Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Monophenolase Inhibitory Activity
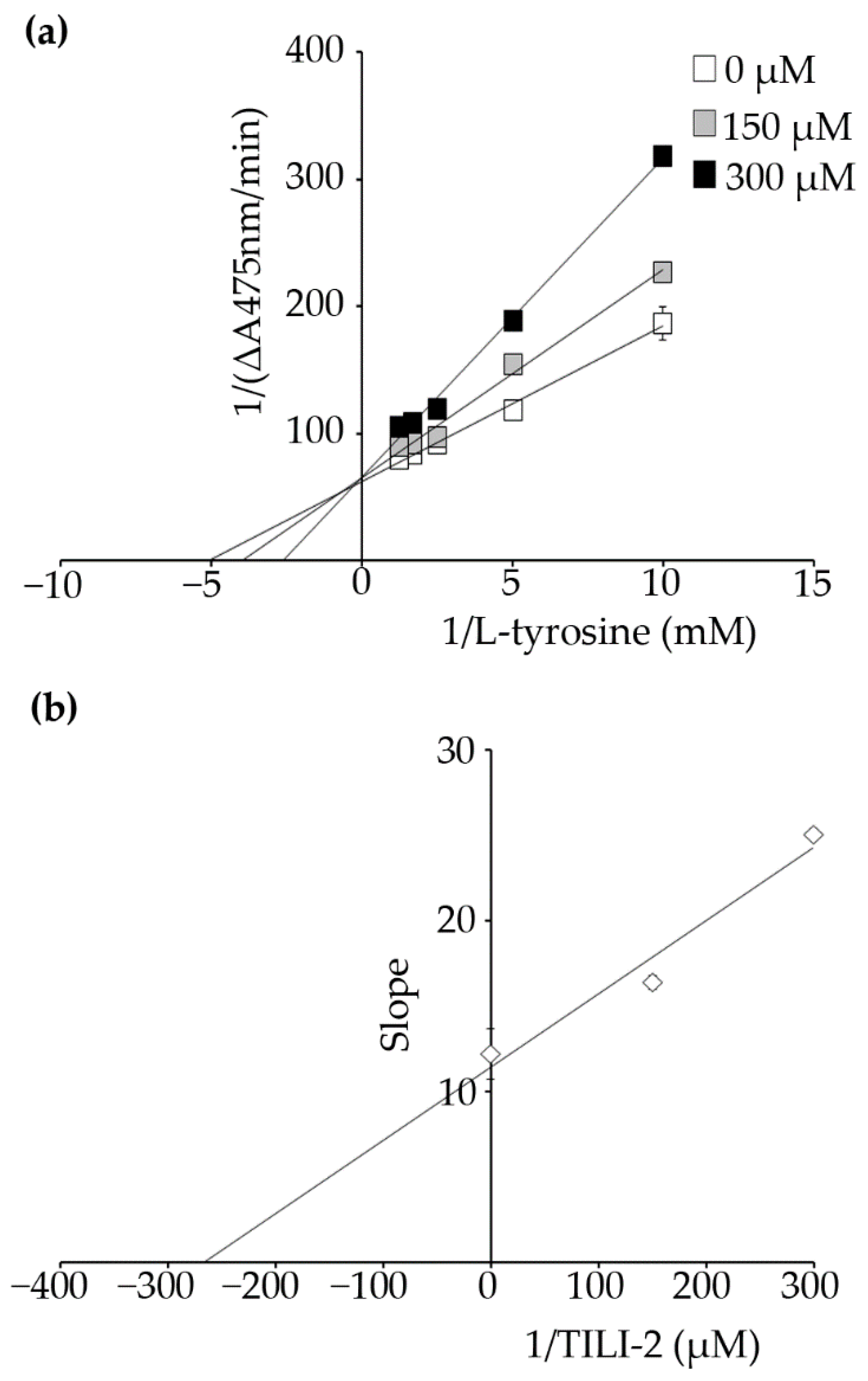
2.2. Kinetic Study
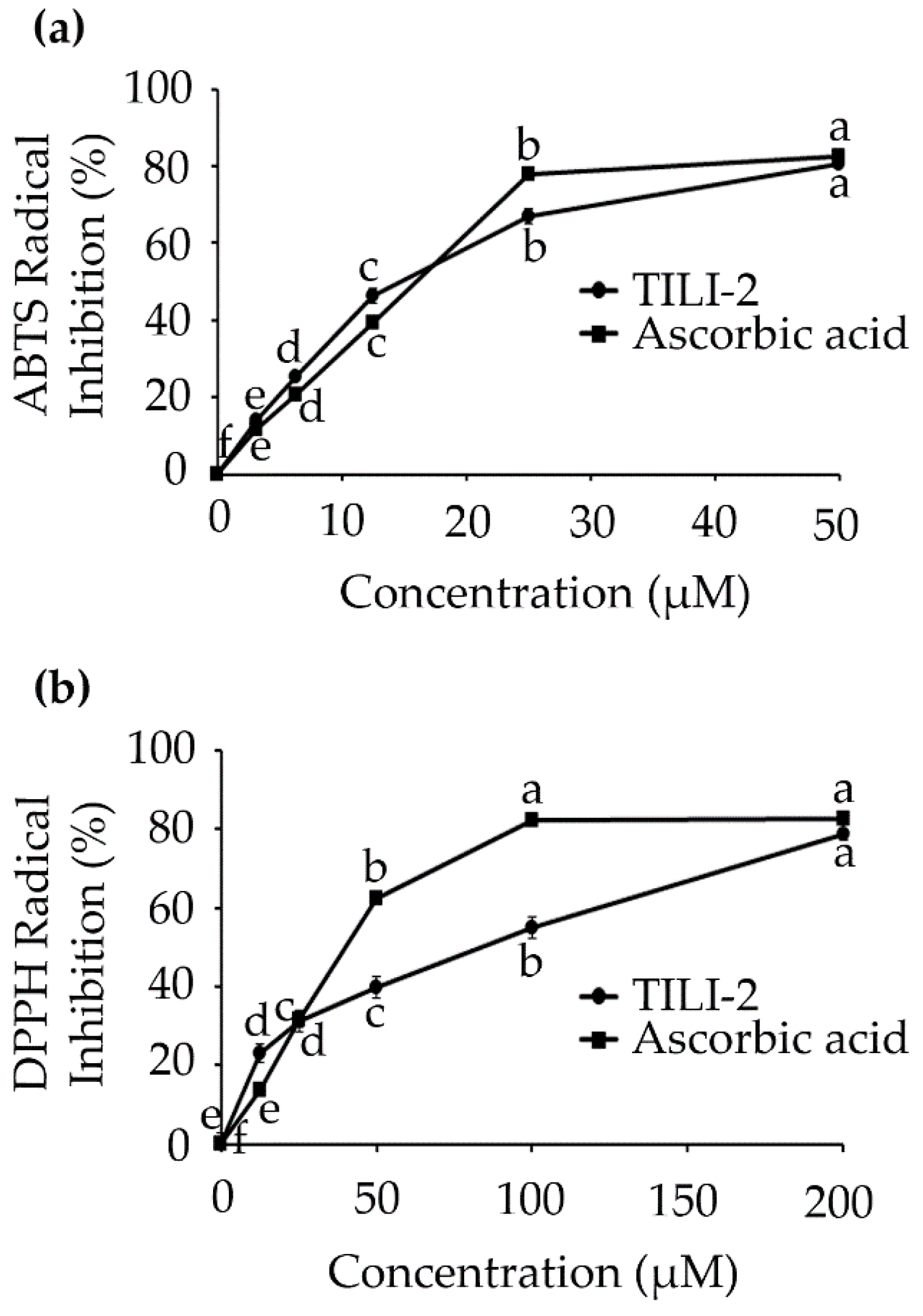
2.3. Antioxidant Activity
2.4. Cytotoxicity
2.5. B16F1 Cell Proteomics Profile Based Biological Processes and Cellular Components
2.6. B16F1 Cell Proteomics Profile Based Cell Signaling Pathway
2.7. Protein-Protein Interaction Prediction by STITCH Online Database
2.8. Gene Expression Using Real-Time PCR
3. Materials and Methods
3.1. Materials and Reagents
3.2. Peptide Synthesis
3.3. Assay for Monophenolase Inhibitory Activity
3.4. Kinetic Study
3.5. ABTS Radical Scavenging Assay
3.6. DPPH Radical Scavenging Assay
3.7. Cell Culture
3.8. Cell Viability Assay
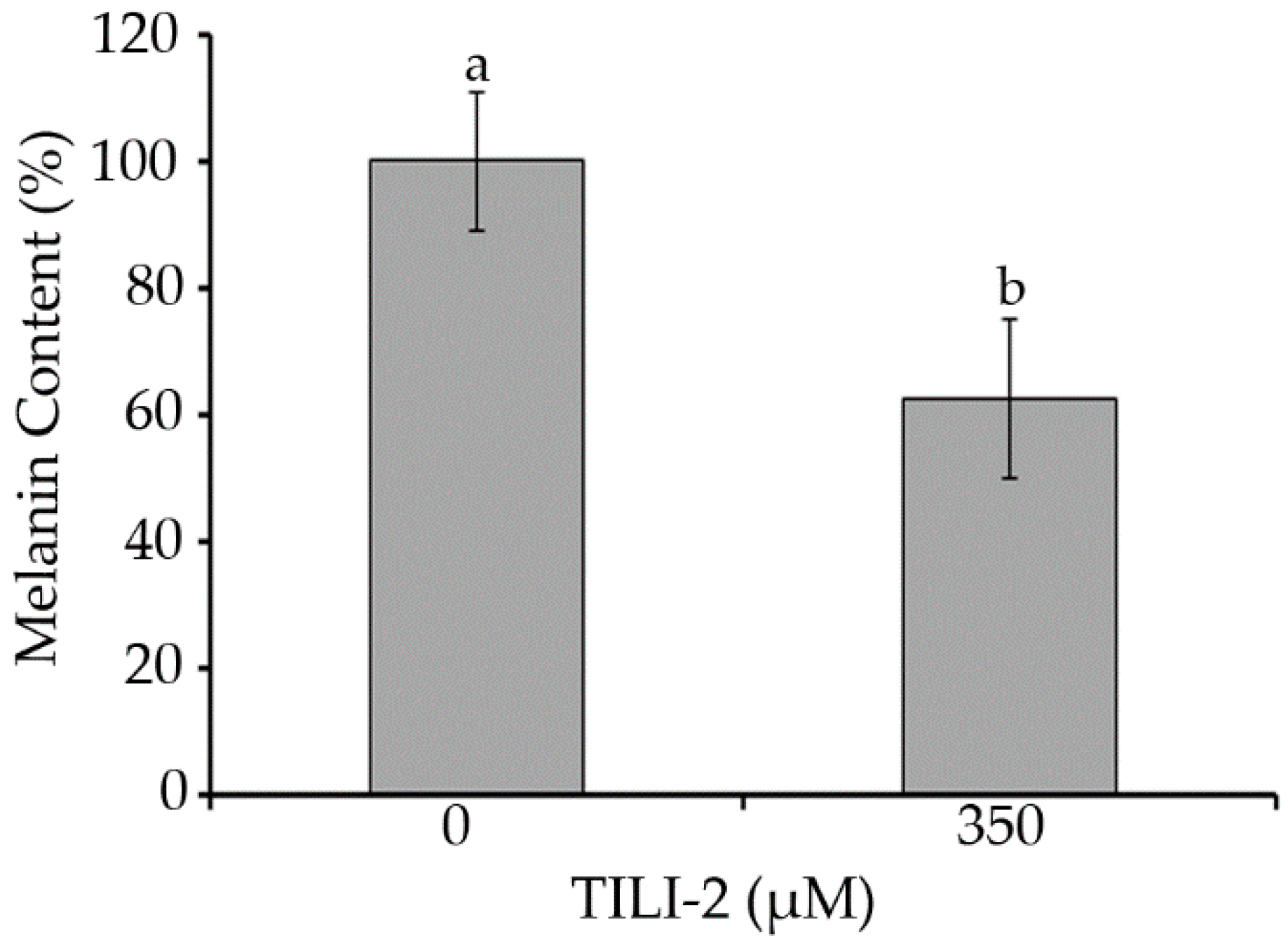
3.9. Melanin Content
3.10. B16F1 Cell Protein Extraction
3.11. B16F1 Cell Protein Trypsinization and Label-Free LC-MS/MS-Based Proteomics
3.12. Protein Quantitation and Identification
3.13. Proteomics Data Bioinformatic Analysis
3.14. Real-Time PCR
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kao, Y.Y.; Chuang, T.F.; Chao, S.H.; Yang, J.H.; Lin, Y.C.; Huang, H.Y. Evaluation of the antioxidant and melanogenesis inhibitory properties of pracparatum mungo (lu-do huang). J. Tradit. Complement. Med. 2013, 3, 163–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kasamatsu, S.; Hachiya, A.; Nakamura, S.; Yasuda, Y.; Fujimori, T.; Takano, K.; Moriwaki, S.; Hase, T.; Suzuki, T.; Matsunaga, K. Depigmentation caused by application of the active brightening material, rhododendrol, is related to tyrosinase activity at a certain threshold. J. Dermatol. Sci. 2014, 76, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; Ding, S.-S.; Deng, Y.-J.; Wang, X.-J. Skin-care effects of dandelion leaf extract and stem extract: Antioxidant properties, tyrosinase inhibitory and molecular docking simulations. Ind. Crops Prod. 2018, 111, 238–246. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef]
- Boo, Y.C. Up- or Downregulation of Melanin Synthesis Using Amino Acids, Peptides, and Their Analogs. Biomedicines 2020, 8, 322. [Google Scholar] [CrossRef]
