardas, R.L.; Khatri, O.P. Thermophysical properties of trioctylalkylammonium bis(salicylato)borate ionic liquids: Effect of alkyl chain length. J. Mol. Liq. 2018, 269, 540–546. [Google Scholar] [CrossRef]
- Lambert, F.L.; Leff, H.S. The Correlation of Standard Entropy with Enthalpy Supplied from 0 to 298.15 K. J. Chem. Educ. 2009, 86, 94–98. [Google Scholar] [CrossRef]
- Tong, J.; Qu, Y.; Li, K.; Chen, T.-F.; Tong, J.; Yang, J.-Z. The molar surface Gibbs energy of the aqueous solution of the ionic liquid [C6mim][OAc]. J. Chem. Thermodyn. 2016, 97, 362–366. [Google Scholar] [CrossRef]
- Myers, R.T. True molar surface energy and alignment of surface molecules. J. Colloid Interface Sci. 2004, 274, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ersfeld, B.; Felderhof, B.U. Retardation correction to the Lorentz-Lorenz formula for the refractive index of a disordered system of polarizable point dipoles. Phys. Rev. E 1998, 57, 1118–1126. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, W.; Liu, L.; Yu, K.; Hong, M.; Tong, J. The molar surface Gibbs energy and polarity of ether-functionalized ionic liquids. J. Chem. Thermodyn. 2019, 138, 313–320. [Google Scholar] [CrossRef]
- Hong, M.; Sun, A.; Liu, C.; Guan, W.; Tong, J.; Yang, J.-Z. Physico-Chemical Properties of 1-Alkyl-3-methylimidazolium Propionate Ionic Liquids {[Cnmim][Pro](n = 3, 4, 5, 6)} from 288.15 K to 328.15 K. Ind. Eng. Chem. Res. 2013, 52, 15679–15683. [Google Scholar] [CrossRef]
- Seoane, R.G.; Corderí, S.; Gómez, E.; Calvar, N.; González, E.J.; Macedo, E.A.; Domínguez, Á. Temperature Dependence and Structural Influence on the Thermophysical Properties of Eleven Commercial Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 2492–2504. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, W.; Cheng, Y.; Dong, J.; Zhu, M.; Zhang, B.; Dubois, A.; Zhu, M.; Jie, W.; Xu, Y. Anisotropic dielectric behavior of layered perovskite-like Cs3Bi2I9 crystals in the terahertz region. Phys. Chem. Chem. Phys. 2020, 22, 24555–24560. [Google Scholar] [CrossRef]
- Weiß, N.; Schmidt, C.H.; Thielemann, G.; Heid, E.; Schröder, C.; Spange, S. The physical significance of the Kamlet–Taft π* parameter of ionic liquids. Phys. Chem. Chem. Phys. 2021, 23, 1616–1626. [Google Scholar] [CrossRef]
- Wei, J.; Fan, B.-H.; Pan, Y.; Xing, N.-N.; Men, S.-Q.; Tong, J.; Guan, W. Vaporization enthalpy and the molar surface Gibbs free energy for ionic liquids C(n)Dmim NTF2 (n = 2, 4). J. Chem. Thermodyn. 2016, 101, 278–284. [Google Scholar] [CrossRef]
- Peter Atkins, P.; De Paula, J. Atkins’ Physical Chemistry; OUP Oxford: Oxford, UK, 2014. [Google Scholar]
- Zhang, D.; Li, B.; Hong, M.; Kong, Y.-X.; Tong, J.; Xu, W.-G. Synthesis and characterization of physicochemical properties of new ether-functionalized amino acid ionic liquids. J. Mol. Liq. 2020, 304, 112718. [Google Scholar] [CrossRef]
