Abstract
Gastropods comprise approximately 80% of molluscans, of which land snails are used variably as food and traditional medicines due to their high protein content. Moreover, different components from land snails exhibit antimicrobial activities. In this study, we evaluated the antifungal activity of soft tissue extracts from Helix aspersa against Candida albicans, Aspergillus flavus, and Aspergillus brasiliensis by identifying extract components using liquid chromatography-tandem mass spectrometry (LC-MS-MS). Two concentrations of three extracts (methanol, acetone, and acetic acid) showed antifungal activity. Both acetone (1 g/3 mL) and acetic acid extracts (1 g/mL) significantly inhibited C. albicans growth (p = 0.0001, 5.2 ± 0.2 mm and p = 0.02, 69.7 ± 0.6 mm, respectively). A. flavus and A. brasiliensis growth were inhibited by all extracts at 1 g/mL, while inhibition was observed for acetic acid extracts against A. brasiliensis (p = 0.02, 50.3 ± 3.5 mm). The highest growth inhibition was observed for A. flavus using acetic acid and acetone extracts (inhibition zones = 38 ± 1.7 mm and 3.1 ± 0.7 mm, respectively). LC-MS-MS studies on methanol and acetone extracts identified 11-α-acetoxyprogesterone with a parent mass of 372.50800 m/z and 287.43500 m/z for luteolin. Methanol extracts contained hesperidin with a parent mass of 611.25400 m/z, whereas linoleic acid and genistein (parent mass = 280.4 and 271.48900 m/z, respectively) were the main metabolites.
1. Introduction
For hundreds of years, land snails have been used as a food source and for medical treatments. Land snails are pharmacologically and medicinally important [1] as they are high in protein, which they use to combat different environmental conditions [2]. Previously, seven crude proteins were extracted from different snails. The most active crude proteins identified in the land snail, Cryptozona bistrialis, were active against different bacterial and fungal pathogens [3]. Therefore, snails should be considered important bioactive compound sources with safe pharmaceutical applications as polypeptides, proteins, and glycans from snail mucus could function as promising candidates for some dermal diseases [4].
Similarly, protein and peptide components from the hemolymph and mucus of garden snails showed antimicrobial activity [5]. Slime from the African giant land snail Archachatina marginata demonstrated antibacterial activity against Escherichia coli (inhibition zone = 15.2 mm), Klebsiella sp. (inhibition zone = 14.2 mm), Pseudomonas aeruginosa (13.0 mm), and Proteus mirabilis (13.3 mm) [6].
Three protein fractions from the marine snail Rapana venosa were effective against Aspergillus niger, Botrytis cinerea, and Candida albicans [7].
Two protein fractions from the mucus of Helix aspersa (>20 kDa) and a peptide fraction from the hemolymph of Helix lucorum (<10 kDa) were isolated, and their antibacterial activities against E. coli and Brevindomonas diminita were characterized. Their minimum inhibitory concentration values ranged from 145 to 682.5 µg/mL for E. coli and B. diminuta [8]. In hemolymph from H. lucorum, nuclear magnetic resonance metabolic analysis and tandem mass spectrometry (MS-MS) were used to detect metabolites (<1 kDa and <3 kDa) with antioxidant and antimicrobial activities [9]. Ref. [10] isolated a 1485.26 Da peptide (Cm-p1, sequence = SRSELIVHQR) from a crude extract of the marine snail, Cenchritis muricatus, which demonstrated antifungal activity against filamentous fungi and yeast. Moreover, the antifungal activity of a crude methanol extract from Cypraea spp. Against C. albicans and A. niger was similarly demonstrated [11]. In other work, ethanol, acetone, and methanol crude extracts from Babylonia spirata were effective against A. flavus and C. albicans [12].
