Abstract
Nitrostilbenes characterized by two different or differently substituted aryl moieties can be obtained from the initial ring-opening of 3-nitrobenzo[b]thiophene with amines. Such versatile building blocks couple the well-recognized double electrophilic reactivity of the nitrovinyl moiety (addition to the double bond, followed by, e.g., intramolecular replacement of the nitro group) with the possibility to exploit a conjugated system of double bonds within an electrocyclization process. Herein, nitrostilbenes are reacted with different aromatic enols provided by a double (carbon and oxygen) nucleophilicity, leading to novel, interesting naphthodihydrofurans. From these, as a viable application, aromatization and electrocyclization lead in turn to valuable polycondensed, fully aromatic O-heterocycles.
1. Introduction
Benzo- and naphtho-fused furans and dihydrofurans are structural motifs abundantly distributed in natural products characterized by a wide spectrum of different biological activities [1]. For instance, Furomollugin and Rubicordifolin, isolated from the roots of Rubia Cordifolia, show activity against the hepatitis B virus and cytotoxicity against some tumoral cell lines [2]; Balsaminone A, isolated from the fruit of Impatiens balsamina L., is used for the treatment of articular rheumatism, bruises, and beriberi in Chinese traditional medicine [3], while Xylarianaphthol-1, isolated from a marine sponge-derived fungus of order Xylariales, is a p21 activator promoter and contributes to cancer prevention and treatment [4] (Figure 1).
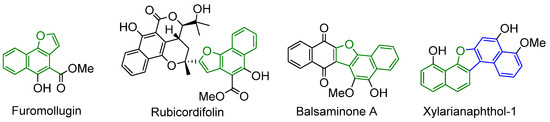
Figure 1.
Representative examples of active natural compounds characterized by fused furans.
Although in nature naphtho[1,2-b]furans and 2,3-dihydronaphtho[1,2-b]furans, such as those in Figure 1, are more common, in synthetic pharmacologically-active compounds, naphtho- and 1,2-dihydronaphtho[2,1-b]furan derivatives are more diffuse and exhibit a broad spectrum of different biological properties, such as anticancer [5,6]; anti-inflammatories [7] or antitubercular agents [8]; regulators of nuclear receptor HNF4-α [9]; and inhibitors of α-chymotrypsin, lipoxygenase, and C17,20 lyase [10,11,12] (Figure 2).
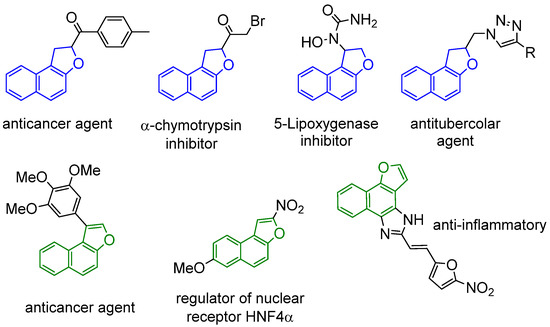
Figure 2.
Examples of pharmacologically relevant naphthofurans.
Furthermore, the importance of these scaffolds is also due to the optical properties of some particular compounds, such as, for instance, vinylidene-naphthofurans, which show photochromism at room temperature in the solid state when adsorbed on silica gel or exposed to UV or sunlight [13], or NDFs (naphthodifurans) and ADFs (anthradifurans), which have been studied for their applications as hole-transporting materials and photosensitizers [14] (Figure 3).
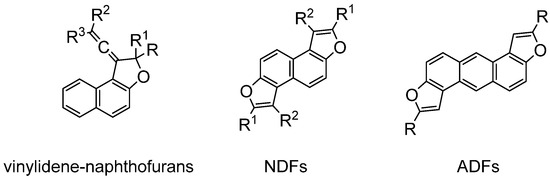
Figure 3.
Optically interesting naphthofurans.
Due to their wide range of applications, many synthetic methods for the preparation of naphtho- and dihydronaphthofuran derivatives have been reported (Scheme 1)—the most common involving either the cyclization of a properly functionalized naphthol [15,16,17] or the two-step intermolecular reaction of a naphthol with a suitable reagent, followed by cyclization to give the final heterocyclic system. More often, basic conditions are employed to improve the reactivity of naphthol as double nucleophile, as, for instance, in the classical approach of the annulation of 2-naphthol with dihalo compounds [18]. However, the use of p-TSA [19,20] or of metal catalysis [21,22,23,24,25,26,27,28] has also been pursued.
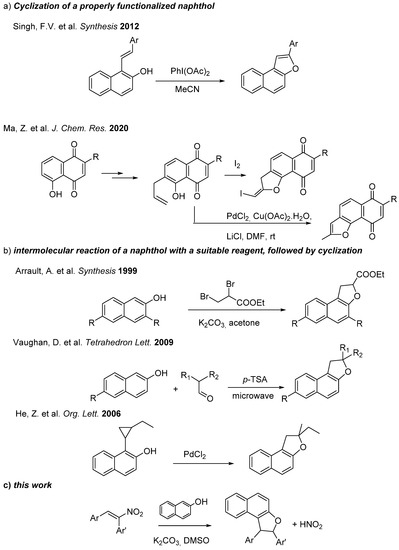
Scheme 1.
Literature-reported methods [15,17,18,19,21] (a), (b) and the approach of the present work (c).
However, since most of the protocols reported in the literature [29,30] suffer from some limitations, such as, for instance, the substrate scope and the possibility to obtain differently functionalized products, the development of more versatile methods is still a topic of interest. In this context, nitrostyrenes have been considered as useful annulation reagents in the reaction with naphthols, because of their ability to react as double electrophiles with the bidentate naphthol enolate, generated in situ under basic conditions. Particular functionalization of the nitro-compound, as, for example, in Morita-Baylis-Hillman [31], together with different reaction conditions, could lead to naphthodihydrofuran or to naphthofuran derivatives, in this case, occasionally with the help of an increase of temperature.
As a matter of fact, starting from a pioneering report [32], during our previous studies, while exploring the reactivity of different nucleophiles towards nitrobutadienes generated by an initial ring-opening of nitrothiophenes [33,34,35], we obtained a great variety of nitrogen-containing heterocycles, such as methyleneazetidines [36], pyrroles [37], pyrazoles [38], carbazoles [39], imidazo[1,2-a]pyridines [40], and nitroindoles [41]. However, the involvement of nucleophiles able to generate O-heterocycles, as, for instance, enols, has not been investigated so far. In order to fill the gap and to obtain furan-fused derivatives, the reaction between nitrobutadienic building blocks and aromatic enolates has been now thoroughly investigated and relevant results are reported hereinafter.
2. Results
Within this context, we considered of particular interest the hypothesizable outcome of the double nucleophilic attack of β-naphthol on 4a (Ar = p-tol). This particular substrate actually is not exactly a nitrobutadiene, but rather a nitrostilbene, deriving from the initial ring-opening of 3-nitrobenzo[b]thiophene with pyrrolidine [42] (Scheme 2). From the ring-opening product 2, sulfane 3a (Ar = p-tol) originates by reaction with p-tolyl magnesium bromide; the following oxidation at sulfur with MCPBA produces sulfone 4a. Therefore, the nitrobutadienes 3a and 4a retain the reactivity of the nitrovinyl moiety, gaining at the same time stability, thanks to the inclusion of the conjugated double bond within a benzene ring.
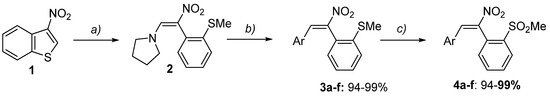
Scheme 2.
Synthetic sequence to prepare sulfanes 3a–f and sulfones 4a–f: (a) Pyrrolidine (2 mol eq.)/AgNO3 (2 mol. eq.), abs. EtOH, rt, overnight; then MeI (excess), 0 °C to rt, 2h; (b) ArMgX or ArLi (1.1 mol eq.), THF, −78 °C, 15–45 min, then acidic quenching; (c) MCPBA (2 mol eq.), DCM, rt.
Quite rewardingly, a TLC analysis of the reaction mixture obtained after 18 h in the conditions of Scheme 2 showed the absence of the substrate and the presence of a single product as a white solid. Spectroscopic analysis (1H and 13C NMR, MS) revealed the structure of 5a (Scheme 3, Ar = p-tol), isolated in excellent yields as a single diastereoisomer, without need of purification by column chromatography.
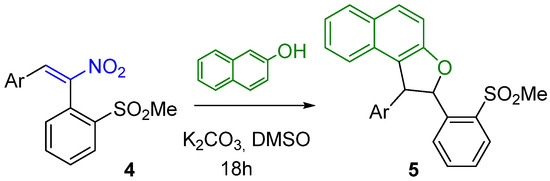
Scheme 3.
Reaction conditions to obtain naphthodihydrofurans 5a–f.