- Qin, L.; Wu, Y.; Liu, Y.; Chen, Y.; Zhang, P. Dual effects of alpha-arbutin on monophenolase and diphenolase activities of mushroom tyrosinase. PLoS ONE 2014, 9, e109398. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. The Dual Antimelanogenic and Antioxidant Activities of the Essential Oil Extracted from the Leaves of Acorus macrospadiceus (Yamamoto) F. N. Wei et Y. K. Li. Evid.-Based Complement. Altern. Med. 2012, 2012, 781280. [Google Scholar] [CrossRef]
- Panich, U.; Tangsupa-a-nan, V.; Onkoksoong, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornsilp, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharm. Res. 2011, 34, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.Y.; Lee, H.Y.; Nam, K.Y.; Park, J.; Lee, S.M.; Kim, J.E.; Lee, J.D.; Hwang, J.S. Hesperidin Suppresses Melanosome Transport by Blocking the Interaction of Rab27A-Melanophilin. Biomol. Ther. 2013, 21, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Upata, M.; Siriwoharn, T.; Makkhun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase Inhibitory and Antioxidant Activity of Enzymatic Protein Hydrolysate from Jellyfish (Lobonema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Ab Aziz, N.A.; Salim, N.; Zarei, M.; Saari, N.; Yusoff, F.M. Extraction, anti-tyrosinase, and antioxidant activities of the collagen hydrolysate derived from Rhopilema hispidum. Prep. Biochem. Biotechnol. 2021, 51, 44–53. [Google Scholar] [CrossRef]
- Ochiai, A.; Tanaka, S.; Imai, Y.; Yoshida, H.; Kanaoka, T.; Tanaka, T.; Taniguchi, M. New tyrosinase inhibitory decapeptide: Molecular insights into the role of tyrosine residues. J. Biosci. Bioeng. 2016, 121, 607–613. [Google Scholar] [CrossRef]
- Joompang, A.; Jangpromma, N.; Choowongkomon, K.; Payoungkiattikun, W.; Tankrathok, A.; Viyoch, J.; Luangpraditkun, K.; Klaynongsruang, S. Evaluation of tyrosinase inhibitory activity and mechanism of Leucrocin I and its modified peptides. J. Biosci. Bioeng. 2020, 130, 239–246. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Tseng, T.S.; Tsai, K.C.; Chen, W.C.; Wang, Y.T.; Lee, Y.C.; Lu, C.K.; Don, M.J.; Chang, C.Y.; Lee, C.H.; Lin, H.H.; et al. Discovery of Potent Cysteine-Containing Dipeptide Inhibitors against Tyrosinase: A Comprehensive Investigation of 20 x 20 Dipeptides in Inhibiting Dopachrome Formation. J. Agric. Food Chem. 2015, 63, 6181–6188. [Google Scholar] [CrossRef]