Fungal infections may occur in the hair, nails, and skin and may cause serious diseases [13]. The total number of individuals who experience different fungal infections is approximately 1,000,000,000. Fungal diseases may kill more than 1.5 million and affect over a billion people. The health consequences of serious fungal infections include asthma, serious chronic illness, blindness and corticosteroid therapies. The modern global estimates have found ~700,000 cases of invasive candidiasis and 3,000,000 cases of chronic pulmonary aspergillosis [14]. C. albicans causes superficial, deep tissue, and invasive candidiasis [15], and its mortality rate is 35–60% due to disseminated candidiasis [16]. Fungal diseases such as aspergillosis cause high mortality rates due to respiratory disorders [17]. Bronchopulmonary aspergillosis caused by A. fumigatus, A. flavus, and A. niger colonies in the lung mucosa spread infection, with a mortality rate of 50–90% [18]. Therefore, antifungal properties from bioactive compounds in invertebrates could provide new directions for medical treatments and scientific research.
A novel neurotoxin (conotoxin TVIIA) was extracted from the sea snail Conus tulipa [19] and was identified as belonging to the conotoxin family, which is composed of six-cysteine/four-loop structures with pharmacological activities. The molecules block ionic calcium, sodium, and potassium channels [20,21]. The contryphan-Vn peptide was extracted from the Mediterranean snail, Conus ventricosus; it contained a D-tryptophan residue and maintained its five-residue intercystine loop. The peptide was bound to potassium channels and displayed distinct molecular targets [22].
In this study, we isolated soft tissue extracts from H. aspersa and identified antifungal activities against C. albicans, A. flavus, and A. brasiliensis. Extract components were identified using liquid chromatography-mass spectrometry (LC-MS-MS).
2. Results
Two concentrations, 1 g/mL and 1 g/3 mL, of three crude extracts (methanol, acetone, and acetic acid extracts) from the soft body (viscera) of H. aspersa were used to test for antifungal effects against C. albicans, A. flavus, and A. brasiliensis.
Individual solvents exerted no effects on fungal growth of C. albicans except for acetic acid (inhibition zone = 26 ± 0.3 mm). All extracts showed antifungal activity when compared with the controls (Figure 1). Methanol and acetone extracts caused insignificant C. albicans growth inhibition (inhibition zone = 20.06 ± 0.1 mm and 59.4 ± 1.4 mm at 1 g/mL). Acetone extracts showed significant inhibition zones against C. albicans (p = 0.0001, 5.2 ± 0.2 mm at 1 g/3 mL). In a concentration-dependent manner, acetic acid generated significant inhibition zones against C. albicans (ANOVA, p = 0.02, 69.7 ± 0.6 mm at 1 g/mL (1:1)) (Figure 2).
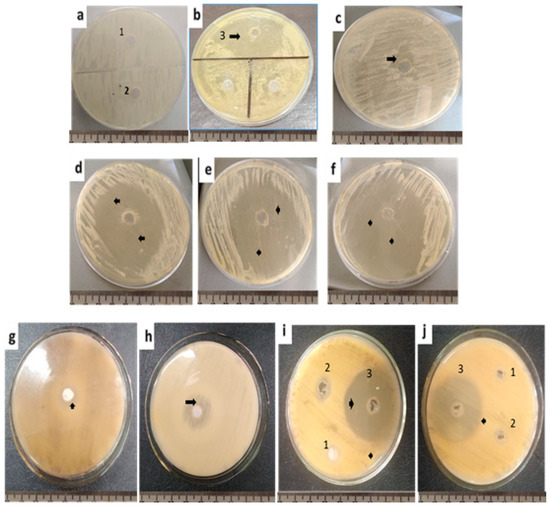
Figure 1.
Antifungal activity of the methanol, acetone and acetic acid viscera extracts of the H. aspersa. (a) C. albicans with methanol (1), acetone (2) and (b) acetic acid (3). (c) C. albicans growth with the antifungal drug, namely fluconazole (25 µg/mL). (d) C. albicans growth with methanolic viscera extract. (e) C. albicans growth with acetone viscera extract. (f) C. albicans growth with acetic acid viscera extract. (g) A. brasiliensis growth with the antifungal drug fluconazole (25 µg/mL). (h) A. flavus growth with the antifungal drug fluconazole (25 µg/mL). (i) A. brasiliensis growth with methanol viscera extract (1), acetone viscera extract (2) and acetic acid viscera extract (3). (j) A. flavus growth with methanol viscera extract (1), acetone viscera extract (2) and acetic acid viscera extract (3). Arrowheads are pointing to the inhibition zone.