We thus considered it worthwhile to investigate the scope of this reaction as to the Ar moiety, introducing groups with different electronic or steric features. Sulfanes 3b–f and sulfones 4b–f were prepared according to Scheme 1, and the latter were initially reacted with β-naphthol (Scheme 3). The obtained results, reported in Table 1, show a generally very good behaviour of 4, whatever the aryl substituent. In each case, the obtained diastereoisomer revealed at the 1HNMR analysis a J of about 5–7 Hz for the dihydrofuran moiety, corresponding presumably to the anti-configuration, as expected on the grounds of a process under thermodynamic control. In order to ascertain without any doubt the configuration of the isolated diastereoisomer, the structure of compound 5a was confirmed by X-ray analysis (Figure 4).

Table 1.
Results obtained in the reactions of sulfones 4a–f with 2-naphthol/K2CO3 in DMSO at rt.
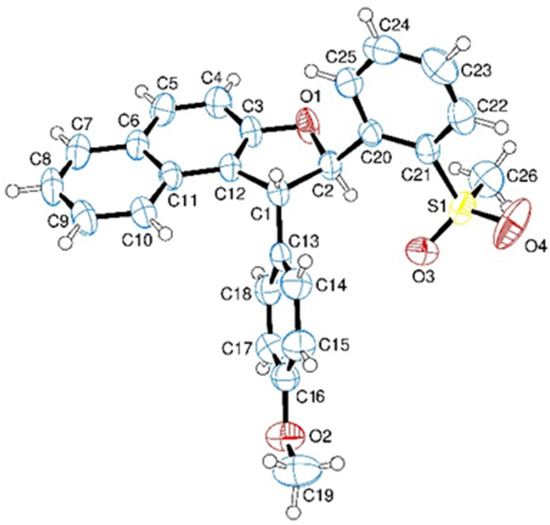
Figure 4.
ORTEP obtained by X-Ray analysis of compound 5a.
Having these (2,3)-diaryl substituted naphthodihydrofurans in hand, we thought it could be of interest to attempt their oxidative aromatization to the corresponding naphthofurans, with the aim of extending the conjugated system, in search of better optical properties. The aromatization to 6a–f proceeded very easily with 3 mol eq. of DDQ in toluene at 50 °C, and furnished generally, after chromatographic separation, rather satisfying yields (Scheme 4).
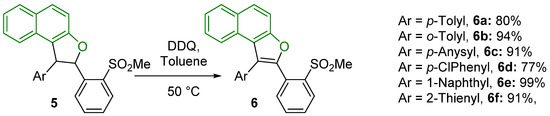
Scheme 4.
Conditions employed for the oxidative aromatization of 5a–f to 6a–f. Yields are given in chromatographically pure compounds (1 spot).
As hoped, after the extension of the conjugated system, we actually observed the appearance of optical properties for the aromatized compounds. The model 6a revealed a good fluorescence when irradiated under the UV lamp (Figure 5), which encourages a deeper study in the future of the fluorescence properties of these new naphthofuran derivatives.
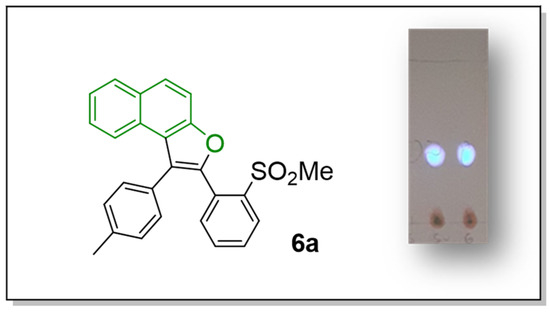
Figure 5.
Fluorescence of compound 6a.
Encouraged to proceed in our study, we changed the aromatic enol moving to a quinoline derivative, so as to introduce a further heteroatom in the system. 8-Hydroxyquinoline was our next choice as promising reagent to obtain angular dihydrofuro- and furo[3,2-h]quinolines, a kind of fusion not so often reported in the literature. Under the same conditions employed for 2-naphthol, the reactions proceeded faster, but yields in the desired product were generally lower (Scheme 5; cf. yields in Table 1), due to the presence in the final mixture of minor components that were necessary to remove by chromatographic purification.
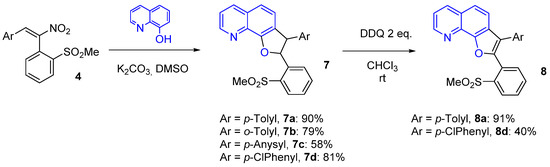
When trying to aromatize the obtained dihydrofuroquinoline 7a according to the conditions reported in Scheme 4, the reaction proceeded quickly, but a TLC analysis of the final reaction mixture revealed an extensive decomposition. Such failure could probably be attributed to the presence of the oxidizable aza group. A search for adequate conditions compatible with the presence of the N-heterocycle brought us to use only 2 mol eq. of the same oxidant DDQ, in a different solvent (CHCl3), at room temperature; the desired furoquinoline was thus obtained within 24 h after chromatography in 91% yields. These successful conditions were then applied to dihydrofuroquinoline 7d, furnishing in this case only a lower yield of 8d (Scheme 5).
It is interesting to note that both dihydrofuroquinoline 7a and furoquinoline 8a show fluorescent properties under UV light, at a different wavelength compared to the naphthofuran derivative 6a (Figure 6). We intend to develop the study of fluorescence for derivatives 7 and 8 in the future.
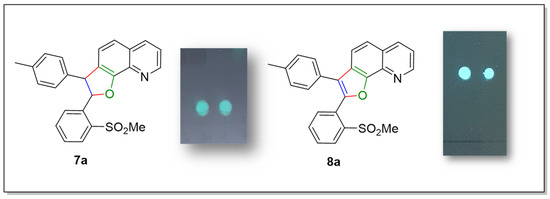
Figure 6.
Fluorescence of compounds 7a and 8a.
In order to obtain a different polyheterocyclic system, we turned our attention to another phenolic nucleophile, e.g., 4-hydroxycoumarin, whose behaviour was studied with the model nitrostilbene 4a. Under the usual reaction conditions (K2CO3 in DMSO) the process proved to be very slow, and the desired product was not present in the final reaction mixture, probably due to the prevalence of alternative pathways, such as, for instance, the hydrolysis of the coumarin ring. Searching for a suitable system, we performed the reaction in the presence of an organic base (DABCO) in a less polar solvent (CHCl3), observing in this case the dihydro-4H-furochromenone derivative 9a in a complex reaction mixture, containing also unreacted starting material (13%); the reaction is still under investigation in order to optimize the conditions and effectively improve the yield (Scheme 6).
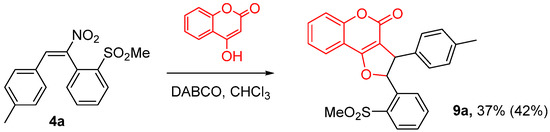
Scheme 6.
Reaction of compound 4a with 4-hydroxycoumarin (42% yield on the reacted substrate).
As a further evolution for naphthofurans 6a–f and furoquinolines 8a,d we devised an electrocyclization process in order to increase the complexity of our polyheterocycles systems. To undergo the pericyclic reaction, the 6π-conjugated system includes three “aromatic” double bonds, e.g., that of the furan ring, and those of the 3-aryl and the 2-(2-methylsulfonyl)phenyl substituents. Due to the presence of two non-equivalent ortho positions in the sulfonylated ring, two different electrocyclization products could in principle be expected (10a and 10′a) (Scheme 7), from which two aromatized systems (11a and 11′a) could in turn originate, via methanesulfinic acid elimination or rather by dehydrogenation, respectively. Both processes seemed to be of potential interest, so we proceeded to find the adequate conditions.
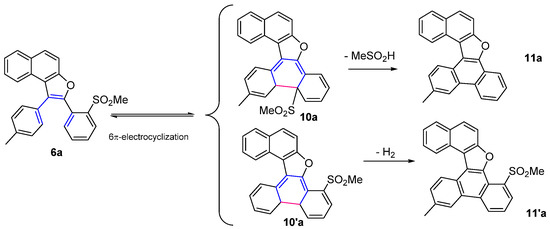
Scheme 7.
Hypothesizable electrocyclized products 10a and 10′a from 6a, and their following evolution to aromatized 11a and 11′a.
A thermic approach on the model substrate 6a, treated in xylene at reflux, after 24 h, revealed by TLC that only starting material was present; evidently, the attainable temperature (140 °C) was not sufficient to induce the pericyclic process. We thus turned to the alternative photoinduction and, on the grounds of our previous experience and of literature data, we dissolved 6a in acetone and introduced the sample in a Rayonet reactor (λ = 300 nm): under these conditions, the pericyclic process went straight on, and, thanks to MeSO2H elimination, the aromatized polyheterocycle 11a was directly generated in satisfactory yield (63%) within the same (24 h) period of time (Scheme 8).
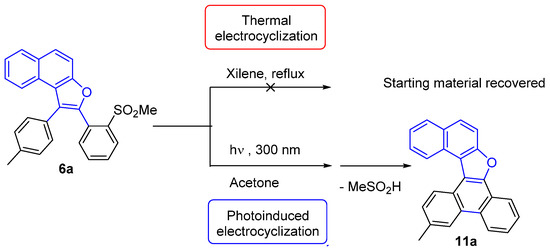
Scheme 8.
First attempts to obtain electrocyclization products from 6a by a thermally or a photochemically induced reaction.