- Zhao, Y.; Lv, J.; Liu, H.; Wu, J.; Tong, J. Study on the polarity and molar surface Gibbs energy of ether-based amino acid ionic liquids [COC4mim][Gly], [COC4mim][Ala] and [COC4mim][Thr]. J. Mol. Liq. 2021, 336, 116094. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Nonelectrolytes; Reinhold Publishing Corporation: New York, NY, USA; London Chapman and Hall: London, UK, 1950. [Google Scholar]
- Lawson, D.D.; Ingham, J. Estimation of solubility parameters from refractive index data. Nature 1969, 223, 614. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R.; Shara, M. Predicting physical properties of ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 642–649. [Google Scholar] [CrossRef]
- Masaki, T.; Nishikawa, K.; Shirota, H. Microscopic Study of Ionic Liquid− H2O Systems: Alkyl-Group Dependence of 1-Alkyl-3-Methylimidazolium Cation. J. Phys. Chem. B 2010, 114, 6323–6331. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Li, Z.; Wang, R.; Hou, A.; Yang, F. Volumetric properties of binary mixtures of ionic liquid with tributyl phosphate and dimethyl carbonate. J. Chem. Thermodyn. 2018, 123, 165–173. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Yermalayeu, A.V.; Schick, C.; Liu, H.; Maginn, E.J.; Bulut, S.; Krossing, I.; Kalb, R. Making sense of enthalpy of vaporization trends for ionic liquids: New experimental and simulation data show a simple linear relationship and help reconcile previous data. J. Phys. Chem. B 2013, 117, 6473–6486. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Zhao, D.; Zhuang, Y.; Wang, R.; Yang, F.; Liu, X.; Chen, Y. Surface tension of binary mixtures of (ionic liquid+ tributyl phosphate). J. Chem. Thermodyn. 2019, 132, 214–221. [Google Scholar] [CrossRef]
- Luo, H.; Baker, G.A.; Dai, S. Isothermogravimetric determination of the enthalpies of vaporization of 1-alkyl-3-methylimidazolium ionic liquids. J. Phys. Chem. B 2008, 112, 10077–10081. [Google Scholar] [CrossRef]
- Xu, W.-G.; Li, L.; Ma, X.-X.; Wei, J.; Duan, W.-B.; Guan, W.; Yang, J.-Z. Density, surface tension, and refractive index of ionic liquids homologue of 1-alkyl-3-methylimidazolium tetrafluoroborate [C n mim][BF4](n = 2, 3, 4, 5, 6). J. Chem. Eng. Data 2012, 57, 2177–2184. [Google Scholar] [CrossRef]
- Tao, G.-H.; Zou, M.; Wang, X.-H.; Chen, Z.-Y.; Evans, D.; Kou, Y. Comparison of Polarities of Room-Temperature Ionic Liquids Using FT-IR Spectroscopic Probes. Aust. J. Chem.—AUST J CHEM 2005, 58, 327–331. [Google Scholar] [CrossRef]
- Park, S.; Kazlauskas, R.J. Improved Preparation and Use of Room-Temperature Ionic Liquids in Lipase-Catalyzed Enantio- and Regioselective Acylations. J. Org. Chem. 2001, 66, 8395–8401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sasaki, T.; Kazushi, K.; Seo, T.; Sakurai, K. Interactions between Spiropyrans and Room-Temperature Ionic Liquids: Photochromism and Solvatochromism. J. Phys. Chem. B 2008, 112, 7530–7536. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Potangale, M.; Tiwari, S. Correlation of the empirical polarity parameters of solvate ionic liquids (SILs) with molecular structure. J. Mol. Liq. 2020, 297, 111882. [Google Scholar] [CrossRef]