- Hsiao, N.W.; Tseng, T.S.; Lee, Y.C.; Chen, W.C.; Lin, H.H.; Chen, Y.R.; Wang, Y.T.; Hsu, H.J.; Tsai, K.C. Serendipitous discovery of short peptides from natural products as tyrosinase inhibitors. J. Chem. Inf. Model. 2014, 54, 3099–3111. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hsiao, N.W.; Tseng, T.S.; Chen, W.C.; Lin, H.H.; Leu, S.J.; Yang, E.W.; Tsai, K.C. Phage display-mediated discovery of novel tyrosinase-targeting tetrapeptide inhibitors reveals the significance of N-terminal preference of cysteine residues and their functional sulfur atom. Mol. Pharmacol. 2015, 87, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, O.; Lee, Y.; Kang, L.J.; Nguyen, C.N.; Ishihara, A.; Kim, H.E. Feruloylserotonin inhibits hydrogen peroxide-induced melanogenesis and apoptosis in B16F10 and SK-Mel-2 melanoma cells. Biochem. Biophys. Res. Commun. 2017, 491, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Lee, S.G. Induction of ATP synthase beta by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int. J. Biochem. Cell Biol. 2013, 45, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef]
- Oh, G.-W.; Ko, S.-C.; Heo, S.-Y.; Nguyen, V.-T.; Kim, G.; Jang, C.H.; Park, W.S.; Choi, I.-W.; Qian, Z.-J.; Jung, W.-K. A novel peptide purified from the fermented microalga Pavlova lutheri attenuates oxidative stress and melanogenesis in B16F10 melanoma cells. Process Biochem. 2015, 50, 1318–1326. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Yanez, A.; Coetzee, S.G.; Olsson, A.; Muench, D.E.; Berman, B.P.; Hazelett, D.J.; Salomonis, N.; Grimes, H.L.; Goodridge, H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 2017, 47, 890–902. [Google Scholar] [CrossRef]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Peterson, D.E.; Collier, J.M.; Katterman, M.E.; Turner, R.A.; Riley, M.R. Cytotoxicity of bacterial-derived toxins to immortal lung epithelial and macrophage cells. Appl. Biochem. Biotechnol. 