Figure 2.
Antifungal activity of viscera (two concentrations) extracted by (methanol, acetone and acetic acid) of the garden snail, H. aspersa, against Candida albicans. The values are expressed asmean ± SD;  indicates a significant difference between tissue extract and concentration;
indicates a significant difference between tissue extract and concentration;  indicates a significant difference between extracting solvents (ANOVA, p ≤ 0.05).
indicates a significant difference between extracting solvents (ANOVA, p ≤ 0.05).
 indicates a significant difference between tissue extract and concentration;
indicates a significant difference between tissue extract and concentration;  indicates a significant difference between extracting solvents (ANOVA, p ≤ 0.05).
indicates a significant difference between extracting solvents (ANOVA, p ≤ 0.05).
The antifungal drug fluconazole (25 µg/mL) inhibited C. albicans growth at 19 ± 0.1 mm (Figure 1).
The antifungal drug fluconazole (25 µg/mL) inhibited A. brasiliensis and A. flavus growth at 17 ± 0.4 mm and 14 ± 0.1 mm, respectively. The solvents had no effects on fungal growth except for acetic acid (inhibition zone = 15 ± 0.1 mm and 10 ± 0.5 mm for A. brasiliensis and A. flavus, respectively) (Figure 1). Both A. flavus and A. brasiliensis growth was inhibited by all extracts at 1 g/mL. Still, inhibition was significant for the acetic acid extract against A. brasiliensis (ANOVA, p = 0.02, 50.3 ± 3.5 mm) (Figure 1 and Figure 3).
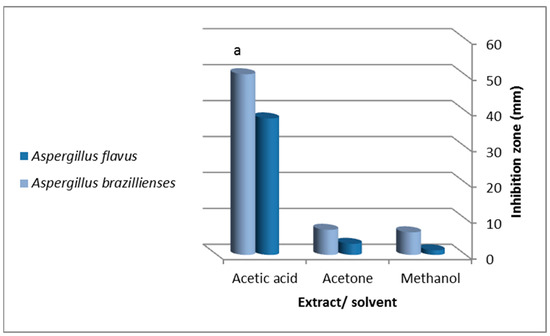
Figure 3.
Antifungal activity of viscera extracted by (methanol, acetone and acetic acid) of the garden snail, Helix aspersa against Aspergillus flavus and Aspergillus brasiliensis. Values are expressed as mean ± SD; “a” indicates a significant difference between extracting solvents (ANOVA, p = 0.02).
The most significant growth inhibition was observed for A. flavus against acetic acid and acetone extracts (inhibition zones = 38 ± 1.7 mm and 3.1 ± 0.7 mm, respectively).
Identified Compounds Using LC-MS-MS Analysis
Plant metabolome mass profiles were screened using the Global Natural Products Social Molecular Networking (GNPS) database (Figure S1, Table 1). Five compounds were identified: genistein, luteolin, and hesperidin are flavonoid compounds, linoleic acid is a fatty acid, and 11-α-acetoxyprogesterone is a steroid compound.

Table 1.
The identified metabolites’ parent masses and fragments from the raw mass spectrum compared with that of the molecular networking database and data published.
3. Discussion
Our data indicated that all extracts from the snail Helix aspersa have bioactive ingredients which exerted antifungal activities against C. albicans, A. flavus, and A. brasiliensis in a concentration-dependent manner. Furthermore, our results were supported by [12], who extracted bioactive compounds from the marine snail, B. spirata. Moreover, the antibacterial activity of crustacean and molluscan methanol extracts was higher when compared with water extracts [3].