The photoinduced process was then extended to the whole 6b–f series, obtaining in each case (but for 6b) good yields in the aromatized products 11c–f (Figure 7), without isolation of the alternative polycycles 11′c–f or of the corresponding cyclohexadiene intermediates 10 or 10′.
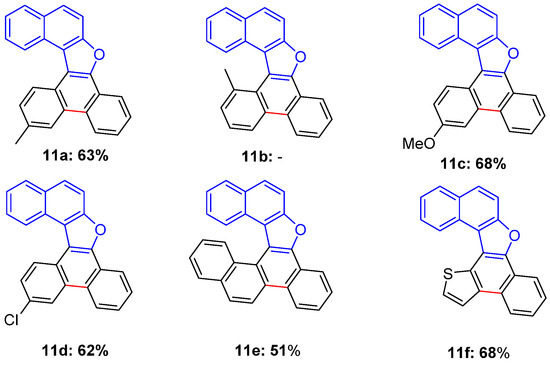
Figure 7.
Products obtained from the photochemically induced electrocyclization of compounds 6a, c–f, followed by aromatization, thanks to MeSO2H elimination. Yields refer to chromatographically purified products (1 spot in TLC).
In order to test the role of the methylsulfonyl group of 6 in the electrocyclization, we prepared the corresponding sulfane derivative 13a by applying to 3a the procedure of Scheme 3 and aromatizing the resulting 12a. Interestingly enough, when we applied the photoinduced process to 13a, the product isolated was identical to that isolated from 6a, e.g., the full aromatic polycycle 11a (Scheme 9).
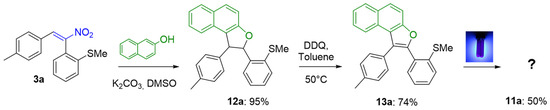
Scheme 9.
Applying the described sequence to sulfane 3a.
3. Materials and Methods
1H NMR and 13C NMR spectra were recorded with a JEOL JNM-ECZR 400 spectrometer, at 400 and 100 MHz, respectively; chemical shifts (TMS as internal reference) are reported as δ values (ppm). Signals are designated as follows: s, singlet; d, doublet; dd, doublet of doublets; ddd, doublet of doublet of doublets; t, triplet; tt, triplet of triplets; m, multiplet; and br, broad. Gas chromatography–mass spectrometry (GC-MS) was performed on HP 5890/5971 (EI 70 eV) system equipped with a HP-1 MS capillary column (12 m × 0.2 mm i.d × 0.33 μm). High-resolution mass spectra (HRMS) were obtained with an Agilent MSD TOF mass spectrometer and recorded in positive ion mode with an electrospray (ESI) source. IR spectra were recorded on a Perkin Elmer Spectrum 65 FT-IR and wave numbers are reported in cm−1. Melting points were determined with a Büchi 535 apparatus and are uncorrected. Petroleum ether and light petroleum refer to the fractions with bp 40–60 °C and 80–100 °C, respectively. Silica gel 230–400 mesh or neutral alluminium oxide Acros Organics (50–200 μm), were used for column chromatography. TLC analysis were performed on commercially prepared 60 F254 silica gel plates and visualized by UV irradiation (eluant: petroleum ether—ethyl acetate). All commercially available reagents were used as received. The 3-nitrobenzothiophene 1 [43], (E)-1-(2-(2-(methylthio)phenyl)-2-nitrovinyl)pyrrolidine 2 [42], nitrovinyl derivatives 3a, b, d–f and 4a, b, d–f were obtained usually in high yields, as already reported [44]. Compounds 3c and 4c were prepared following the same procedure.
(E)-(2-(2-(4-Methoxyphenyl)-1-nitrovinyl)phenyl)(methyl)sulfane (3c)
Yellow solid. M.p. 87.3–88.4 °C (taken-up with petroleum ether/DCM). 1H NMR (400 MHz, CDCl3) δ 2.41 (s, 3H), 3.78 (s, 3H), 6.76 (AA’ of AA’BB’, J = 8.0 Hz, 2H), 7.05 (BB’ of AA’BB’, J = 8.0 Hz, 2H), 7.21 (app dd, 1H), 7.27 (app td, 1H), 7.42 (app dd, 1H), 7.51 (app td, 1H), 8.30 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 15.98 (CH3), 55.58 (OCH3), 114.57 (Ar CH), 114.89 (Ar CH), 116.11 (Ar CH), 123.59 (Ar C), 126.01 (Ar CH), 126.71 (Ar CH), 130.31 (Ar C), 130.85 (Ar CH), 131.27 (Ar CH), 133.23(Ar CH), 136.49 (Ar CH), 140.28 (Ar C), 162.21 (Ar C-OMe). HRMS (ESI) m/z calculated [M + H]+ C16H16NO3S+ 302.0846, found 302.0932.
(E)-1-(2-(4-Methoxyphenyl)-1-nitrovinyl)-2-(methylsulfonyl)benzene (4c)
Yellow solid. M.p. 142.3–143.5 °C (ethanol). 1H NMR (400 MHz, CDCl3) δ 2.96 (s, 3H), 3.78 (s, 3H), 6.75 (AA’ of AA’BB’, J = 8.0 Hz, 2H), 6.95 (BB’ of AA’BB’, J = 8.0 Hz, 2H), 737–7.42 (m, 1H), 7.72–7.80 (m, 2H), 8.21–8.25 (m, 1H), 8.35 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 44.08, 55.63, 114.88, 123.16, 130.95, 131.06, 131.52, 133.63, 133.66, 134.91, 136.40, 140.51, 144.45, 162.37. HRMS (ESI) m/z calculated [M + H]+ C16H16NO5S+ 334.0744, found 334.0623.
3.1. General Procedure for the Reactions of Substrates 4 with β-Naphthol
In a flask, the relevant nitrostilbene 4 (0.30 mmol) was dissolved in DMSO (2.0 mL), then 2-naphthol (0.30 mmol, 43.4 mg) and K2CO3 (0.38 mmol, 53 mg) were added as solids under magnetic stirring. The reaction was stirred at room temperature and monitored by TLC. Upon completion (24 h), the mixture was diluted with 20 mL of ethyl acetate, washed with HCl 1M (2 × 5 mL), NaHCO3 (2 × 5 mL), water (1 × 5 mL) and brine (1 × 5 mL). The organic phase was dried with anhydrous Na2SO4, and the solvent evaporated, to give the products 5a–f, with no need of further purification.
2-(2-(Methylsulfonyl)phenyl)-1-(p-tolyl)-1,2-dihydronaphtho[2,1-b]furan (5a)
White solid. M.p. 171.2–173.0 °C (taken-up with petroleum ether/DCM). 1H NMR (400 MHz, CDCl3) δ 2.29 (s, 3H), 2.85 (s, 3H), 5.03 (d, J = 5.9 Hz, 1H), 6.66 (d, J = 5.9 Hz, 1H), 7.11 (q, J = 7.9 Hz, 4H), 7.23–7.34 (m, 5H), 7.49–7.56 (m, 1H), 7.57–7.64 (m, 1H), 7.64–7.70 (m, 1H), 7.79–7.87 (m, 2H), 8.10 (d, J = 7.9 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 21.28, 45.48, 57.52, 88.07, 112.07, 120.84, 122.97, 123.34, 127.05, 128.10, 128.73, 129.00, 129.22, 129.53, 129.75, 130.27, 130.63, 130.76, 134.52, 137.18, 138.40, 139.14, 141.19, 157.39. HRMS (ESI) m/z calculated [M + H]+ C26H23O3S+ 415.1368, found 415.1380.
2-(2-(Methylsulfonyl)phenyl)-1-(o-tolyl)-1,2-dihydronaphtho[2,1-b]furan (5b)
White solid. M.p. 224.0–226.4 °C (taken-up with petroleum ether/DCM). 1H NMR (400 MHz, CDCl3) δ 2.20 (s, 3H), 2.84 (s, 3H), 5.28–5.52 (m, 1H), 6.53–6.69 (m, 1H), 7.01 (s, 1H), 7.08 (t, J = 6.9 Hz, 1H), 7.11–7.21 (m, 3H), 7.22–7.33 (m, 3H), 7.55 (td, J = 7.7, 1.4 Hz, 1H), 7.65 (td, J = 7.6, 1.5 Hz, 1H), 7.77 (dd, J = 7.9, 1.4 Hz, 1H), 7.82–7.88 (m, 2H), 8.13 (dd, J = 8.1, 1.5 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 20.04, 45.35, 53.12, 87.84, 112.17, 120.82, 123.05, 123.39, 127.12, 127.37, 128.05, 128.87, 129.04, 129.40, 129.53, 130.25, 130.47, 130.72, 134.65, 135.76, 138.60, 140.21, 141.19, 154.96, 157.69 (two carbons are accidentally isochronous). HRMS (ESI) m/z calculated [M + H]+ C26H23O3S+ 415.1362, found 415.1393.