2010, 160, 751–763. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Suzuki, M.; Igras, V.; Du, J.; Lonning, S.; Miyachi, Y.; Roes, J.; Beermann, F.; Fisher, D.E. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 2010, 6, 130–140. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Nishimura, E.K.; Xin, H.; Zhou, A.; Guo, Y.; Dong, L.; Denning, M.F.; Nickoloff, B.J.; Cui, R. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol. Cell 2008, 32, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Matsuzaki, F.; Funaba, M. Regulation of melanin synthesis by the TGF-beta family in B16 melanoma cells. Mol. Biol. Rep. 2009, 36, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.I. Effect of Chrysanthemum indicum Linne extract on melanogenesis through regulation of TGF-beta/JNK signaling pathway. Food Sci. Biotechnol. 2019, 28, 1577–1582. [Google Scholar] [CrossRef]
- Park, H.Y.; Wu, C.; Yaar, M.; Stachur, C.M.; Kosmadaki, M.; Gilchrest, B.A. Role of BMP-4 and Its Signaling Pathways in Cultured Human Melanocytes. Int. J. Cell Biol. 2009, 2009, 750482. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.R.; Jung, J.M.; Kim, S.Y.; Song, Y.; Chang, S.E. TGF-beta3 suppresses melanogenesis in human melanocytes cocultured with UV-irradiated neighboring cells and human skin. J. Dermatol. Sci. 2020, 99, 100–108. [Google Scholar] [CrossRef]
- Kubic, J.D.; Young, K.P.; Plummer, R.S.; Ludvik, A.E.; Lang, D. Pigmentation PAX-ways: The role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment. Cell Melanoma Res. 2008, 21, 627–645. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Chae, J.K.; Subedi, L.; Jeong, M.; Park, Y.U.; Kim, C.Y.; Kim, H.; Kim, S.Y. Gomisin N Inhibits Melanogenesis through Regulating the PI3K/Akt and MAPK/ERK Signaling Pathways in Melanocytes. Int. J. Mol. Sci. 2017, 18, 471. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Oh, S.W.; Park, S.H.; Lee, J.