The antifungal activity of extracts may have been related to their metabolite components, as demonstrated by LC-MS-MS; the most common component was hesperidin, with a parent mass = 611.25400. This flavanone glycoside induced apoptosis and cell cycle arrest [27,28] and showed antifungal, antiviral, antihelminthic, antioxidant, and molluscicidal activities toward Schistosoma mansoni. Genistein is also present in plants and humans and is an isoflavone with antihelminthic qualities [29]. Genistein was speculated to inhibit fungal growth due to its apoptosis-inducing characteristics [30]. Genistein also induced apoptosis in human cancer cells by triggering both caspase-3 and caspase-9 activity, causing cell death by inhibiting NF-κB signaling and altering levels of the antiapoptotic protein Bcl-2 and proapoptotic protein. In addition, genistein excited oxidative stress-induced apoptosis by increasing nitric oxide production and its bioavailability [31,32,33]. Furthermore, genistein is an estrogenic isoflavone found in the molluscan tissues, and its receptors were isolated from the cerebral ganglia of the gastropod Thais clavigera [34].
The mass of 11-α-acetoxyprogesterone was 372.50800. Progesterone is a steroid hormone that functions as a substantial metabolic intermediate during corticosteroid and sex hormone production and also functions as a neurosteroid [35,36]. The antifungal effects of this compound are facilitated by the presence of high-affinity progesterone-binding sites in the plasma membrane, as confirmed by [37] in Rhizopus nigricans. A corticosteroid-binding protein and steroid receptor were identified by [38] in C. albicans [39] as well as other fungal species. Progesterone is considered an effective fungal growth inhibitor [40]. Its growth inhibition is related to reduced intracellular cyclic adenosine monophosphate(cAMP) levels that are crucial arbiters of fungal growth and responses to nutritional stress [41].
Luteolin is a tetrahydroxyflavone and exerts antifungal effects against C. albicans [42]. The fungal growth inhibition was reported whereby plasma membrane disruption led to membrane permeability changes and excess reactive oxygen species (ROS) production [43]. The ROS effects are mainly directed toward membrane lipids in C. albicans, which generate lipid hydroperoxides and thus lipid peroxidation [44]. The induction of mitochondrial dysfunction via inhibited mitochondrial electron transport chain reduces ATP synthesis and causes cell death by inhibiting cell wall formation, cell division, and RNA and protein synthesis [45].
Linoleic acid is a polyunsaturated essential fatty acid that reduces biomass production in Rhizoctonia solani, Pythium ultimum, Pyrenophora avenae, and Crinipellis perniciosa, while 1000 µM linoleic acid reduces mycelial growth in R. solani, P. ultimum, and P. avenae [46]. Linoleic acid is a structural component related to membrane fluidity functions and directly interacts with fungal cell membranes by entering lipid bilayers, increasing membrane fluidity, disorganizing cell membranes, and causing cell disintegration [47]. Antifungal fatty acids can replace synthetic agrochemicals that control fungal pathogens [48]. The antifungal activity of linoleic acid was reported against Aspergillus amylovorus (NRRL 5813) and A. flavus (NRRL 3518) [49,50]) reported its antifungal activity against C. albicans and Candida parapsilosis [50].
In conclusion, soft tissue extract metabolites from H. aspersa are promising antifungal agents.
4. Materials and Methods
4.1. Sample Collection and Care
Wild H. aspersa were collected from infested ornamental fruit and grass plants in Menoufia governorate, Egypt. Snails were maintained under the following laboratory conditions: 12 h:12 h light/dark photoperiod, room temperature, and relative humidity, 85%. Approximately 10–15 individuals (average weight = 4 g) were placed in glass containers (15 × 15 × 22 cm) filled with moist soil (sandy loam) and covered in muslin for ventilation. Fresh lettuce leaves (Lactuca sativa) and water for soil humidity were supplied daily. Waste food and fecal matter were removed at the end of every other day. Snails were acclimatized under laboratory conditions for at least 4 weeks.