- Spange, S.; Lienert, C.; Friebe, N.; Schreiter, K. Complementary interpretation of ET (30) polarity parameters of ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 9954–9966. [Google Scholar] [CrossRef]
- Tong, J.; Zheng, X.; Li, H.; Tong, J.; Liu, Q. Densities and Viscosities of Aqueous Amino Acid Ionic Liquids [Cnmim][Ala](n = 3, 4, 5). J. Chem. Eng. Data 2017, 62, 2501–2508. [Google Scholar] [CrossRef]
- Liu, Q.; Janssen, M.H.; van Rantwijk, F.; Sheldon, R.A. Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem. 2005, 7, 39–42. [Google Scholar] [CrossRef]
- Ma, X.-X.; Wei, J.; Zhang, Q.-B.; Tian, F.; Feng, Y.-Y.; Guan, W. Prediction of Thermophysical Properties of Acetate-Based Ionic Liquids Using Semiempirical Methods. Ind. Eng. Chem. Res. 2013, 52, 9490–9496. [Google Scholar] [CrossRef]
- Wei, J.; Bu, X.; Guan, W.; Xing, N.; Fang, D.; Wu, Y. Measurement of vaporization enthalpy by isothermogravimetrical method and prediction of the polarity for 1-alkyl-3-methylimidazolium acetate {[C n mim][OAc](n= 4, 6)} ionic liquids. RSC Adv. 2015, 5, 70333–70338. [Google Scholar] [CrossRef]
- Tong, J.; Yang, H.X.; Liu, R.J.; Li, C.; Xia, L.X.; Yang, J.Z. Determination of the Enthalpy of Vaporization and Prediction of Surface Tension for Ionic Liquid 1-Alkyl-3-methylimidazolium Propionate C(n)mim Pro (n=4, 5, 6). J. Phys. Chem. B 2014, 118, 12972–12978. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Hong, M.; Liu, C.; Sun, A.; Guan, W.; Yang, J.-Z. Estimation of Properties of Ionic Liquids 1-Alkyl-3-methylimidazolium Lactate Using a Semiempirical Method. Ind. Eng. Chem. Res. 2013, 52, 4967–4972. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Gu, C.; Pan, Y.; Xing, N.-N.; Tong, J.; Guan, W. Determination of vaporization enthalpy for ionic liquids C(n)mim Lact (n=2, 3, 5) and applications of the molar surface Gibbs free energy. J. Therm. Anal. Calorim. 2016, 125, 547–556. [Google Scholar] [CrossRef]
- Wei, J.; Ma, T.; Ma, X.; Guan, W.; Liu, Q.; Yang, J. Study on thermodynamic properties and estimation of polarity of ionic liquids { C(n)mmim NTf2 (n=2, 4)}. Rsc Adv. 2014, 4, 30725–30732. [Google Scholar] [CrossRef]
- Verevkin, S.P. Predicting enthalpy of vaporization of ionic liquids: A simple rule for a complex property. Angew. Chem.-Int. Ed. 2008, 47, 5071–5074. [Google Scholar] [CrossRef]
- Hong, M.; Sun, A.; Yang, Q.; Guan, W.; Tong, J.; Yang, J.-Z. Studies on properties of ionic liquids 1-alkyl-3-methylimidazolium lactate at temperatures from (288.15 to 333.15)K. J. Chem. Thermodyn. 2013, 67, 91–98. [Google Scholar] [CrossRef]
- Fang, D.-W.; Tong, J.; Guan, W.; Wang, H.; Yang, J.-Z. Predicting Properties of Amino Acid Ionic Liquid Homologue of 1-Alkyl-3-methylimidazolium Glycine. J. Phys. Chem. B 2010, 114, 13808–13814. [Google Scholar] [CrossRef]