1-(4-Methoxyphenyl)-2-(2-(methylsulfonyl)phenyl)-1,2-dihydronaphtho[2,1-b]furan (5c)
White solid. M.p. 178.2–178.5 (ethanol). 1H NMR (400 MHz, CDCl3) δ 2.87 (s, 3H), 3.76 (s, 3H), 5.03 (d, J = 6.0 Hz, 1H), 6.65 (d, J = 6.0 Hz, 1H), 6.82 (d, J = 8.7 Hz, 2H), 7.16 (d, J = 8.7 Hz, 2H), 7.23–7.33 (m, 4H), 7.53 (ddd, J = 7.8, 7.2, 1.5 Hz, 1H), 7.60 (td, J = 7.5, 1.4 Hz, 1H), 7.66 (dd, J = 7.8, 1.5 Hz, 1H), 7.80–7.86 (m, 2H), 8.11 (dd, J = 8.0, 1.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 45.54, 55.33, 57.16, 88.12, 112.09, 114.41, 120.89, 122.99, 123.37, 127.06, 128.76, 129.03, 129.23, 129.32, 129.57, 130.33, 130.65, 130.79, 134.26, 134.53, 138.48, 141.22, 157.36, 158.98. HRMS (ESI) m/z calculated [M + H]+ C26H23O4S+ 431.1317, found 431.1332.
1-(4-Chlorophenyl)-2-(2-(methylsulfonyl)phenyl)-1,2-dihydronaphtho[2,1-b]furan (5d)
Whitish solid. M.p. 140.1–142.5 °C (ethanol/dioxane). 1H NMR (400 MHz, CDCl3) δ 2.98 (s, 3H), 4.99 (d, J = 5.1 Hz, 1H), 6.59 (d, J = 5.1 Hz, 1H), 7.17 (d, J = 8.5 Hz, 2H), 7.22–7.32 (m, 6H), 7.49–7.62 (m, 3H), 7.80–7.87 (m, 2H), 8.07–8.12 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 45.58, 57.01, 87.88, 112.00, 120.49, 122.81, 123.58, 127.30, 128.36, 129.10, 129.16, 129.36, 129.58, 129.70, 130.34, 130.50, 131.13, 133.30, 134.58, 138.16, 140.83, 140.97, 157.46. HRMS (ESI) m/z calculated [M + H]+ C25H20ClO3S+ 435.0822, found 435.0844.
2-(2-(Methylsulfonyl)phenyl)-1-(naphthalen-1-yl)-1,2-dihydronaphtho[2,1-b]furan (5e)
Waxy solid. 1H NMR (400 MHz, CDCl3) δ 2.52 (s, 3H), 6.07 (d, J = 6.2 Hz, 1H), 6.69 (d, J = 6.2 Hz, 1H), 7.10–7.24 (m, 3H), 7.27–7.37 (m, 4H), 7.47 (t, J = 7.5 Hz, 1H), 7.56–7.63 (m, 1H), 7.63–7.74 (m, 2H), 7.77 (d, J = 8.2 Hz, 1H), 7.83–7.95 (m, 4H), 8.10 (d, J = 7.8 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 45.16, 51.85, 87.99, 112.48, 120.30, 122.85, 123.43, 125.99, 126.15, 126.23, 126.57, 127.12, 128.07, 128.85, 129.10, 129.27, 129.61, 129.77, 130.32, 130.54, 130.96, 131.59, 134.05, 134.81, 137.94, 139.07, 140.92, 157.89 (one carbon is accidentally isochronous). HRMS (ESI) m/z calculated [M + H]+ C29H23O3S+ 451.1367, found 451.1390.
2-(2-(Methylsulfonyl)phenyl)-1-(thiophen-2-yl)-1,2-dihydronaphtho[2,1-b]furan (5f)
White solid. M.p. 126.1–127.7 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 2.97 (s, 3H), 5.40 (d, J = 5.8 Hz, 1H), 6.72 (d, J = 5.9 Hz, 1H), 6.89–6.99 (m, 2H), 7.19 (dd, J = 4.9, 1.4 Hz, 1H), 7.24 (d, J = 8.9 Hz, 1H), 7.27–7.38 (m, 2H), 7.45 (dd, J = 8.1, 1.5 Hz, 1H), 7.50–7.64 (m, 3H), 7.85 (d, J = 8.3 Hz, 2H), 8.13 (dd, J = 7.8, 1.4 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.52, 52.44, 88.04, 112.10, 120.13, 122.70, 123.53, 125.25, 125.95, 127.14, 127.25, 128.65, 129.06, 129.42, 129.71, 130.28, 130.60, 131.26, 134.52, 138.60, 140.42, 145.48, 157.12. HRMS (ESI) m/z calculated [M + H]+ C23H19O3S2+ 407.0775, found 407.0799.
3.2. General Procedure for the Oxidative Aromatization Reaction of Dihydronaphthofurans 5a–f to Naphthofurans 6a–f
The relevant dihydrofuran 5 (0.17 mmol) was dissolved in toluene (3.0 mL) and DDQ (0.51 mmol, 116 mg) was added. The reaction was stirred at 50 °C in an oil bath and monitored by TLC. After the disappearance of the starting material (24 h), the mixture was filtered, and then the filtrate was washed with dichloromethane and toluene, and then with saturated Na2CO3 (2 × 5 mL), water (1 × 5 mL) and brine (1 × 5 mL). The organic phase was dried with anhydrous Na2SO4 and the solvent evaporated to give the crude 7, which was purified by column chromatography (petroleum ether/ethyl acetate 1:1 to 1:2).
2-(2-(Methylsulfonyl)phenyl)-1-(p-tolyl)naphtho[2,1-b]furan (6a)
White solid. M.p. 208.5–209.5 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 2.41 (s, 3H), 3.41 (s, 3H), 7.21 (d, J = 7.7 Hz, 2H), 7.28–7.37 (m, 2H), 7.39–7.50 (m, 4H), 7.51–7.57 (m, 1H), 7.67 (d, J = 8.9 Hz, 1H), 7.80 (d, J = 8.7 Hz, 1H), 7.87 (dd, J = 8.4, 0.7 Hz, 1H), 7.94 (dd, J = 8.1, 0.7 Hz, 1H), 8.21 (dd, J = 7.9, 1.4 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 21.52, 45.37, 112.00, 122.26, 122.58, 123.52, 124.63, 126.23, 126.58, 128.69, 129.06, 129.52, 129.62, 129.94, 130.38, 130.54, 130.79, 131.19, 133.19, 133.90, 137.72, 140.70, 148.61, 152.16. HRMS (ESI) m/z calcd. For C26H21O3S [M + H]+: 413.1211, found 413.1230.
2-(2-(Methylsulfonyl)phenyl)-1-(o-tolyl)naphtho[2,1-b]furan (6b)
White solid. M.p. 221.4–222.9 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 2.21 (s, 3H), 3.51 (s, 3H), 7.22 (td, J = 6.9, 6.4, 2.6 Hz, 1H), 7.27–7.36 (m, 4H), 7.38–7.55 (m, 5H), 7.69 (d, J = 8.9 Hz, 1H), 7.81 (d, J = 8.9 Hz, 1H), 7.94 (d, J = 8.8 Hz, 1H), 8.21 (dd, J = 7.8, 1.4 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 20.28, 45.57, 112.03, 121.37, 122.78, 122.88, 124.68, 126.28, 126.63, 128.54, 128.78, 128.96, 129.54, 130.33, 130.70, 131.03, 131.54, 132.45, 132.89, 133.23, 138.43, 140.13, 148.36, 152.14 (two carbons are accidentally isochronous). HRMS (ESI) m/z calcd. For C26H21O3S [M + H]+: 413.1211, found 413.1242.
1-(4-Methoxyphenyl)-2-(2-(methylsulfonyl)phenyl)naphtho[2,1-b]furan (6c)
White solid. M.p. 199.8–200.2 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 3.42 (s,3H), 3.86 (s, 3H), 6.94 (d, J = 7.9 Hz, 2H), 7.29 (dt, J = 7.7, 1.2 Hz, 1H), 7.31–7.37 (m, 1H), 7.40–7.50 (m, 4H), 7.50–7.58 (m, 1H), 7.67 (dd, J = 8.9, 0.9 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.87 (dt, J = 8.4, 1.1 Hz, 1H), 7.94 (d, J = 8.2 Hz, 1H), 8.21 (d, J = 7.8 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.39, 55.38, 112.01, 114.25, 121.93, 122.67, 123.46, 124.64, 125.09, 126.27, 126.56, 128.70, 129.07, 129.61, 130.40, 130.57, 131.18, 132.09, 133.20, 133.90, 140.71, 148.69, 152.14, 159.39. HRMS (ESI) m/z calcd. for C26H21O4S [M + H]+: 429.1161, found 429.1182.
1-(4-Chlorophenyl)-2-(2-(methylsulfonyl)phenyl)naphtho[2,1-b]furan (6d)
White solid. M.p. 215.0–216.3 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 3.42 (s, 3H), 7.23–7.27 (m, 1H), 7.34–7.41 (m, 3H), 7.43–7.53 (m, 4H), 7.58 (td, J = 7.7, 1.4 Hz, 1H), 7.67 (d, J = 9.0 Hz, 1H), 7.78–7.84 (m, 2H), 7.96 (dt, J = 8.2, 1.0 Hz, 1H), 8.22 (dd, J = 7.9, 1.4 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.47, 111.97, 121.09, 122.20, 123.26, 124.82, 126.47, 126.84, 128.44, 129.10, 129.21, 129.99, 130.11, 130.52, 131.22, 131.69, 132.32, 133.37, 133.80, 134.11, 140.83, 148.93, 152.24. HRMS (ESI) m/z calcd. for C25H18ClO3S [M + H]+: 433.0665, found 433.0696.