4.2. Tissue Extraction
Snails were rinsed, and the shells removed. The soft body (viscera) was cut into small pieces and homogenized in different solvents, methanol, acetone, and acetic acid [3,12]. Solvent tissue homogenization was performed on ice. Two concentrations of the three solvents (methanol, acetone and acetic acid) were used: 1 and 3 mL for one gram of tissue (viscera). Homogenates were centrifuged at maximum speed in a refrigerated centrifuge, added to clean tubes, and maintained on ice till the inculcation of samples. Crude extracts were used for antifungal assays against the fungal pathogens, C. albicans, A. flavus, and A. brasiliensis.
4.3. Antifungal Assays
Two concentrations (1 g/mL and 1 g/3 mL) of the three crude extracts (methanol, acetone and acetic acid extracts) were prepared to test the growth inhibition of C. albicans, A. flavus, and A. brasiliensis (ATCC 16404) clinical isolates.
Antifungal activity was determined using the agar well diffusion method [51]. Nutrient agar plates were aseptically spread with 24 h cultures from respective pathogens and incubated for 15 min in a laminar chamber to facilitate absorption. Next, 5 mm wells were aseptically cut in the agar for visceral extract addition—100 µL of each extract was added, and plates were left for 1 h for infusion. Then, plates were incubated at 37 °C for 24 h, after which extract inhibition zone diameters were measured in mm. Fluconazole (25 µg/mL) was used as a positive control. All tests were performed in triplicate, and mean values were recorded for statistical analysis.
4.4. Chemical Analysis of Extracts
Methanol and acetone extracts were analyzed using LC-MS-MS. A Shimadzu LC-10 high-performance liquid chromatography instrument with a Grace Vydac Everest Narrowbore C18 column (100 mm × 2.1 mm i.d., 5 µm, 300 Å) was connected to an LCQ electrospray ion trap MS (Thermo Finnigan, San Jose, CA, USA). Raw data files were converted to mzXML format using MSConvert from the ProteoWizard suite (http://proteowizard.sourceforge.net/tools.shtml, (accessed on 1 March 2022).). A molecular network was created using the GNPS online workflow. Spectra in the network were then searched against GNPS spectral libraries and published data [52].
4.5. Statistical Analysis
Data were expressed as the mean ± standard deviation and analyzed using Statgraphics Centurion XVI (Stat-Point Technologies Inc., Warrenton, VA, USA). Statistical analysis was conducted using a two-way analysis of variance to identify differences between tissue extracts and solvents and tissue extracts and concentrations. A probability p ≤ 0.05 level was accepted as significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27103170/s1, Figure S1: The identified metabolites’ parent masses and fragments of the methanol and acetone viscera extracts of the Helix aspersa from the raw mass spectrum; (a) genistein; (b) luteolin; (c) linoleic acid; (d) 11-alpha-acetoxyprogesterone and (e) hesperidin
Author Contributions
Conceptualization, H.H.A.-E.A.; methodology, H.H.A.-E.A.; writing—original draft preparation, H.H.A.-E.A. and H.R.E.-S.; writing—review and editing, G.Y.O., S.A.M.K., M.M.G. and A.M.F.; funding acquisition, A.M.F. and H.R.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