- Wei, J.; Chang, C.; Zhang, Y.; Hou, S.; Fang, D.; Guan, W. Prediction of thermophysical properties of novel ionic liquids based on serine C(n)mim Ser (n=3,4) using semiempirical methods. J. Chem. Thermodyn. 2015, 90, 310–316. [Google Scholar] [CrossRef]
- Clara, R.A.; Marigliano, A.C.G.; Solimo, H.N. Density, viscosity, isothermal (vapour plus liquid) equilibrium, excess molar volume, viscosity deviation, and their correlations for chloroform plus methyl isobutyl ketone binary system. J. Chem. Thermodyn. 2007, 39, 261–267. [Google Scholar] [CrossRef]
- Dragoescu, D. Refractive indices and their related properties for several binary mixtures containing cyclic ketones and chloroalkanes. J. Mol. Liq. 2015, 209, 713–722. [Google Scholar] [CrossRef]
- Fenclová, D.; Vrbka, P.; Dohnal, V.; Řehák, K.; García-Miaja, G. (Vapour + liquid) equilibria and excess molar enthalpies for mixtures with strong complex formation. Trichloromethane or 1-bromo-1-chloro-2,2,2-trifluoroethane (halothane) with tetrahydropyran or piperidine. J. Chem. Thermodyn. 2002, 34, 361–376. [Google Scholar] [CrossRef]
- Helm, R.V.; Lanum, W.J.; Cook, G.L.; Ball, J.S. Purification and Properties of Pyrrole, Pyrrolidine, Pyridine and 2-Methylpyridine. J. Phys. Chem. 1958, 62, 858–862. [Google Scholar] [CrossRef]
- Lagemann, R.T.; McMillan, D.R., Jr.; Woolf, W.E. Temperature Variation of Ultrasonic Velocity in Liquids. J. Chem. Phys. 1949, 17, 369–373. [Google Scholar] [CrossRef]
- Colnay, M.E.; Vasseur, A.; Guerin, M. The influence of non-polar solvents on molecular dielectric polarization. 1: An attempt to select by experiment appropriate expressions. J. Chem. Res 1983, 9, 220–221. [Google Scholar]
- Gill, D.S.; Singh, P.; Singh, J.; Singh, P.; Senanayake, G.; Hefter, G.T. Ultrasonic velocity, conductivity, viscosity and calorimetric studies of copper(i) and sodium perchlorates in cyanobenzene, pyridine and cyanomethane. J. Chem. Soc.-Faraday Trans. 1995, 91, 2789–2795. [Google Scholar] [CrossRef]
- Sharma, B.R.; Singh, P.P. Excess Gibbs energies of mixing for some binary mixtures. J. Chem. Eng. Data 1975, 20, 360–363. [Google Scholar] [CrossRef]
- Kyte, C.; Jeffery, G.; Vogel, A. 864. Physical properties and chemical constitution. Part XXVIII. Pyridine derivatives. J. Chem. Soc. (Resumed) 1960, 4454–4472. [Google Scholar] [CrossRef]
- Ortega, J. Densities and refractive-indexes of pure alcohols as a function of temperature. J. Chem. Eng. Data 1982, 27, 312–317. [Google Scholar] [CrossRef]
- Vijande, J.; Piñeiro, M.M.; García, J.; Valencia, A.J.L.; Legido, J.L. Density and Surface Tension Variation with Temperature for Heptane + 1-Alkanol. J. Chem. Eng. Data 2006, 51, 1778–1782. [Google Scholar] [CrossRef]
- Das, K.N.; Habibullah, M.; Rahman, I.M.M.; Hasegawa, H.; Uddin, M.A.; Saifuddin, K. Thermodynamic Properties of the Binary Mixture of Hexan-1-ol with m-Xylene at T = (303.15, 313.15, and 323.15) K. J. Chem. Eng. Data 2009, 54, 3300–3302. [Google Scholar] [CrossRef]