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Yu, E.; Cho, J.Y.; et al. Melanogenic Effects of Maclurin Are Mediated through the Activation of cAMP/PKA/CREB and p38 MAPK/CREB Signaling Pathways. Oxid. Med. Cell. Longev. 2019, 2019, 9827519. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-alpha/p38 MAPK-mediated pathways. Sci. Rep. 2018, 8, 14958. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Park, S.H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Nho, Y.H.; Lee, S.; Han, B.S.; Cho, J.Y.; Lee, J. Anti-melanogenic effects of resorcinol are mediated by suppression of cAMP signaling and activation of p38 MAPK signaling. Biosci. Biotechnol. Biochem. 2018, 82, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Yaar, M.; Wu, C.; Park, H.Y.; Panova, I.; Schutz, G.; Gilchrest, B.A. Bone morphogenetic protein-4, a novel modulator of melanogenesis. J. Biol. Chem. 2006, 281, 25307–25314. [Google Scholar] [CrossRef]
- Jin, E.J.; Erickson, C.A.; Takada, S.; Burrus, L.W. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev. Biol. 2001, 233, 22–37. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Itman, C.; Mendis, S.; Barakat, B.; Loveland, K.L. All in the family: TGF-beta family action in testis development. Reproduction 2006, 132, 233–246. [Google Scholar] [CrossRef][Green Version]
- Liu, S.H.; Pan, I.H.; Chu, I.M. Inhibitory effect of p-hydroxybenzyl alcohol on tyrosinase activity and melanogenesis. Biol. Pharm. Bull. 2007, 30, 1135–1139. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural Melanogenesis Inhibitors Acting Through the Down-Regulation of Tyrosinase Activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- An, S.M.; Kim, H.J.; Kim, J.E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jandaruang, J.; Siritapetawee, J.; Thumanu, K.; Songsiriritthigul, C.; Krittanai, C.; Daduang, S.; Dhiravisit, A.; Thammasirirak, S. The effects of temperature and pH on secondary structure and antioxidant activity of Crocodylus siamensis hemoglobin. Protein J. 2012, 31, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Phosri, S.; Mahakunakorn, P.; Lueangsakulthai, J.; Jangpromma, N.; Swatsitang, P.; Daduang, S.; Dhiravisit, A.; Thammasirirak, S. An