Taif University research supporting Project number (TURSP-2020/46), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to Taif University research supporting Project number (TURSP-2020/46), Taif University, Taif, Saudi Arabia for financial support. Authors are grateful to Mohamed El Sayied, Demonstrator and Esraa Mohamed, Assistant lecturer (Botany and Microbiology Department, Faculty of Science, Menoufia University) for providing help regarding the microbial experiments. Hesham El-Seedi thanks International Research Center for Food Nutrition and Safety, Jiangsu University, Zhenjiang, 212013, China and International Joint Research Laboratory of Intelligent Agriculture and Agri-products Processing (Jiangsu University), Jiangsu Education Department, China for Adjunct Professor fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bonnemain, B. Helix and Drugs: Snails for Western Health Care from Antiquity to the Present. Evid. Based Complement. Altern. Med. 2005, 2, 583043. [Google Scholar] [CrossRef] [PubMed]
- González, Y.; Tanaka, A.S.; Hirata, I.Y.; del Rivero, M.A.; Oliva, M.L.; Araujo, M.S.; Chávez, M.A. Purification and partial characterization of human neutrophil elastase inhibitors from the marine snail Cenchritis muricatus (Mollusca). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Ulagesan, S.; Kim, H.J. Antibacterial and Antifungal Activities of Proteins Extracted from Seven Different Snails. Appl. Sci. 2018, 8, 1362. [Google Scholar] [CrossRef]
- Dhiman, V.; Pant, D. Human health and snails. J. Immunoass. Immunochem. 2021, 42, 211–235. [Google Scholar] [CrossRef]
- Gentili, V.; Bortolotti, D.; Benedusi, M.; Alogna, A.; Fantinati, A.; Guiotto, A.; Turrin, G.; Cervellati, C.; Trapella, C.; Rizzo, R.; et al. HelixComplex snail mucus as a potential technology against O3 induced skin damage Valentina. PLoS ONE 2020, 15, e0229613. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Ali, M.; Muazu, L.; Abdallah, M.S.; Zungum, I.U. Assessment of antibacterial activity of snail Archachatina marginata slime on some clinical wound isolate. Innov. Int. J. Med. Pharm. Sci. 2021, 6, 8–11. [Google Scholar]
- Krumova, E.; Dolashka, P.; Abrashev, R.; Velkova, L.; Dolashki, A.; Daskalova, A.; Dishliyska, V.; Atanasov, V.; Kaynarov, D.; Angelova, M. Antifungal activity of separated fractions from the hemolymph of marine snail Rapana venosa. Bulg. Chem. Commun. 2021, 53, 42–48. [Google Scholar] [CrossRef]
- Aleksova, M.; Velkova, L.P.; Dolashka, P.; Radeva, G. Antibacterial activity of bioactive fractions from mucus and hemolymph of different snails species and crab. Bulg. Chem. Commun. 2021, 53, 22–26. [Google Scholar] [CrossRef]
- Vassilev, N.; Simova, S.; Dangalov, M.; Velkova, L.; Atanassov, V.; Dolashki, A.; Dolashka, P. A 1H NMR based study of metabolites profiling of garden snail Helix lucorum hemolymph. Bulg. Chem. Commun. 2021, 53, 49–56. [Google Scholar] [CrossRef]
- López-Abarrategui, C.; Alba, A.; Silva, O.N.; Reyes-Acosta, O.; Vasconcelos, I.M.; Oliveira, J.T.; Migliolo, L.; Costa, M.P.; Costa, C.R.; Silva, M.R.; et al. Functional characterization of a synthetic hydrophilic antifungal peptide derived from the marine snail Cenchritis muricatus. Biochimie 2012, 94, 968–974. [Google Scholar] [CrossRef]
- Subavathy, P.; Thilaga, R.D.; Shibana, C. Screening of marine gastropod cypraea arabica for antibacterial and antifungal activity. Int. J. Curr. Res. 2015, 7, 19995–20001. [Google Scholar]
- Periyasamy, N.; Srinivasan, M.; Balakrishnan, S. Antimicrobial activities of the tissue extracts of Babylonia spirata Linnaeus, 1758 (Mollusca: Gastropoda) from Thazhanguda, southeast coast of India. Asian Pac. J. Trop. Biomed. 2012, 2, 36–40. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dylazg, A. A global view on fungal infections in humans and animals: Infections caused by dimorphic fungi and dermatophytoses. J. Appl. Microbiol. 2021, 131, 2688–2704. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Antifungal activity of selected Indian medicinal plant salts. J. Glob. Pharma Technol. 2010, 2, 71–74. [Google Scholar]