- Neyband, R.S.; Yousefi, A.; Zarei, H. Experimental and Computational Thermodynamic Properties of (Benzyl Alcohol plus Alkanols) Mixtures. J. Chem. Eng. Data 2015, 60, 2291–2300. [Google Scholar] [CrossRef]
- Venkatramana, L.; Sivakumar, K.; Gardas, R.; Reddy, K.D. Effect of chain length of alcohol on thermodynamic properties of their binary mixtures with benzylalcohol. Thermochim. Acta 2014, 581, 123–132. [Google Scholar] [CrossRef]
- Weissler, A. Ultrasonic Investigation of Molecular Properties of Liquids. II. 1 The Alcohols1a. J. Am. Chem. Soc. 1948, 70, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Yeh, C.-T.; Tu, C.-H. Densities, Viscosities, Refractive Indices, and Surface Tensions for the Ternary Mixtures of 2-Propanol + Benzyl Alcohol + 2-Phenylethanol at T = 308.15 K. J. Chem. Eng. Data 2008, 53, 1203–1207. [Google Scholar] [CrossRef]
- Hiers, G.S.; Adams, R. Omega-cyclohexyl derivatives of various normal aliphatic acids. IV. J. Am. Chem. Soc. 1926, 48, 2385–2393. [Google Scholar] [CrossRef]
- Hovorka, S.; Roux, A.H.; Roux-Desgranges, G.; Dohnal, V. Limiting partial molar excess heat capacities and volumes of selected organic compounds in water at 25 degrees C. J. Solut. Chem. 1999, 28, 1289–1305. [Google Scholar] [CrossRef]
- Shinomiya, T. Dielectric Relaxation and Intermolecular Association of Alicyclic Alcohols in Liquid and Solid States. Bull. Chem. Soc. Jpn. 1990, 63, 1087–1092. [Google Scholar] [CrossRef]
- Lasich, M.; Moodley, T.; Bhownath, R.; Naidoo, P.; Ramjugernath, D. Liquid–Liquid Equilibria of Methanol, Ethanol, and Propan-2-ol with Water and Dodecane. J. Chem. Eng. Data 2011, 56, 4139–4146. [Google Scholar] [CrossRef]
- Zarei, H.A.; Shahvarpour, S. Volumetric Properties of Binary and Ternary Liquid Mixtures of 1-Propanol (1) + 2-Propanol (2) + Water (3) at Different Temperatures and Ambient Pressure (81.5 kPa). J. Chem. Eng. Data 2008, 53, 1660–1668. [Google Scholar] [CrossRef]
- Ashcroft, S.J.; Clayton, A.D.; Shearn, R.B. Isothermal vapor-liquid equilibriums for the systems toluene-n-heptane, toluene-propan-2-ol, toluene-sulfolane, and propan-2-ol-sulfolane. J. Chem. Eng. Data 1979, 24, 195–199. [Google Scholar] [CrossRef]
- Ritzoulis, G. Excess properties of the binary liquid systems dimethylsulfoxide + isopropanol and propylene carbonate + isopropanol. Can. J. Chem. 1989, 67, 1105–1108. [Google Scholar] [CrossRef]
- Almasi, M. Densities and viscosities of binary mixtures of ethylmethylketone and 2-alkanols; application of the ERAS model and cubic EOS. Thermochim. Acta 2013, 554, 25–31. [Google Scholar] [CrossRef]
- Ruostesuo, P.; Pirilahonkanen, P. Thermodynamic and spectroscopic properties of 2-pyrrolidinones. 2. Dielectric-Properties of 2-pyrrolidinone in binary-mixtures. J. Solut. Chem. 1990, 19, 473–482. [Google Scholar] [CrossRef]