investigation of antioxidant and anti-inflammatory activities from blood components of Crocodile (Crocodylus siamensis). Protein J. 2014, 33, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Maijaroen, S.; Klaynongsruang, S.; Roytrakul, S.; Konkchaiyaphum, M.; Taemaitree, L.; Jangpromma, N. An Integrated Proteomics and Bioinformatics Analysis of the Anticancer Properties of RT2 Antimicrobial Peptide on Human Colon Cancer (Caco-2) Cells. Molecules 2022, 27, 1426. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Maijaroen, S.; Klaynongsruang, S.; Reabroi, S.; Chairoungdua, A.; Roytrakul, S.; Daduang, J.; Taemaitree, L.; Jangpromma, N. Proteomic profiling reveals antitumor effects of RT2 peptide on a human colon carcinoma xenograft mouse model. Eur. J. Pharmacol. 2022, 917, 174753. [Google Scholar] [CrossRef]
- Thorsell, A.; Portelius, E.; Blennow, K.; Westman-Brinkmalm, A. Evaluation of sample fractionation using micro-scale liquid-phase isoelectric focusing on mass spectrometric identification and quantitation of proteins in a SILAC experiment. Rapid Commun. Mass Spectrom. 2007, 21, 771–778. [Google Scholar] [CrossRef]
- Johansson, C.; Samskog, J.; Sundstrom, L.; Wadensten, H.; Bjorkesten, L.; Flensburg, J. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics 2006, 6, 4475–4485. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. PANTHER version 11: Expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017, 45, D183–D189. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Omotehara, T.; Hirano, T.; Kubota, N.; Yanai, S.; Hasegawa, C.; Takada, T.; Mantani, Y.; Hoshi, N. Identification of reference genes for quantitative PCR analyses in developing mouse gonads. J. Vet. Med. Sci. 2018, 80, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Svingen, T.; Spiller, C.M.; Kashimada, K.; Harley, V.R.; Koopman, P. Identification of suitable normalizing genes for quantitative real-time RT-PCR analysis of gene expression in fetal mouse gonads. Sex. Dev. 2009, 3, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
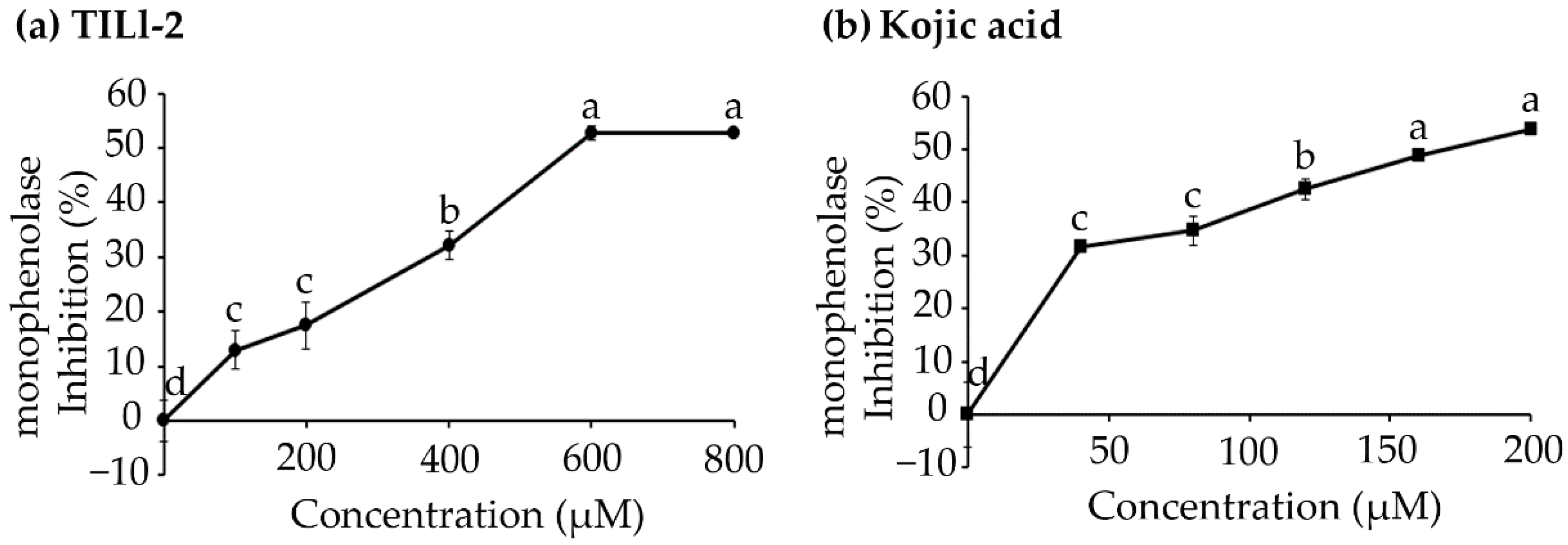