- Saranya, S.; Moorthy, K.; Malar, S.S.A.S.; Punitha, T.; Vinodhini, R.; Bhuvaneshwari, M.; Kanimozhi, C. Prevalence and antifungal susceptibility pattern of Candida albicans from low socio-economic group. Int. J. Pharm. Pharm. Sci. 2014, 6, 158–162. [Google Scholar]
- Casadevall, A. Fungal Diseases in the 21st Century: The Near and Far Horizons. Pathog. Immun. 2018, 3, 183–196. [Google Scholar] [CrossRef]
- Lin, S.; Schranz, J.; Teutsch, S.M. Aspergillosis Case-Fatality Rate: Systematic Review of the Literature. Clin. Infect. Dis. 2001, 32, 358–366. [Google Scholar] [CrossRef]
- Hill, J.M.; Atkins, A.R.; Loughnan, M.L.; Jones, A.; Adams, D.A.; Martin, R.C.; Lewis, R.J.; Craik, D.J.; Alewood, P.F. Conotoxin TVIIA, a novel peptide from the venom of Conus tulipa. Eur. J. Biochem. 2000, 267, 4642–4648. [Google Scholar] [CrossRef]
- Myers, R.A.; Cruz, L.J.; Rivier, J.E.; Olivera, B.M. Conus peptides as chemical probes for receptors and ion channels. Chem. Rev. 1993, 93, 1923–1936. [Google Scholar] [CrossRef]
- Terlau, H.; Shon, K.J.; Grilley, M.; Stocker, M.; StuÈhmer, W.; Olivera, B. Strategy for rapid immobilization of prey by a fish-hunting marine snail. Nature 1996, 381, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Massilia, G.R.; Schininà, M.; Ascenzi, P.; Polticelli, F. Contryphan-Vn: A Novel Peptide from the Venom of the Mediterranean Snail Conus ventricosus. Biochem. Biophys. Res. Commun. 2001, 288, 908–913. [Google Scholar] [CrossRef]
- Hussain, F.; Jahan, N.; Rahman, K.-U.; Sultana, B.; Jamil, S. Identification of Hypotensive Biofunctional Compounds of Coriandrum sativum and Evaluation of Their Angiotensin-Converting Enzyme (ACE) Inhibition Potential. Oxidative Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). Biol. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Vanhaecke, L.; Bussche, J.V.; Wille, K.; Bekaert, K.; De Brabander, H.F. Ultra-high performance liquid chromatography–tandem mass spectrometry in high-throughput confirmation and quantification of 34 anabolic steroids in bovine muscle. Anal. Chim. Acta 2011, 700, 70–77. [Google Scholar] [CrossRef]
- Fang, T.; Wang, Y.; Ma, Y.; Su, W.; Bai, Y.; Zhao, P. A rapid LC/MS/MS quantitation assay for naringin and its two metabolites in rats plasma. J. Pharm. Biomed. Anal. 2006, 40, 454–459. [Google Scholar] [CrossRef]
- Allam, G.; Abuelsaad, A.S.A. In vitro and in vivo effects of hesperidin treatment on adult worms of Schistosoma mansoni. J. Helminthol. 2014, 88, 362–370. [Google Scholar] [CrossRef]
- Lahlou, M. Study of the molluscicidal activity of some phenolic compounds: Structure-activity relationship. Pharm. Biol. 2004, 42, 258–261. [Google Scholar] [CrossRef]
- Fedoreyev, S.; Pokushalova, T.; Veselova, M.; Glebko, L.; Kulesh, N.; Muzarok, T.; Seletskaya, L.; Bulgakov, V.; Zhuravlev, Y. Isoflavonoid production by callus cultures of Maackia amurensis. Fitoterapia 2000, 71, 365–372. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.-X.; Zhou, Z.-Q.; Wang, Z.; Zhang, Y.-G.; Zhang, Y.; Zhao, P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumor Biol. 2014, 35, 11483–11488. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.-Y.; Xia, X.; Che, L.; Song, Y.-T. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of Nrf2 expression in ovariectomized rats. Neurol. Res. 2018, 40, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, D.; Marimuthu, P.; Hörmann, V.; Kumi-Diaka, J.; Rathinavelu, A. Induction of apoptosis in HeLa cells via caspase activation by resveratrol and genistein. J. Med. Food 2013, 16, 139–146. [Google Scholar] [CrossRef]