2-(2-(Methylsulfonyl)phenyl)-1-(naphthalen-1-yl)naphtho[2,1-b]furan (6e)
Waxy solid. 1H NMR (CDCl3, 400 MHz) δ 3.52 (s, 3H), 7.02–7.20 (m, 4H), 7.35 (ddt, J = 8.3, 3.4, 1.5 Hz, 2H), 7.41–7.53 (m, 3H), 7.62 (dd, J = 7.0, 1.3 Hz, 1H), 7.75 (d, J = 8.9 Hz, 1H), 7.82–7.98 (m, 5H), 8.17 (dd, J = 8.0, 1.3 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.63, 100.06, 112.01, 120.10, 123.51, 124.62, 125.93, 126.31, 126.34, 126.40, 126.80, 126.94, 128.38, 128.49, 128.81, 128.86, 129.19, 129.50, 129.65, 130.29, 130.54, 131.13, 132.82, 133.22, 133.68, 140.22, 149.64, 152.23. HRMS (ESI) m/z calcd. for C29H21O3S [M + H]+: 449.1211, found 449.1246.
2-(2-(Methylsulfonyl)phenyl)-1-(thiophen-2-yl)naphtho[2,1-b]furan (6f)
Waxy solid. 1H NMR (CDCl3, 400 MHz) δ 3.34 (s, 3H), 7.11 (dd, J = 5.2, 3.4 Hz, 1H), 7.22 (dd, J = 3.5, 1.2 Hz, 1H), 7.36–7.52 (m, 4H), 7.52–7.63 (m, 2H), 7.68 (d, J = 8.9 Hz, 1H), 7.82 (d, J = 8.9 Hz, 1H), 7.90–7.98 (m, 2H), 8.22 (dd, J = 7.6, 1.6 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.27, 111.99, 115.12, 122.74, 123.32, 124.89, 126.52, 126.92, 127.28, 127.64, 128.38, 129.09, 129.69, 129.89, 130.12, 130.33, 131.23, 133.10, 133.23, 133.73, 140.78, 150.17, 152.09. HRMS (ESI) m/z calcd. for C23H17O3S2 [M + H]+: 405.0619, found 405.0656.
3.3. General Procedure for the Reaction of Substrates 4 with 8-Hydroxyquinoline
In a flask, the relevant nitrostilbene 4 (0.30 mmol) was dissolved in DMSO (2 mL) and 8-hydroxyquinoline (0.30 mmol, 44 mg) and K2CO3 (0.38 mmol, 53 mg) were added as solids under magnetic stirring. The reaction was stirred at room temperature and monitored by TLC. Upon completion (4–5 h), the mixture was diluted with 20 mL of ethyl acetate, washed with HCl 1M (2 × 5 mL), NaHCO3 (2 × 5 mL), water (1 × 5 mL), and brine (1 × 5 mL). The organic phase was dried with anhydrous Na2SO4, and the solvent evaporated. The crude was purified by column chromatography (petroleum ether/ethyl acetate 1:1 to 1:2), thus obtaining compounds 7a–d.
2-(2-(Methylsulfonyl)phenyl)-3-(p-tolyl)-2,3-dihydrofuro[3,2-h]quinoline (7a)
White solid. M.p. 202.2–203.0 °C (taken-up with petroleum ether/DCM). 1H NMR (CDCl3, 400 MHz) δ 2.33 (s, 3H), 3.04 (s, 3H), 4.95 (d, J = 7.3 Hz, 1H), 6.71 (d, J = 7.3 Hz, 1H), 7.08–7.17 (m, 4H), 7.21 (dd, J = 8.2, 0.8 Hz, 1H), 7.40–7.47 (m, 2H), 7.48–7.66 (m, 3H), 8.12 (dt, J = 7.8, 1.1 Hz, 1H), 8.18 (dd, J = 8.3, 1.7 Hz, 1H), 8.88–8.93 (m, 1H). 13C NMR (CDCl3, 101 MHz) δ 21.30, 45.73, 58.92, 89.57, 121.38, 121.55, 123.79, 128.35, 129.17, 129.24, 129.54 129.72, 129.98, 134.31, 135.90, 136.37, 137.27, 138.51, 138.65, 140.84, 150.24 (two carbons are accidentally isochronous). HRMS (ESI) m/z calcd. for C25H22NO3S [M + H]+: 416.1320, found 416.1346.
2-(2-(Methylsulfonyl)phenyl)-3-(o-tolyl)-2,3-dihydrofuro[3,2-h]quinoline (7b)
White solid. M.p. 147.0–148.0 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 2.09 (s, 3H), 3.02 (s, 3H), 5.35 (d, J = 7.7 Hz, 1H), 6.66 (d, J = 7.1 Hz, 1H), 7.16 (s, 4H), 7.21 (d, J = 8.2 Hz, 1H), 7.40–7.48 (m, 2H), 7.54 (td, J = 7.6, 1.5 Hz, 1H), 7.61 (td, J = 7.6, 1.5 Hz, 1H), 7.70 (d, J = 7.7 Hz, 1H), 8.12 (dd, J = 7.9, 1.5 Hz, 1H), 8.19 (dd, J = 8.4, 1.7 Hz, 1H), 8.91 (dd, J = 4.2, 1.7 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 19.94, 29.83, 31.37, 45.62, 121.41, 121.54, 123.67, 126.93, 127.49, 128.37, 129.20, 129.34, 129.48, 129.94, 130.61, 134.41, 135.93, 136.38, 136.53, 138.47, 140.90, 150.25, 154.84 (two carbons are accidentally isochronous). HRMS (ESI) m/z calcd. for C25H22NO3S [M + H]+: 416.1320, found 416.1358.
2-(2-(Methylsulfonyl)phenyl)-3-(p-anysyl)-2,3-dihydrofuro[3,2-h]quinoline (7c)
Yellow solid. 1H NMR (CDCl3, 400 MHz) δ 3.05 (s, 3H), 3.78 (s, 3H), 4.95 (d, J = 7.4 Hz, 1H), 6.68 (d, J = 7.4 Hz, 1H), 6.85 (d, J = 8.7 Hz, 2H), 7.14 (d, J = 8.7 Hz, 2H), 7.21 (dd, J = 8.2, 0.7 Hz, 1H), 7.43 (dt, J = 8.4, 2.2 Hz, 2H), 7.49–7.65 (m, 3H), 8.12 (dd, J = 7.8, 1.4 Hz, 1H), 8.19 (dd, J = 8.4, 1.7 Hz, 1H), 8.90 (dd, J = 4.3, 1.7 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.72, 55.34, 58.51, 89.63, 114.32, 121.38, 121.55, 123.76, 128.41, 129.17, 129.23, 129.50, 129.53, 129.96, 133.64, 134.30, 135.89, 136.37, 138.45, 140.61, 150.24, 154.52, 159.09. HRMS (ESI) m/z calcd. for C25H22NO4S [M + H]+: 432.1269, found 432.1297.
3-(4-Chlorophenyl)-2-(2-(methylsulfonyl)phenyl)-2,3-dihydrofuro[3,2-h]quinoline (7d)
Yellow solid. M.p. 147.0–148.3 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 3.10 (s, 3H), 4.92 (d, J = 6.7 Hz, 1H), 6.63 (d, J = 6.7 Hz, 1H), 7.12–7.20 (m, 3H), 7.23–7.33 (m, 3H), 7.39–7.48 (m, 2H), 7.48–7.61 (m, 2H), 8.10 (dt, J = 7.5, 1.2 Hz, 1H), 8.19 (dd, J = 8.4, 1.7 Hz, 1H), 8.91 (dd, J = 4.2, 1.6 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 45.72, 58.60, 89.31, 121.65, 121.74, 123.62, 127.70, 128.86, 129.14, 129.39, 129.63, 129.72, 130.04, 133.51, 134.40, 135.85, 136.43, 138.21, 140.34, 150.38, 154.63 (two carbons are accidentally isochronous). HRMS (ESI) m/z calcd. for C24H19ClNO3S [M + H]+: 436.0774, found 436.0798.
3.4. General Procedure for the Oxidative Aromatization Reaction of Dihydrofuroquinolines 7a, d to Furoquinolines 8a, d
The relevant dihydrofuran 6 (0.12 mmol) was dissolved in chloroform (3.0 mL) and DDQ (0.24 mmol, 54.5 mg) was added. The reaction was maintained under magnetic stirring at room temperature, until completion (24 h, monitored by TLC). Then, an aqueous saturated NaHCO3 solution (8 mL) was added, and the resulting mixture was extracted with CHCl3 (3 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The reaction mixture was purified by column chromatography (petroleum ether/ethyl acetate 1:1) to afford the desired products 8a–d.