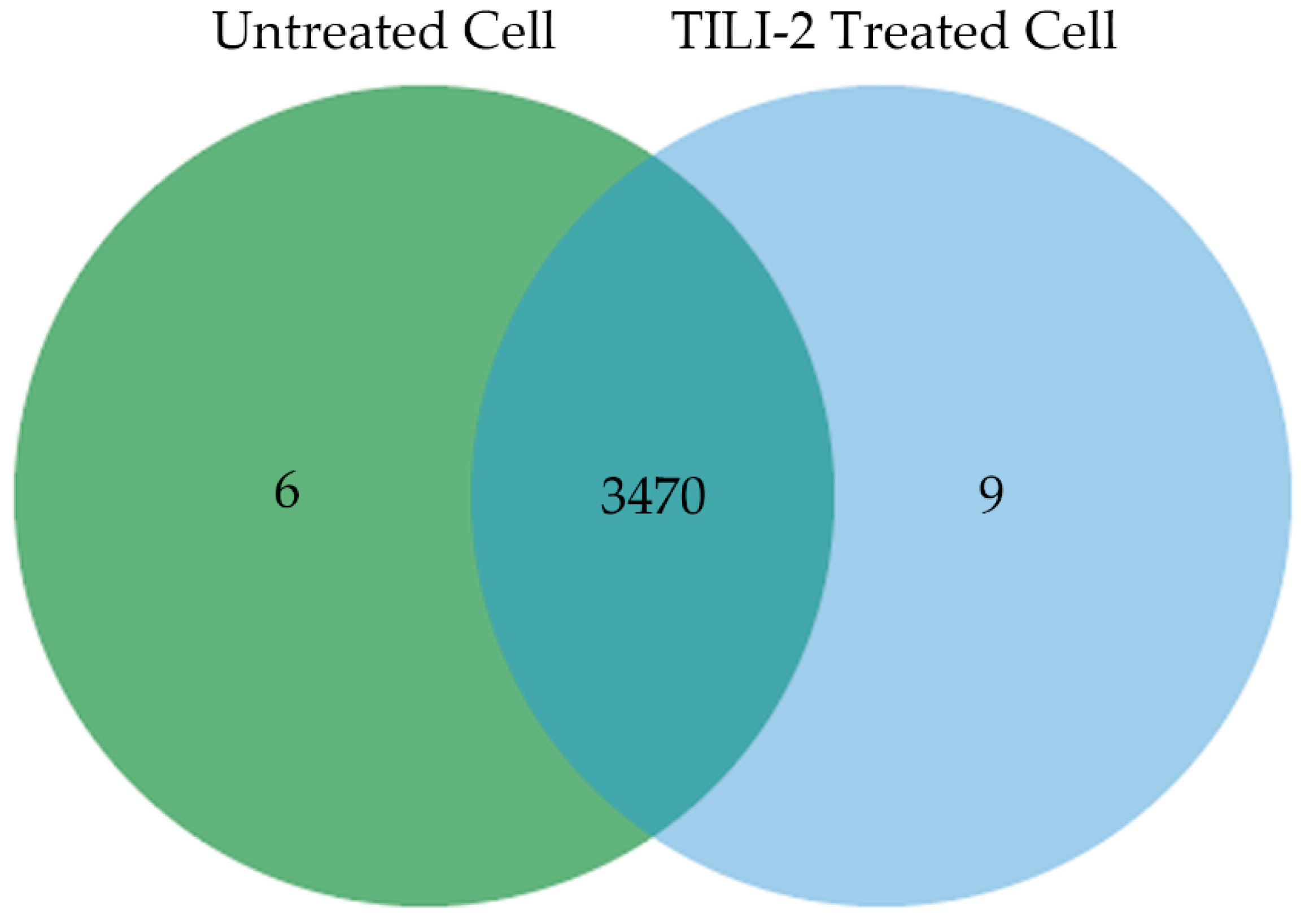
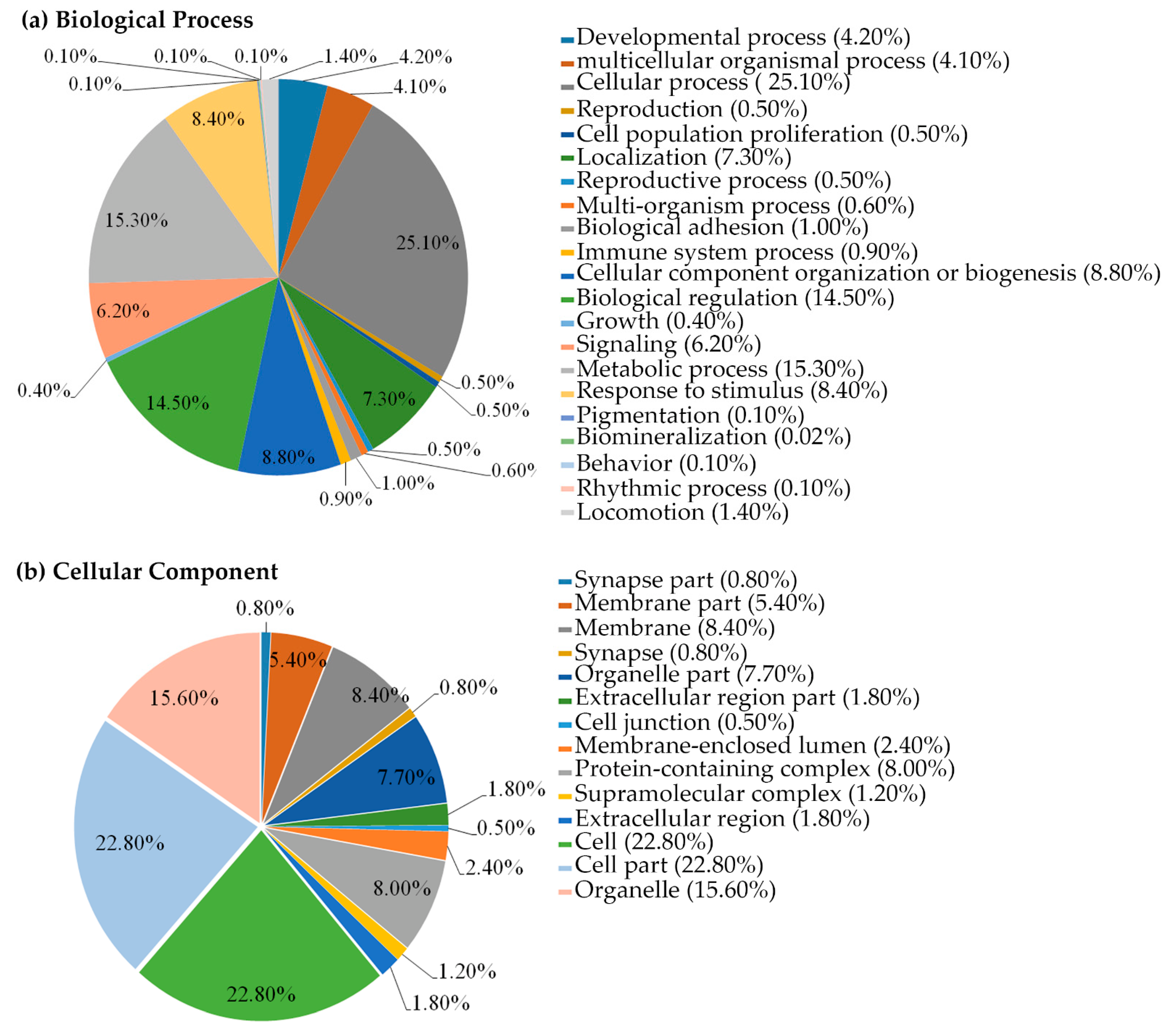
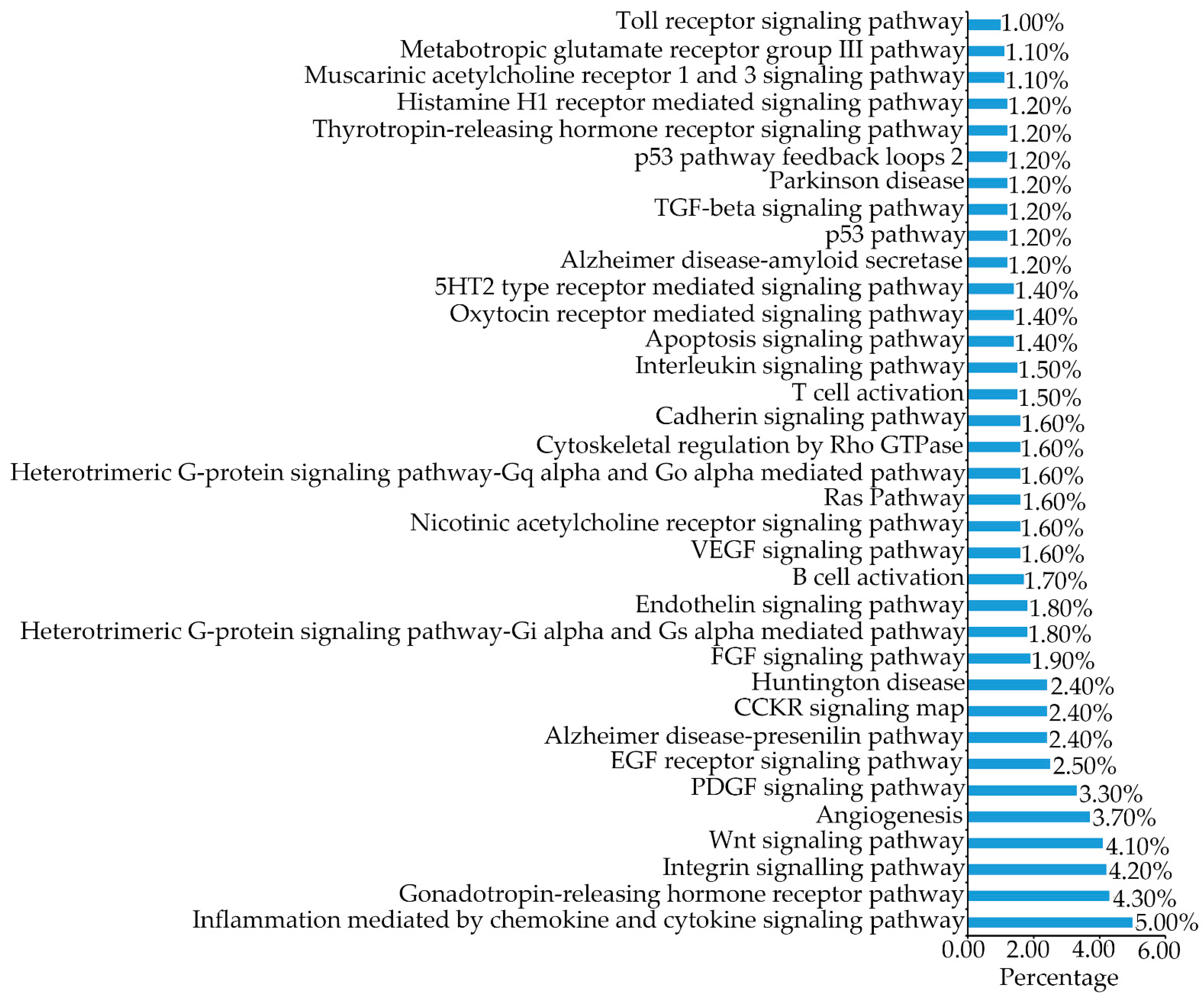
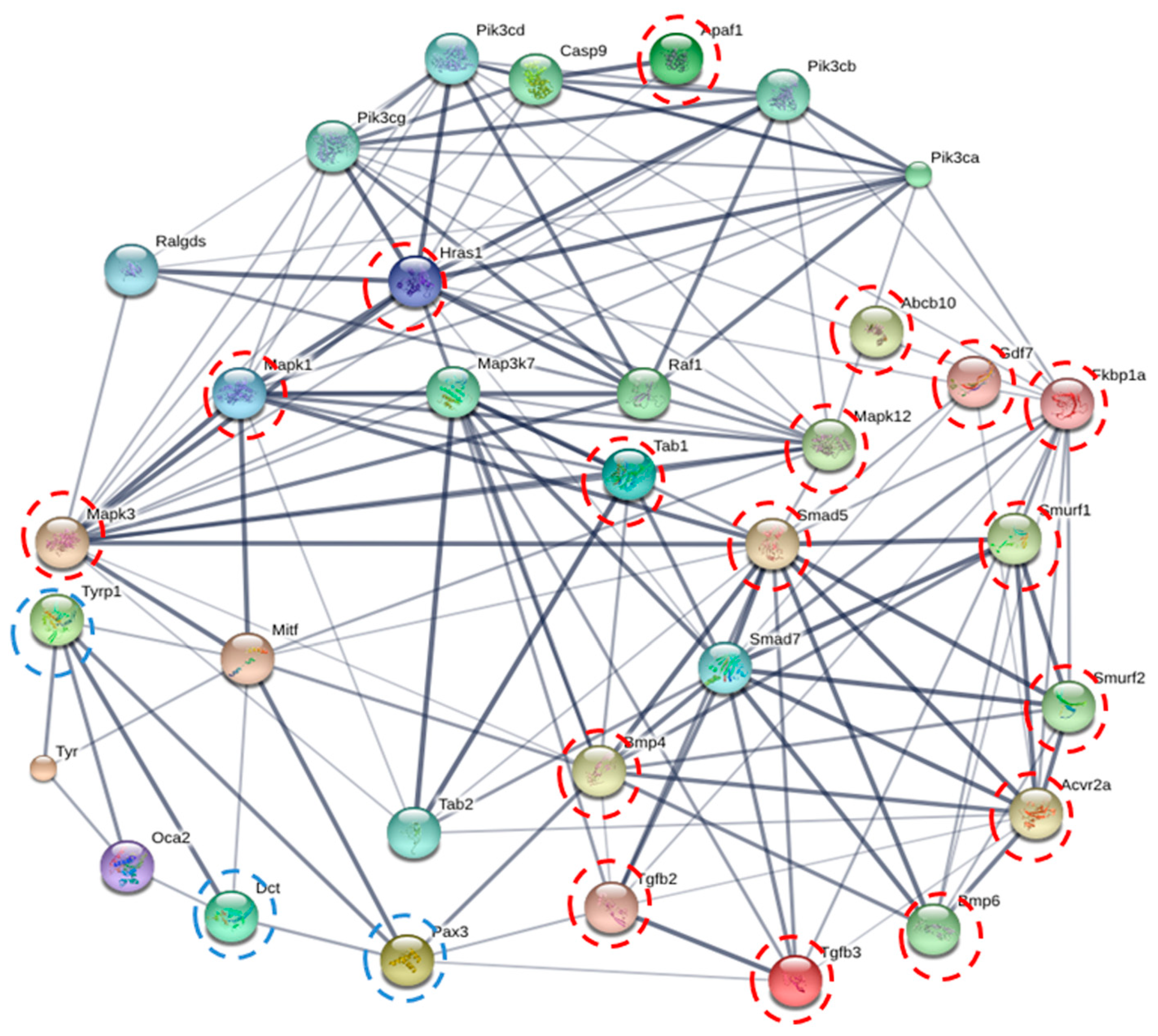

| Peptide Name | Sequence | IC50 (µM) |
|---|---|---|
| TILI-2 | CNGVQPK | 582.92 ± 6.44 a |
| Kojic acid | - | 173.20 ± 6.44 b |
| Name | Concentration (µM) | Km or K’m (µM) | Vmax or V’max (△A475/min) | Mode of Inhibition | Ki (µM) |
|---|---|---|---|---|---|
| TILI-2 | 0 | 196.58 ± 36.64 a | 0.016 ± 0.0010 a | competitive | 273.39 ± 72.90 |
| 150.00 | 250.09 ± 10.65 b | 0.015 ± 0.0003 a | |||
| 300.00 | 381.30 ± 10.77 c | 0.015 ± 0.0002 a |
| Peptide Name | Sequence | IC50 (µM) | |
|---|---|---|---|
| ABTS Radical | DPPH Radical | ||
| TILI-2 | CNGVQPK | 16.78 ± 0.48 a | 89.83 ± 2.72 a |
| Ascorbic acid | - | 15.86 ± 0.16 a | 40.09 ± 0.21 b |
| Function | Order | Protein Name | Gene Name | t-Test (p) | Average Log2 Expression Value | |
|---|---|---|---|---|---|---|
| Untreated Cell | TILI-2 Treated Cell | |||||
| TGF-beta pathway | 1 | Mitogen-activated protein kinase 1 | MK01_MOUSE | 0.9510 | 14.99 | 15.01 |
| 2 | Mitogen-activated protein kinase kinase kinase 7-interacting protein 1 | TAB1_MOUSE | 0.9878 | 15.62 | 15.63 | |
| 3 | Mothers against decapentaplegic homolog 5 | SMAD5_MOUSE | 0.7259 | 15.18 | 15.26 | |
| 4 | Transforming growth factor beta-3 | TGFB3_MOUSE | 0.8527 | 14.79 | 14.86 | |
| 5 | GTPase HRas | RASH_MOUSE | 0.3821 | 14.07 | 14.25 | |
| 6 | Mitogen-activated protein kinase 3 | MK03_MOUSE | 0.6866 | 14.79 | 14.61 | |
| 7 | E3 ubiquitin-protein ligase SMURF1 | SMUF1_MOUSE | 0.5889 | 12.76 | 14.39 | |
| 8 | E3 ubiquitin-protein ligase SMURF2 | SMUF2_MOUSE | 0.0265 | 15.33 | 15.58 | |
| 9 | Growth/differentiation factor 5 | ABCBA_MOUSE | 0.3343 | 16.03 | 16.39 | |
| 10 | Bone morphogenetic protein 6 | BMP6_MOUSE | 0.3300 | 15.96 | 16.51 | |
| 11 | Growth/differentiation factor 7 | GDF7_MOUSE | 0.5095 | 15.48 | 13.72 | |
| 12 | Bone morphogenetic protein 4 | BMP4_MOUSE | 0.6398 | 15.86 | 16.00 | |
| 13 | Activin receptor type-2A | AVR2A_MOUSE | 0.6189 | 15.52 | 15.71 | |