- Kajiwara, M.; Kuraku, S.; Kurokawa, T.; Kato, K.; Toda, S.; Hirose, H.; Takahashi, S.; Shibata, Y.; Iguchi, T.; Matsumoto, T.; et al. Tissue preferential expression of estrogen receptor gene in the marine snail, Thais clavigera. Gen. Comp. Endocrinol. 2006, 148, 315–326. [Google Scholar] [CrossRef]
- Baulieu, E.; Schumacher, M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Hum. Reprod. 2000, 15, 1–13. [Google Scholar] [CrossRef][Green Version]
- Brucker, M.C.; King, T.L. Pharmacology for Women’s Health; Issue Jones & Bartlett Publishers: Burlington, MA, USA, 2017. [Google Scholar]
- Lenasi, H.; Bavec, A.; Zorko, M. Membrane-bound progesterone receptors coupled to G proteins in the fungus Rhizopus nigricans. FEMS Microbiol. Lett. 2002, 213, 97–101. [Google Scholar] [CrossRef][Green Version]
- Loose, D.S.; Schurman, D.J.; Feldman, D. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature 1981, 293, 477–479. [Google Scholar] [CrossRef]
- Feldman, D.; Toktst, L.G.; Stathis, P.A.; Miller, S.C.; Kurzt, W.; Harveyt, D. Identification of 17fi-estradiol as the estrogenic substance in Saccharomyces cerevisiae (yeast/steroid hormone/estrogen receptor). Biochemistry 1984, 81, 4722–4726. [Google Scholar]
- Clemons, K.V.; Schär, G.; Stover, E.P.; Stevens, D.A.F. Dermatophyte-hormone relationships: Characterization of progesterone-binding specificity and growth inhibition in the genera Trichophyton and Microsporum. J. Clin. Microbiol. 1988, 26, 2110–2115. [Google Scholar] [CrossRef]
- Jeraj, N.; Lenasi, H.; Breskvar, K. The involvement of cAMP in the growth inhibition of filamentous fungus Rhizopus nigricans by steroids. FEMS Microbiol. Lett. 2005, 242, 147–154. [Google Scholar] [CrossRef][Green Version]
- Alves, C.T.; Ferreira, I.; Barros, L.; Sónia Silva, S.; Azeredo, J.; Henriques, M.; Azeredo, J.; Henriques, M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Res. 2011, 11, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. J. Mycol. Med. 2017, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against Plant Pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef]
- Avis, T.J.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Juárez, Z.N.; Hernández, L.R.; Bach, H.; Sánchez-Arreola, E.; Bach, H. Antifungal activity of essential oils extracted from Agastache mexicana ssp. xolocotziana and Porophyllum linaria against post-harvest pathogens. Ind. Crops Prod. J. 2015, 74, 178–182. [Google Scholar] [CrossRef]
- Erdogan Eliuz, E.A.; Yabalak, E.; Gökşen, G.; Ayas, D. Chemical composition, antifungal activity, antifungal mechanism and interaction manner of the fatty acid of Prunus mahaleb L. with fluconazole. Int. J. Environ. Health Res. 2021, 31, 1–13. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 3, 1066–1069. [Google Scholar] [CrossRef]
- Elrasoul, A.S.A.; Mousa, A.A.; Orabi, S.H.; Mohamed, M.A.E.-G.; Gad-Allah, S.M.; Almeer, R.; Abdel-Daim, M.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Eldaim, M.A.A. Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Azolla pinnata Ethanolic Extract against Lead-Induced Hepatotoxicity in Rats. Antioxidants 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).