2-(2-(Methylsulfonyl)phenyl)-3-(p-tolyl)furo[3,2-h]quinoline (8a)
White solid. M.p. 203.2–205.2 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 2.37 (s, 3H), 3.63 (s, 3H), 7.19 (d, J = 7.8 Hz, 2H), 7.35–7.43 (m, 3H), 7.46 (dd, J = 8.2, 4.3 Hz, 1H), 7.54 (td, J = 7.6, 1.4 Hz, 1H), 7.62 (td, J = 7.7, 1.5 Hz, 1H), 7.70 (d, J = 8.6 Hz, 1H), 7.85 (d, J = 8.5 Hz, 1H), 8.29 (d, J = 8.1 Hz, 2H), 8.93 (dd, J = 4.4, 1.7 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 21.44, 46.15, 120.12, 120.83, 120.95, 123.58, 126.87, 128.01, 128.43, 129.57, 129.72, 130.02, 130.71, 130.82, 133.42, 133.49, 136.49, 137.10, 137.58, 141.15, 149.38, 149.42, 150.33. HRMS (ESI) m/z calcd. For C25H20NO3S [M + H]+: 414.1163, found 414.1187.
3-(4-Chlorophenyl)-2-(2-(methylsulfonyl)phenyl)furo[3,2-h]quinoline (8d)
Pale yellow solid. M.p. 227.3–229.0 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 3.63 (s, 3H), 7.26 (s, 2H), 7.33–7.40 (m, 3H), 7.43 (d, J = 8.5 Hz, 2H), 7.46–7.51 (m, 1H), 7.57 (td, J = 7.5, 1.4 Hz, 1H), 7.65 (td, J = 7.8, 1.4 Hz, 1H), 7.73 (d, J = 8.6 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 8.30 (dd, J = 8.2, 1.5 Hz, 2H), 8.94 (dd, J = 4.3, 1.7 Hz, 1H).13C NMR (CDCl3, 101 MHz) δ 46.19, 119.69, 119.94, 121.02, 123.92, 126.97, 127.58, 129.30, 130.01, 130.36, 130.83, 130.99, 133.40, 133.58, 133.82, 136.57, 137.06, 141.27, 149.48, 149.87, 150.50. HRMS (ESI) m/z calcd. For C24H17ClN2O3S [M + H]+: 434.0612, found 434.0658.
3.5. Procedure for the Reaction of Substrate 4a with 4-Hydroxycoumarin
In a round-bottom flask the substrate 4a (0.157 mmol) was dissolved in chloroform (1.5 mL), then 4-hydroxycoumarin (0.157 mmol) and DABCO (0.047 mmol, 30%) were added. The reaction was stirred at room temperature for four days. The crude residue was purified by preparative TLC (petroleum ether/ethyl acetate 1:1).
2-(2-(Methylsulfonyl)phenyl)-3-(p-tolyl)-2,3-dihydro-4H-furo[3,2-c]chromen-4-one (9a)
Waxy solid. 1H NMR (CDCl3, 400 MHz) δ 2.31 (s, 3H), 2.75 (s, 3H), 4.67 (d, J = 6.4 Hz, 1H), 6.99 (d, J = 6.4 Hz, 1H), 7.11–7.22 (m, 4H), 7.35 (dd, J = 7.6, 1.1 Hz, 1H), 7.43 (dd, J = 8.5, 1.0 Hz, 1H), 7.63 (ddt, J = 9.5, 8.1, 1.3 Hz, 3H), 7.73 (ddd, J = 18.7, 7.6, 1.5 Hz, 2H), 8.13 (dd, J = 8.0, 1.4 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 21.29, 45.59, 55.39, 90.14, 105.69, 112.30, 117.28, 123.25, 124.31, 127.65, 128.54, 129.92, 129.99, 130.06, 133.10, 134.84, 136.46, 137.95, 138.50, 138.96, 155.59, 159.55, 166.13. HRMS (ESI) m/z calcd. for C25H21O5S [M + H]+: 433.1109, found 433.1125.
3.6. General Procedure for the 6π-Electrocyclization of Naphthofurans 6a–f
The relevant naphthofuran 6a–f (0.072 mmol) was introduced in a 25 mL quartz tube and dissolved in 3.5 mL of acetone (spectroscopic grade); the tube was sealed and placed within a Rayonet photochemical reactor equipped with fourteen 300 nm lamps. The reaction, followed by TLC, required 24–48 h; at the end, the solvent was removed, and the crude residue was dissolved in DCM, washed with a NaHCO3 saturated solution and water, and then dried over Na2SO4; after filtration, the solvent was removed under reduced pressure and the residue purified by chromatography on column (petroleum ether/ethyl acetate mixtures as eluent).
13-Methylnaphtho[2,1-b]phenanthro[9,10-d]furan (11a)
White solid. M.p. 137.2–138.4 °C (taken-up with petroleum ether/DCM). 1H NMR (CDCl3, 400 MHz) δ 2.70 (s, 3H), 7.55–7.67 (m, 2H), 7.68–7.77 (m, 3H), 7.91 (d, J = 8.9 Hz, 1H), 7.95 (d, J = 8.9 Hz, 1H), 8.08 (dd, J = 8.1, 1.4 Hz, 1H), 8.55 (dd, J = 7.1, 2.0 Hz, 1H), 8.63 (s, 1H), 8.77 (dd, J = 7.4, 1.9 Hz, 1H), 9.04 (d, J = 8.3 Hz, 1H), 9.12 (d, J = 8.5 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 22.06, 112.92, 116.82, 120.20, 121.58, 122.52, 123.38, 124.08, 124.55, 125.75,126.04, 126.10, 126.27, 126.91, 127.28, 127.81, 128.20, 128.71, 129.01, 129.60, 130.28, 131.50, 134.92, 150.88, 154.25. HRMS (ESI) m/z calcd. for C25H17O [M + H]+: 333.1279, found 333.1297.
13-Methoxynaphtho[2,1-b]phenanthro[9,10-d]furan (11c)
White solid. M.p. 188.7–190.1 °C (taken-up with petroleum ether/DCM). 1H NMR (CDCl3, 400 MHz) δ 4.09 (s, 3H), 7.44 (dd, J = 9.0, 2.6 Hz, 1H), 7.59 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 7.66–7.78 (m, 3H), 7.90 (d, J = 8.9 Hz, 1H), 7.95 (d, J = 8.9 Hz, 1H), 8.08 (dd, J = 8.2, 1.6 Hz, 1H), 8.24 (d, J = 2.7 Hz, 1H), 8.55 (dd, J = 7.7, 1.8 Hz, 1H), 8.69 (dd, J = 8.0, 1.4 Hz, 1H), 9.08 (dd, J = 8.7, 4.0 Hz, 2H).13C NMR (CDCl3, 101 MHz) δ 55.74, 106.60, 112.93, 115.56, 116.86, 120.07, 121.65, 122.51, 122.71, 123.44, 124.54, 125.58, 126.16, 126.75, 127.52, 127.68, 127.81, 128.70, 129.62, 129.94, 130.46, 131.48, 150.12, 154.25, 157.36. HRMS (ESI) m/z calcd. For C25H17O2 [M + H]+: 349.1228, found 349.1259.
13-Chloronaphtho[2,1-b]phenanthro[9,10-d]furan (11d)
White solid. M.p. 231.4–232.1 °C (ethanol).1H NMR (CDCl3, 400 MHz) δ 7.59 (app t,1 H), 7.66–7.85 (m, 4H), 7.88–7.99 (m, 2H), 8.07 (d, J = 7.6 Hz, 1H), 8.54 (d, J = 7.6 Hz, 1H), 8.67 (d, J = 8.0 Hz, 1H), 8.76 (s, 1H), 8.99 (d, J = 8.0 Hz, 1H), 9.05 (d, J = 8.8 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 100.12, 105.98, 112.95, 116.57, 119.88, 121.78, 122.74, 123.53, 123.95, 124.82, 125.48, 126.45, 127.01, 127.41, 127.69, 128.12, 128.30, 128.57, 129.57, 129.81, 130.29, 131.31, 131.62, 151.31, 154.44. HRMS (ESI) m/z calcd. for C24H14ClO [M + H]+: 353.0728, found 353.0752.
Chryseno[6,5-b]naphtho[1,2-d]furan (11e)
White solid. M.p. 212.1–213.6 °C (taken-up with petroleum ether/DCM). 1H NMR (CDCl3, 400 MHz) δ 7.28–7.40 (m, 2H), 7.48 (ddd, J = 8.1, 6.8, 1.2 Hz, 1H), 7.62 (ddd, J = 8.0, 6.8, 1.2 Hz, 1H), 7.70–7.82 (m, 3H), 7.95–8.15 (m, 5H), 8.42 (d, J = 8.3 Hz, 1H), 8.60–8.73 (m, 1H), 8.74–8.85 (m, 2H). 13C NMR (CDCl3, 101 MHz) δ 112.79, 114.96, 120.66, 121.48, 121.65, 121.94, 123.69, 124.41, 124.74, 124.92, 125.83, 126.31, 126.74, 127.11, 127.14, 127.31, 127.61, 128.25, 128.52, 128.60, 129.01, 129.90, 130.09, 130.51, 131.22, 132.63, 152.38, 154.20. HRMS (ESI) m/z calcd. for C28H17O [M + H]+: 369.1279, found 369.1299.