- Olivieri, G.V.; da Cunha, C.S.; dos Santos Martins, L.; Paegle, P.A.M.; Nuncio, S.D.; de Araújo Morandim-Giannetti, A.; Torres, R.B. Thermodynamic and spectroscopic study of binary mixtures of n-butylammonium oleate ionic liquid plus alcohol at T=288.15-308.15 K. J. Therm. Anal. Calorim. 2018, 131, 2925–2942. [Google Scholar] [CrossRef]
- Rafiee, H.R.; Frouzesh, F.; Miri, S. Volumetric properties for binary mixtures of ethyl acetate, vinyl acetate and tert-butyl acetate with 1-propanol and iso-butanol at T = (293.15–313.15) K and P=0.087 MPa. J. Mol. Liq. 2016, 213, 255–267. [Google Scholar] [CrossRef]
- Zaoui-Djelloul-Daouadji, M.; Mokbel, I.; Bahadur, I.; Negadi, A.; Jose, J.; Ramjugernath, D.; Ebenso, E.E.; Negadi, L. Vapor-liquid equilibria, density and sound velocity measurements of (water or methanol or ethanol+1,3-propanediol) binary systems at different temperatures. Thermochim. Acta 2016, 642, 111–123. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Bahadur, I.; Redhi, G.G.; Gengan, R.M.; Anand, K. Synthesis, characterization and thermophysical properties of ionic liquid N-methyl-N-(2′,3′-epoxypropyl)-2-oxopyrrolidinium chloride and its binary mixtures with water or ethanol at different temperatures. J. Mol. Liq. 2016, 219, 685–693. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, S.; Wang, J.; Zhou, Q.; Dong, H.; Zhang, X. Densities and Viscosities of the Binary Mixtures of 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide with N-Methyl-2-pyrrolidone or Ethanol at T = (293.15 to 323.15) K. J. Chem. Eng. Data 2012, 57, 875–881. [Google Scholar] [CrossRef]
- Yan, J.-H.; Dai, L.-Y.; Wang, X.-Z.; Chen, Y.-Q. Densities and Viscosities of Binary Mixtures of Cyclopropanecarboxylic Acid with Methanol, Ethanol, Propan-1-ol, and Butan-1-ol at Different Temperatures. J. Chem. Eng. Data 2009, 54, 1147–1152. [Google Scholar] [CrossRef]
- Zarei, H.A.; Mirhidari, N.; Zangeneh, Z. Densities, Excess Molar Volumes, Viscosity, and Refractive Indices of Binary and Ternary Liquid Mixtures of Methanol (1) + Ethanol (2) + 1,2-Propanediol (3) at P = 81.5 kPa. J. Chem. Eng. Data 2009, 54, 847–854. [Google Scholar] [CrossRef]
- Fan, W.; Zhou, Q.; Zhang, S.; Yan, R. Excess molar volume and viscosity deviation for the methanol plus methyl methacrylate binary system at T = (283.15 to 333.15) K. J. Chem. Eng. Data 2008, 53, 1836–1840. [Google Scholar] [CrossRef]
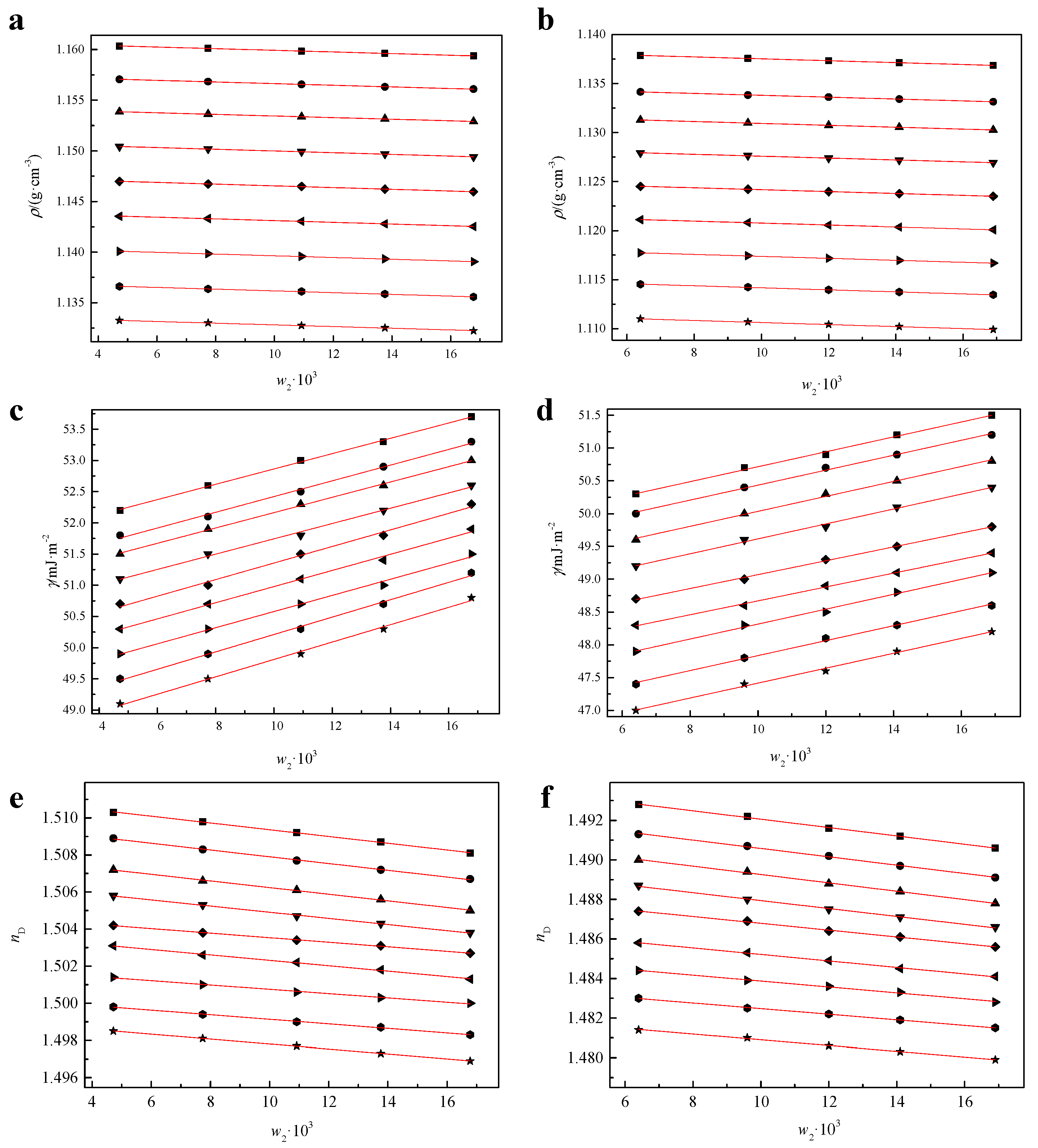
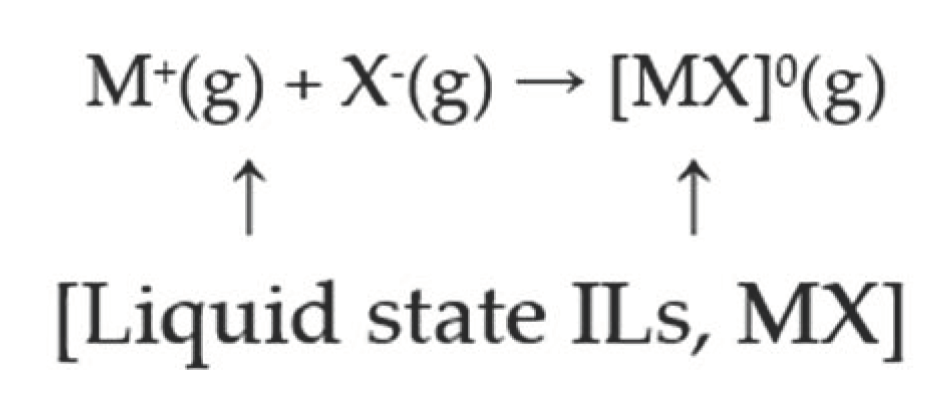
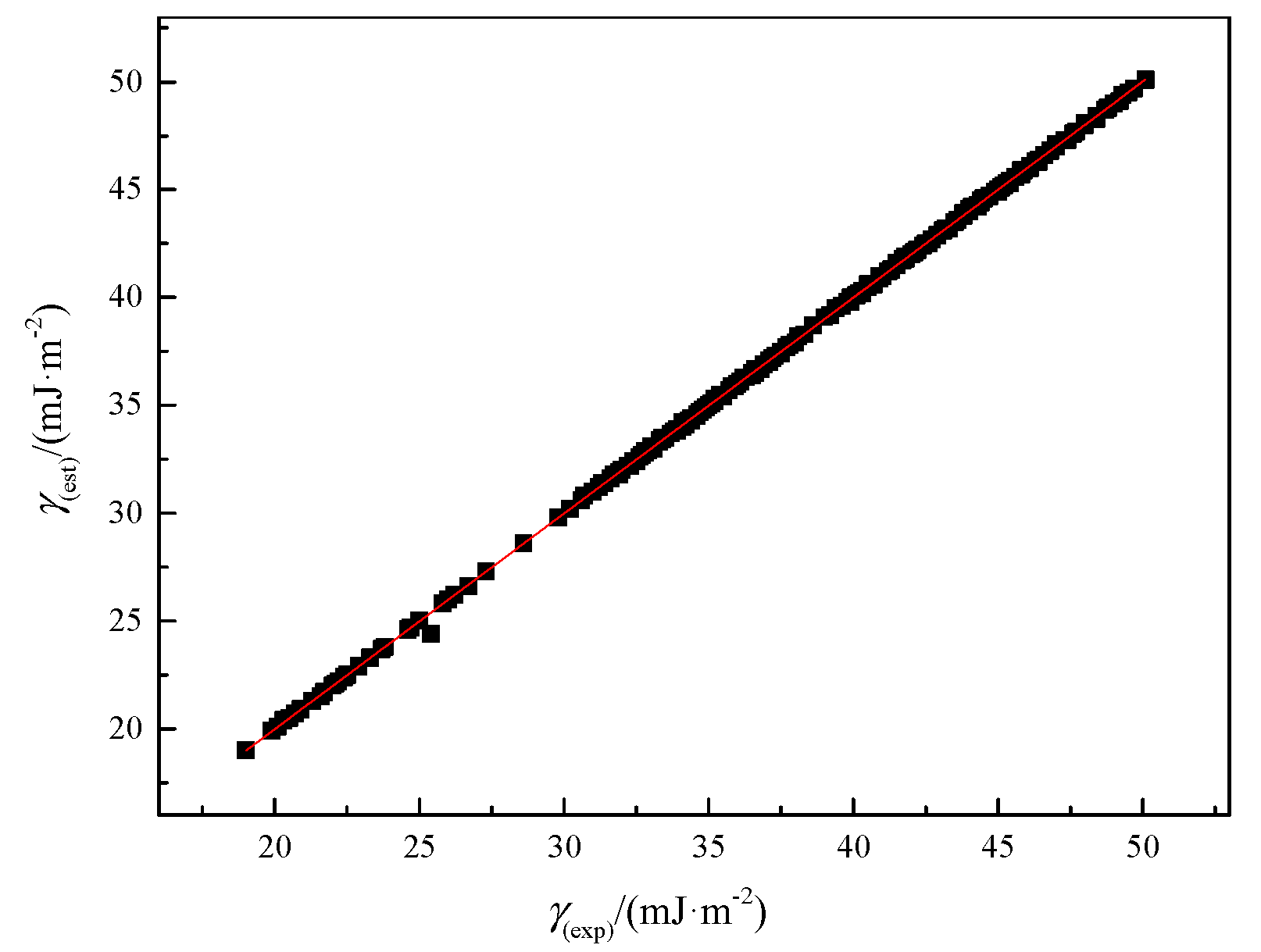
| T (K) | ρ (g·cm−3) | γ (mJ·m−2) | nD | α (K−1 × 104) |
|---|---|---|---|---|
| [C1OC2mim][Ala] | ||||
| 288.15 | 1.16073 | 51.6 | 1.5112 | 5.8555 |
| 293.15 | 1.15744 | 51.2 | 1.5097 | 5.8722 |
| 298.15 | 1.15423 | 50.9 | 1.5080 | 5.8885 |
| 303.15 | 1.15082 | 50.5 | 1.5066 | 5.9060 |
| 308.15 | 1.14739 | 50.0 | 1.5048 | 5.9236 |
| 313.15 | 1.14395 | 49.7 | 1.5038 | 5.9414 |