| 14 | Apoptotic protease-activating factor 1 | APAF_MOUSE | 0.9578 | 14.13 | 13.99 | |
| 15 | Mitogen-activated protein kinase 12 | MK12_MOUSE | 0.5613 | 13.22 | 13.55 | |
| 16 | Peptidyl-prolyl cis-trans isomerase FKBP1A | FKB1A_MOUSE | 0.1541 | 14.53 | 13.37 | |
| 17 | Transforming growth factor beta-2 | TGFB2_MOUSE | 0.0009 | 15.47 | 16.10 | |
| Melanogenesis | 18 | L-dopachrome tautomerase | TYRP2_MOUSE | 0.1481 | 14.26 | 14.65 |
| 19 | 5,6-dihydroxyindole-2-carboxylic acid oxidase | TYRP1_MOUSE | 0.4880 | 16.09 | 16.28 | |
| 20 | Paired box protein Pax-3 | PAX3_MOUSE | 0.3759 | 13.35 | 11.55 | |
| Gene Name | Primer Sequence (5’→3’) | Annealing Temperature (°C) | Product Size (bp) | Reference Sequence |
|---|---|---|---|---|
| TGF-β3 | F_TCTCTGTCCACTTGCACCAC | 57 | 137 | NM_009368.3 |
| R_TGATAGGGGACGTGGGTCAT | ||||
| BMP4 | F_AGGGATCTTTACCGGCTCCA | 57 | 111 | NM_007554.3 |
| R_ACTCCTCACAGTGTTGGCTC | ||||
| PAX3 | F_ACATCTCAGCCCTATTGTCCC | 57 | 105 | NM_008781.4 |
| R_CGTCCAAGGCTTACTTTGTCC | ||||
| GAPDH | F_CGTCCCGTAGACAAAATGGT | 57 | 110 | NM_008084.3 [62,63] |
| R_TTGATGGCAACAATCTCCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joompang, A.; Anwised, P.; Klaynongsruang, S.; Roytrakul, S.; Taemaitree, L.; Jangpromma, N. Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach. Molecules 2022, 27, 3228. https://doi.org/10.3390/molecules27103228
Joompang A, Anwised P, Klaynongsruang S, Roytrakul S, Taemaitree L, Jangpromma N. Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach. Molecules. 2022; 27(10):3228. https://doi.org/10.3390/molecules27103228
Chicago/Turabian StyleJoompang, Anupong, Preeyanan Anwised, Sompong Klaynongsruang, Sittiruk Roytrakul, Lapatrada Taemaitree, and Nisachon Jangpromma. 2022. "Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach" Molecules 27, no. 10: 3228. https://doi.org/10.3390/molecules27103228
APA StyleJoompang, A., Anwised, P., Klaynongsruang, S., Roytrakul, S., Taemaitree, L., & Jangpromma, N. (2022). Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach. Molecules, 27(10), 3228. https://doi.org/10.3390/molecules27103228