Naphtho[2,1-b]thieno[2’,3’:3,4]naphtho[2,1-d]furan (11f)
White solid. M.p. 200.2–201.3 °C (taken-up with petroleum ether/DCM). 1H NMR (CDCl3, 400 MHz) δ 7.55–7.73 (m, 4H), 7.80 (t, J = 7.7 Hz, 1H), 7.91 (d, J = 8.9 Hz, 1H), 7.96 (d, J = 8.9 Hz, 1H), 8.06 (d, J = 8.1 Hz, 1H), 8.16 (d, J = 5.5 Hz, 1H), 8.39–8.48 (m, 1H), 8.55–8.63 (m, 1H), 9.47 (d, J = 8.4 Hz, 1H). 13C NMR (CDCl3, 101 MHz) δ 112.94, 115.65, 118.96, 120.24, 121.76, 122.92, 122.98, 124.24, 124.67, 125.57, 126.09, 126.67, 126.84, 128.17, 128.25, 128.39, 129.53, 130.78, 131.17, 133.49, 150.39, 153.88. HRMS (ESI) m/z calcd. for C22H13OS [M + H]+: 325.0687, found 325.0698.
2-(2-(Methylthio)phenyl)-1-(p-tolyl)-1,2-dihydronaphtho[2,1-b]furan (12a)
White solid. M.p. 75.2–76.1 °C (ethanol). 1H NMR (DMSO, 400 MHz) δ 7.93–7.86 (2 part. overlapped d, 2H), 7.47–7.19 (m, 9H), 7.15–7.08 (m, 3H), 5.96 (d, J = 4.4 Hz, 1H), 4.80 (d, J = 4.4 Hz, 1H), 2.47 (s, 3H), 2.25 (s, 3H). 13C NMR (DMSO, 101 MHz) δ 15.54, 20.54, 54.53, 89.40, 111.85, 121.18, 122.41, 122.92, 124.80, 124.84, 126.22, 126.70, 127.36, 128.49, 128.65, 129.13, 129.49, 129.84, 130.24, 135.79, 135.87, 138.29, 140.12, 156.50. HRMS (ESI) m/z calcd. for C26H23OS [M + H]+: 383.1470, found 383.1498.
2-(2-(Methylthio)phenyl)-1-(p-tolyl)naphtho[2,1-b]furan (13a)
White solid. M.p. 92.5–93.6 °C (ethanol). 1H NMR (CDCl3, 400 MHz) δ 2.42 (s, 6H), 7.06 (td, J = 8.00 and 4.00 Hz), 7.20–7.45 (m, 10H), 7.77 (AB system, J = 8.8 Hz), 7.90 (d, J = 8.8 Hz), 7.95 (d, J = 8.0 Hz). 13C NMR (CDCl3, 101 MHz) δ 16.23, 21.62, 112.73, 121.81, 122.47, 123.55, 124.40, 124.49, 125.75, 126.01, 126.03, 128.64, 129.10, 129.42, 129.62, 129.67, 130.63, 130.67, 131.09, 131.95, 137.41, 140.58, 150.96, 152.21. HRMS (ESI) m/z calcd. for C26H21OS [M + H]+: 381.1313, found 383.1320.
The described NMR spectra plots of all synthesized compounds are collected in the Supplementary Materials.
3.7. Crystal Structure of 5a
A single crystal of compound 5a was submitted to X-ray data collection on an Oxford-Diffraction Xcalibur Sapphire 3 diffractometer with a graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) at 293 K. The structure was solved by direct methods implemented in SHELXS-97 program [45]. The refinement was carried out by full-matrix anisotropic least-squares on F2 for all reflections for non-H atoms by means of the SHELXL-97 program (Version 2019/2) [46]. Crystallographic data for this structure have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2166047. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; (Fax: +44-(0)-1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27103147/s1, 1H and 13C NMR spectra for all compounds.
Author Contributions
Conceptualization, L.B., C.T. and G.P.; resources, L.B., C.T. and G.P.; writing—original draft preparation, C.T.; writing-review and editing, C.T., G.P. and D.S.; investigation, A.B. and L.B.; methodology, A.B., G.G. and M.M.; supervision, L.B.; C.T; G.P. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by grants from Genoa University (FRA 2019 and 2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The skillful experimental contribution of G. Marcantoni Taddei and of G. De Santis is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of all of the compounds are available from the authors.
References
- Boto, A.; Alvarez, L. Furan and its derivatives. In Heterocycles in Natural Product Synthesis; Wiley: Weinheim, Germany, 2011; pp. 97–152. [Google Scholar]
- Ho, L.-K.; Don, M.-J.; Chen, H.-C.; Yeh, S.-F.; Chen, J.-M. Inhibition of Hepatitis B Surface Antigen Secretion on Human Hepatoma Cells. Components from Rubia cordifolia. J. Nat. Prod. 1996, 59, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Ohira, Y.; Oku, H. Antipruritic Dinaphthofuran-7,12-dione Derivatives from the Pericarp of Impatiens balsamina. J. Nat. Prod. 1998, 61, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Higashimoto, K.; Kurioka, M.; Arai, M.; Fukuda, A.; Sumii, Y.; Sowa, Y.; Sakai, T.; Kobayashi, M. Xylarianaphthol-1, a novel dinaphthofuran derivative, activates p21 promoter in a p53-independent manner. Bioorganic Med. Chem. Lett. 2014, 24, 3389–3391. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Faridi, U.; Sisodia, B.S.; Darokar, M.P.; Luqman, S.; Khanuja, S.P.S. Synthesis of 1-(3′,4′,5′-trimethoxy) phenyl naphtho[2,1-b]furan as a novel anticancer agent. Bioorg. Med. Chem. Lett. 2006, 16, 911–914. [Google Scholar] [CrossRef]
- Islam, K.; Pal, K.; Debnath, U.; Basha, R.S.; Khan, A.T.; Jana, K.; Misra, A.K. Anti-cancer potential of (1,2-dihydronaphtho[2,1-b]furan-2-yl)methanone derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 127476. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Lin, C.-S.; Shih, P.-K.; Tsao, L.-T.; Wang, J.-P.; Cheng, C.-M.; Tzeng, C.-C.; Chen, Y.-L. Furo[3′,2′:3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg. Med. Chem. Lett. 2009, 17, 6773–6779. [Google Scholar] [CrossRef]
- Tripathi, R.P.; Yadav, A.K.; Ajay, A.; Bisht, S.S.; Chaturvedi, V.; Sinha, S.K. Application of Huisgen (3 + 2) cycloaddition reaction: Synthesis of 1-(2,3-dihydrobenzofuran-2-yl-methyl [1,2,3]-triazoles and their antitubercular evaluations. Eur. J. Med. Chem. 2010, 45, 142–148. [Google Scholar] [CrossRef]
- Guével, R.L.; Oger, F.; Lecorgne, A.; Dudasova, Z.; Chevance, S.; Bondon, A.; Barath, P.; Simonneaux, G.; Salbert, G. Identification of small molecule regulators of the nuclear receptor HNF4α based on naphthofuran scaffolds. Bioorg. Med. Chem. Lett. 2009, 17, 7021–7030. [Google Scholar] [CrossRef]
- Pattabiramin, T.N.; Lawson, W.B. Stereochemistry of the active site of α-chymotrypsin: The effect of some tricyclic bromomethyl ketones on α-chymotrypsin. Biochim. Biophys. Acta 1972, 258, 548–553. [Google Scholar] [CrossRef]
- Adams, J.L.; Garigipati, R.S.; Sorenson, M.; Schmidt, S.J.; Brian, W.R.; Newton, F.J.; Tyrrell, K.A.; Garver, E.; Yodis, L.A.; Chabot-Fletcher, M.; et al. Bicyclic N-Hydroxyurea Inhibitors of 5-Lipoxygenase: Pharmacodynamic, Pharmacokinetic, and in Vitro Metabolic Studies Characterizing N-Hydroxy-N-(2,3-dihydro-6-(phenylmethoxy)-3-benzofuranyl)urea. J. Med. Chem. 1996, 39, 5035–5046. [Google Scholar] [CrossRef]
- Matsunaga, N.; Kaku, T.; Ojida, A.; Tanaka, T.; Hara, T.; Yamaoka, M.; Kusaka, M.; Tasaka, A. C17,20-lyase inhibitors. Part 2: Design, synthesis and structure–activity relationships of (2-naphthylmethyl)-1H-imidazoles as novel C17,20-lyase inhibitors. Bioorg. Med. Chem. 2004, 12, 4313–4336. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Sousa, C.M.; Coelho, P.J. Colour switching with photochromic vinylidene-naphthofurans. Tetrahedron 2018, 74, 7372–7379. [Google Scholar] [CrossRef]