| 318.15 | 1.14049 | 49.3 | 1.5019 | 5.9595 |
| 323.15 | 1.13703 | 48.8 | 1.5004 | 5.9776 |
| 328.15 | 1.13365 | 48.4 | 1.4991 | 5.9954 |
| [C2OC2mim][Ala] | ||||
| 288.15 | 1.13849 | 49.6 | 1.4942 | 5.8490 |
| 293.15 | 1.13475 | 49.3 | 1.4927 | 5.8683 |
| 298.15 | 1.13190 | 48.9 | 1.4914 | 5.8830 |
| 303.15 | 1.12854 | 48.5 | 1.4899 | 5.9005 |
| 308.15 | 1.12514 | 48.0 | 1.4885 | 5.9184 |
| 313.15 | 1.12175 | 47.6 | 1.4869 | 5.9363 |
| 318.15 | 1.11838 | 47.2 | 1.4854 | 5.9541 |
| 323.15 | 1.11521 | 46.7 | 1.4839 | 5.9711 |
| 328.15 | 1.11166 | 46.3 | 1.4824 | 5.9901 |
| T (K) | gs (kJ·mol−1) | G0 | G1 | gs(est) (kJ·mol−1) |
|---|---|---|---|---|
| [C1OC2mim][Ala] | ||||
| 288.15 | 14.78 | 19,803 | 17.4 | 14.79 |
| 293.15 | 14.69 | 19,803 | 17.4 | 14.70 |
| 298.15 | 14.63 | 19,803 | 17.4 | 14.62 |
| 303.15 | 14.55 | 19,803 | 17.4 | 14.53 |
| 308.15 | 14.43 | 19,803 | 17.4 | 14.44 |
| 313.15 | 14.37 | 19,803 | 17.4 | 14.35 |
| 318.15 | 14.29 | 19,803 | 17.4 | 14.27 |
| 323.15 | 14.17 | 19,803 | 17.4 | 14.18 |
| 328.15 | 14.08 | 19,803 | 17.4 | 14.09 |
| [C2OC2mim][Ala] | ||||
| 288.15 | 14.88 | 20,658 | 19.9 | 14.92 |
| 293.15 | 14.82 | 20,658 | 19.9 | 14.82 |
| 298.15 | 14.73 | 20,658 | 19.9 | 14.72 |
| 303.15 | 14.63 | 20,658 | 19.9 | 14.63 |
| 308.15 | 14.51 | 20,658 | 19.9 | 14.53 |
| 313.15 | 14.42 | 20,658 | 19.9 | 14.43 |
| 318.15 | 14.33 | 20,658 | 19.9 | 14.33 |
| 323.15 | 14.20 | 20,658 | 19.9 | 14.23 |
| 328.15 | 14.11 | 20,658 | 19.9 | 14.13 |
| T (K) | Rm | αp × 1024 | γ(est) |
|---|---|---|---|
| [C1OC2mim][Ala] | |||
| 288.15 | 57.74 | 22.91 | 51.6 |
| 293.15 | 57.77 | 22.92 | 51.2 |
| 298.15 | 57.76 | 22.92 | 50.8 |
| 303.15 | 57.80 | 22.93 | 50.4 |
| 308.15 | 57.82 | 22.94 | 50.0 |
| 313.15 | 57.84 | 22.95 | 49.6 |
| 318.15 | 57.89 | 22.97 | 49.2 |
| 323.15 | 57.90 | 22.97 | 48.8 |
| 328.15 | 57.89 | 22.97 | 48.4 |
| [C2OC2mim][Ala] | |||
| 288.15 | 62.24 | 24.69 | 49.4 |
| 293.15 | 62.28 | 24.71 | 49.0 |
| 298.15 | 62.30 | 24.72 | 48.6 |
| 303.15 | 62.32 | 24.73 | 48.2 |
| 308.15 | 62.36 | 24.74 | 47.7 |
| 313.15 | 62.37 | 24.75 | 47.3 |
| 318.15 | 62.39 | 24.76 | 46.9 |
| 323.15 | 62.41 | 24.76 | 46.5 |
| 328.15 | 62.43 | 24.77 | 46.1 |
| IL | s (J·mol−1·K−1) | V × 104 (m3·mol−1) |
|---|---|---|
| [C1OC2mim]Cl [38] | 15.99 | 1.52 |
| [C1OC2mim][Ala] | 17.39 | 1.99 |
| [C1OC2mim][Thr] [45] | 26.60 | 2.18 |
| [C1OC2mim][NTf2] [19] | 17.80 | 2.80 |
| [C2OC2mim]Cl [38] | 17.81 | 1.68 |
| [C2OC2mim][Ala] | 19.94 | 2.15 |
| [C2OC2mim][Thr] [45] | 28.12 | 2.37 |
| [C2OC2mim][NTf2] [19] | 19.32 | 2.99 |
| [C1OC4mim][Gly] [46] | 19.56 | 2.19 |
| [C1OC4mim][Ala] [46] | 20.54 | 2.29 |
| [C1OC4mim][Thr] [46] | 22.082 | 2.51 |
| [C2OC1mim][NTf2] [19] | 18.20 | 2.81 |
| [C1OC3mim][NTf2] [19] | 18.49 | 2.97 |
| [C3OC2mim][NTf2] [19] | 20.57 | 3.16 |
| IL | PN (J·mol−1·K−1) | Reference |
|---|---|---|
| [COC2mim]Cl | 12.86 | [37] |
| [C2OC2mim]Cl | 16.76 | [37] |
| [C1OC2mim][Ala] | 12.26 | This work |
| [C2OC2mim][Ala] | 18.33 | This work |
| [COC4mim][Ala] | 21.83 | [39] |
| [COC2mim][Thr] | 22.18 | [38] |
| [C2OC2mim][Thr] | 25.46 | [38] |
| [COC4mim][Thr] | 24.52 | [39] |
| [COC2mim][NTf2] | 31.31 | [13] |
| [C2OCmim][NTf2] | 35.40 | [13] |
| [C1OC3mim][NTf2] | 35.57 | [13] |
| [C2OC2mim][NTf2] | 42.66 | [13] |
| [C3OC2mim][NTf2] | 55.1 | [13] |
| [COC4mim][Gly] | 19.64 | [39] |
| IL | PN (J·mol−1·K−1) |
|---|---|
| [C6mim]OAC | 35.73 |
| [C4mim]OAC | 23.24 |
| [C4mim]NTf2 [51,53] | 22.03 |
| [C4mmim]NTf2 | 19.96 |
| [C4mim]BF4 | 14.50 |
| [C2mmim]NTf2 | 14.01 |
| [C5mim]Lact | 12.87 |
| [C2mim]BF4 | 8.24 |
| [C2mim]Lact | 8.16 |
| Molecular Liquid | PN | ε |
|---|---|---|
| Ethyl acetate | 169.14 | 6.1 |
| Chloroform | 167.19 | 4.8 |
| Tetrahydrofuran | 124.29 | 7.5 |
| Pyridine | 60.64 | 12.3 |
| Benzyl alcohol | 48.13 | 13.0 |
| 1-Hexanol | 27.06 | 13.0 |
| Cyclohexanol | 25.09 | 15.0 |
| 1-Propanol | 8.48 | 20.3 |
| Ethanol | 5.52 | 25.3 |
| Methanol | 2.40 | 33.0 |
| Reagent Name | CAS No. | Source | Purification | Mass Fraction Purity | Analysis |
|---|---|---|---|---|---|
| Anion exchange resin 717 | 122560-63-8 | SRC | None | Granularity > 0.950 | GC |
| N-Methylimidazole | 616-47-7 | ACROS | Distillation | >0.990 | FM |
| 2-Chloroethyl methyl ether | 627-42-9 | SRC | None | >0.995 | FM |
| 2-Chloroethyl ethyl ether | 628-34-2 | SRC | None | >0.995 | FM |
| DL-Alanine | 302-72-7 | SRC | Recrystallization | >0.990 | FM |
| Acetonitrile | 75-05-8 | SRC | None | >0.995 | FM |
| Ethyl acetate | 141-78-6 | SRC | None | >0.995 | FM |
| Anhydrous ethanol | 64-17-5 | SRC | None | >0.995 | FM |
| Sodium hydroxide | 1310-73-2 | SRC | None | >0.960 | FM |
| [C1OC2mim][Ala] | - | Synthesis | Solvent extraction, vacuum drying | >0.990 | 1H, 13C NMR |
| [C2OC2mim][Ala] | - | Synthesis | Solvent extraction, vacuum drying | >0.990 | 1H, 13C NMR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Guo, C.; Wu, W.; Tong, J. A New Compartmentalized Scale (PN) for Measuring Polarity Applied to Novel Ether-Functionalized Amino Acid Ionic Liquids. Molecules 2022, 27, 3231. https://doi.org/10.3390/molecules27103231
Zheng X, Guo C, Wu W, Tong J. A New Compartmentalized Scale (PN) for Measuring Polarity Applied to Novel Ether-Functionalized Amino Acid Ionic Liquids. Molecules. 2022; 27(10):3231. https://doi.org/10.3390/molecules27103231
Chicago/Turabian StyleZheng, Xu, Chun Guo, Wenqing Wu, and Jing Tong. 2022. "A New Compartmentalized Scale (PN) for Measuring Polarity Applied to Novel Ether-Functionalized Amino Acid Ionic Liquids" Molecules 27, no. 10: 3231. https://doi.org/10.3390/molecules27103231
APA StyleZheng, X., Guo, C., Wu, W., & Tong, J. (2022). A New Compartmentalized Scale (PN) for Measuring Polarity Applied to Novel Ether-Functionalized Amino Acid Ionic Liquids. Molecules, 27(10), 3231. https://doi.org/10.3390/molecules27103231