- Tsuji, H.; Nakamura, E. Design and Functions of Semiconducting Fused Polycyclic Furans for Optoelectronic Applications. Acc. Chem. Res. 2017, 50, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.V.; Wirth, T. Hypervalent iodine mediated oxidative cyclization of o-hydroxystilbenes into benzo-and naphthofurans. Synthesis 2012, 44, 1171–1177. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Prasad, C.V.; Shrivastava, S.; Naidu, V.G.M. Synthesis and anticancer activity of some novel 5,6-fused hybrids of juglone based 1,4-naphthoquinones. Eur. J. Med. Chem. 2014, 83, 84–91. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, M.; Ma, L.; Zhang, M. Synthesis of benzofurans from the cyclodehydration of α-phenoxy ketones mediated by Eaton’s reagent. J. Chem. Res. 2020, 44, 426–436. [Google Scholar] [CrossRef]
- Arrault, A.; Touzeau, F.; Guillaumet, G.; Mérour, J.-Y. A Straightforward Synthesis of 1, 2-Dihydronaphtho [2, 1-b] furans from 2-Naphthols. Synthesis 1999, 1241–1245. [Google Scholar] [CrossRef]
- Vaughan, D.; Jha, A. Convenient synthesis of novel 2,2-dialkyl-1,2-dihydronaphtho[2,1-b]furans. Tetrahedron Lett. 2009, 50, 5709–5712. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Luo, J.; Gan, Z.; Jiang, W.; Tang, Q. One-Step Regioselective Synthesis of Benzofurans from Phenols and α-Haloketones. Molecules 2019, 24, 2187. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Yudin, A.K. Palladium-Catalyzed Oxidative Activation of Arylcyclopropanes. Org. Lett. 2006, 8, 5829–5832. [Google Scholar] [CrossRef]
- Liu, G.; Lu, X. Palladium(II)-catalyzed intramolecular addition of arylboronic acids to ketone. Tetrahedron 2008, 64, 7324–7330. [Google Scholar] [CrossRef]
- van Otterlo, W.A.L.; Morgans, G.L.; Madeley, L.G.; Kuzvidza, S.; Moleele, S.S.; Thornton, N.; de Koning, C.B. An isomerization-ring-closing metathesis strategy for the synthesis of substituted benzofurans. Tetrahedron 2005, 61, 7746–7755. [Google Scholar] [CrossRef]
- Martin-Matute, C.B.; Nevado, C.; Cardenas, D.J.; Echavarren, A.M. Intramolecular Reactions of Alkynes with Furans and Electron Rich Arenes Catalyzed by PtCl2: The Role of Platinum Carbenes as Intermediates. J. Am. Chem. Soc. 2003, 125, 5757–7566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, L.; Shaq, Z.; Wu, Y.-C.; Wang, D.; Chen, Y.-J. InCl3-catalyzed propargylation of indoles and phenols with propargylic acetates: Application to the syntheses of benzofurans and naphthofurans. Synthesis 2007, 1961–1969. [Google Scholar] [CrossRef]
- Kundu, D.; Samim, M.; Majee, A.; Hajra, A. Indium triflate-catalyzed coupling between nitroalkenes and phenol/naphthols: A simple and direct synthesis of arenofurans by a cyclization reaction. Chem. Asian J. 2011, 6, 406–409. [Google Scholar] [CrossRef]
- Rao, V.K.; Shelke, G.M.; Tiwari, R.; Parang, K.; Kumar, A. A Simple and Efficient Synthesis of 2,3-Diarylnaphthofurans Using Sequential Hydroarylation/Heck Oxyarylation. Org. Lett. 2013, 15, 2190–2193. [Google Scholar] [CrossRef] [Green Version]
- Jana, R.; Paul, S.; Biswas, A.; Ray, J.K. Copper-catalyzed addition of water affording highly substituted furan and unusual formation of naphthofuran ring from 3-(1-alkenyl)-2-alkene-1-al. Tetrahedron Lett. 2010, 51, 273–276. [Google Scholar] [CrossRef]
- Olyaei, A.; Sadeghpourb, M. Dihydronaphthofurans: Synthetic strategies and applications. RSC Adv. 2020, 10, 5794–5826. [Google Scholar] [CrossRef]
- Omelchuk, O.A.; Tikhomirov, A.S.; Shchekotikhin, A.E. Annelation of furan rings to arenes. Russ. Chem. Rev. 2016, 85, 817–835. [Google Scholar] [CrossRef]
- Mane, V.; Pandey, J.; Ayyagari, N.; Dey, C.; Kale, R.; Namboothiri, I.N.N. Synthesis of hydrazinoheterocycles from Morita–Baylis–Hillman adducts of nitroalkenes with azodicarboxylates. Org. Biomol. Chem. 2016, 14, 2427–2438. [Google Scholar] [CrossRef]
- Dell’Erba, C.; Spinelli, D.; Leandri, G. Ring-opening reaction in the thiophen series: Reaction between 3,4-dinitrothiophen and secondary amines. J. Chem. Soc. Chem. Commun. 1969, 10, 549. [Google Scholar] [CrossRef]
- Bianchi, L.; Maccagno, M.; Petrillo, G.; Rizzato, E.; Sancassan, F.; Severi, E.; Spinelli, D.; Tavani, C.; Viale, M. (Eds.) Versatile nitrobutadienic building-blocks from the ring opening of 2- and 3-nitrothiophenes. In Targets in Heterocyclic System-Chemistry and Properties; Italian Society of Chemistry: Rome, Italy, 2007; Volume 11, pp. 1–20. [Google Scholar]
- Bianchi, L.; Maccagno, M.; Petrillo, G.; Sancassan, F.; Spinelli, D. (Eds.) Tavani,2,3-Dinitro-1,3-butadienes: Versatile building-blocks from the ring opening of 3,4-dinitrothiophene. In Targets in Heterocyclic Systems-Chemistry and Properties; Italian Society of Chemistry: Rome, Italy, 2006; Volume 10, pp. 1–23. [Google Scholar]
- Petrillo, G.; Benzi, A.; Bianchi, L.; Maccagno, M.; Pagano, A.; Tavani, C.; Spinelli, D. Recent advances in the use of conjugated nitro or dinitro-1,3-butadienes as building-blocks for the synthesis of heterocycles. Tetrahedron Lett. 2020, 61, 152297–152309. [Google Scholar] [CrossRef]
- Tavani, C.; Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G. Densely Functionalized 2-Methylideneazetidines from Nitrodienic Building Blocks. Eur. J. Org. Chem. 2018, 126–136. [Google Scholar] [CrossRef]
- Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Scapolla, C.; Tavani, C. On the behavior of bis(sulfonyl)nitrobutadienes towards primary amines: A convenient access to 1-alkyl-2-aryl-4-(phenylsulfonyl)pyrroles. Tetrahedron 2016, 72, 7050–7058. [Google Scholar] [CrossRef]
- Bianchi, L.; Carloni-Garaventa, A.; Maccagno, M.; Pani, M.; Petrillo, G.; Scapolla, C.; Tavani, C. Synthesis of poly-functionalized pyrazoles and pyridazines from nitrobutadienes: An interesting dichotomy of practical relevance. Tetrahedron 2015, 71, 7550–7561. [Google Scholar] [CrossRef]
- Bianchi, L.; Maccagno, M.; Pani, M.; Petrillo, G.; Scapolla, C.; Tavani, C. A straight access to functionalized carbazoles by tandem reaction between indole and nitrobutadienes. Tetrahedron 2015, 71, 7421–7435. [Google Scholar] [CrossRef]
- Benzi, A.; Bianchi, L.; Giorgi, G.; Maccagno, M.; Petrillo, G.; Tavani, C. 2-Aryl-3-Vinyl Substituted Imidazo[1,2-a]pyridines and Fluorescent Electrocyclization Derivatives therefrom. ChemistrySelect 2020, 5, 4552–4558. [Google Scholar] [CrossRef]
- Benzi, A.; Bianchi, L.; Maccagno, M.; Pagano, A.; Petrillo, G.; Tavani, C. Sequential Annulations to Interesting Novel Pyrrolo[3,2-c]carbazoles. Molecules 2019, 24, 3802. [Google Scholar] [CrossRef] [Green Version]
- Dell’Erba, C.; Gabellini, A.; Novi, M.; Petrillo, G.; Tavani, C.; Cosimelli, B.; Spinelli, D. Ring opening of 2-substituted 4-nitrothiophenes with pyrrolidine. Access to new functionalized nitro-unsaturated building blocks. Tetrahedron 2001, 57, 8159–8165. [Google Scholar] [CrossRef]
- Armstrong, K.J.; Martin-Smith, M.; Brown, N.M.D.; Brophy, G.C.; Sternhell, S. Benzo[b]thiophen derivatives. Part IX. Nitration of benzo[b]thiophen and the isomeric nitrobenzo[b]thiophens. J. Chem. Soc. C Org. 1969, 1766–1775. [Google Scholar] [CrossRef]
- Bianchi, L.; Dell’Erba, C.; Maccagno, M.; Morganti, S.; Novi, M.; Petrillo, G.; Rizzato, E.; Spinelli, D.; Tavani, C. Easy access to 4-nitrothiochroman S,S-dioxides via ring-enlargement from 3-nitrobenzo[b]thiophene. Tetrahedron 2004, 60, 4967–4973. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).