Hepatoprotective Effects of Ixeris chinensis on Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice: An Integrated Gut Microbiota and Metabolomic Analysis
Abstract
1. Introduction
2. Results
2.1. IC Extract Reduced Body Weight, Liver Index, and Fat Tissue in HFD Diet-Induced NAFLD Mice
2.2. Effect of IC Extract on Serum Biochemical Markers
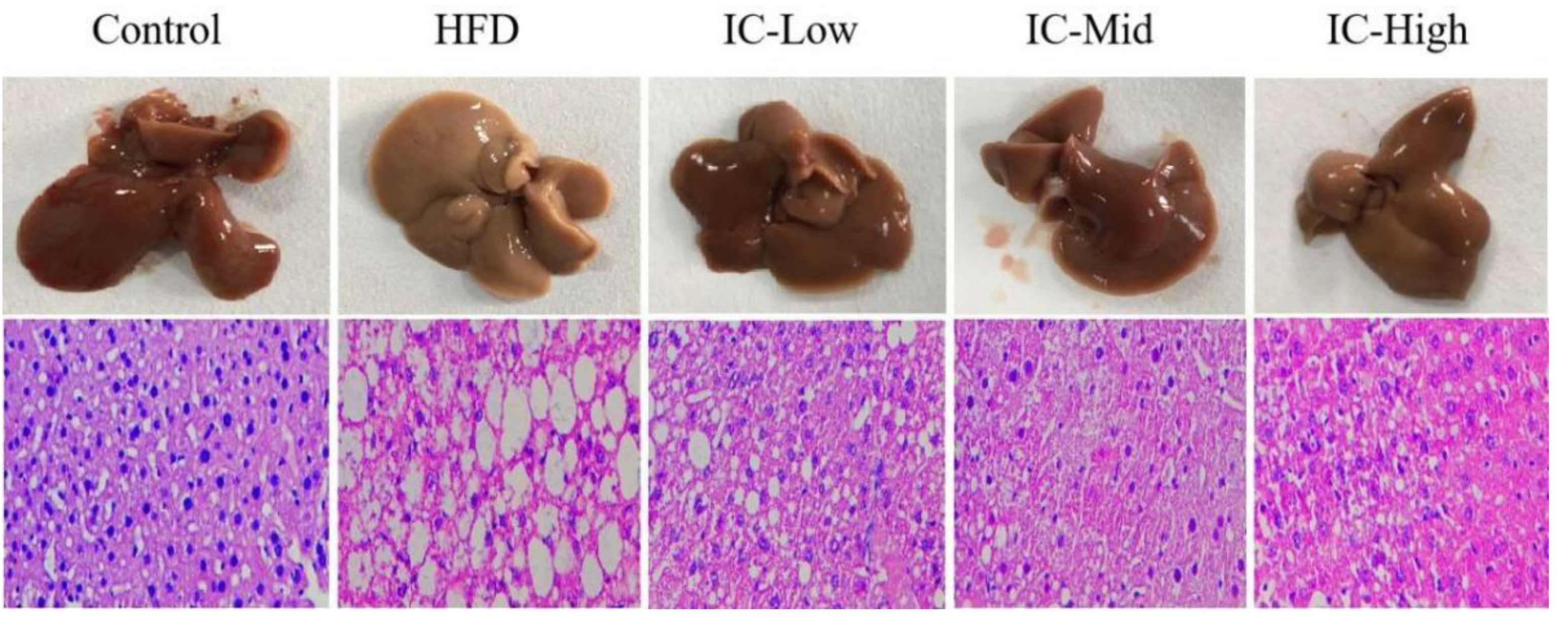
2.3. Effect of IC Extract on Liver Histopathology
2.4. Effect of IC Extract on Liver IL-6, IL-1β, and TNF-α
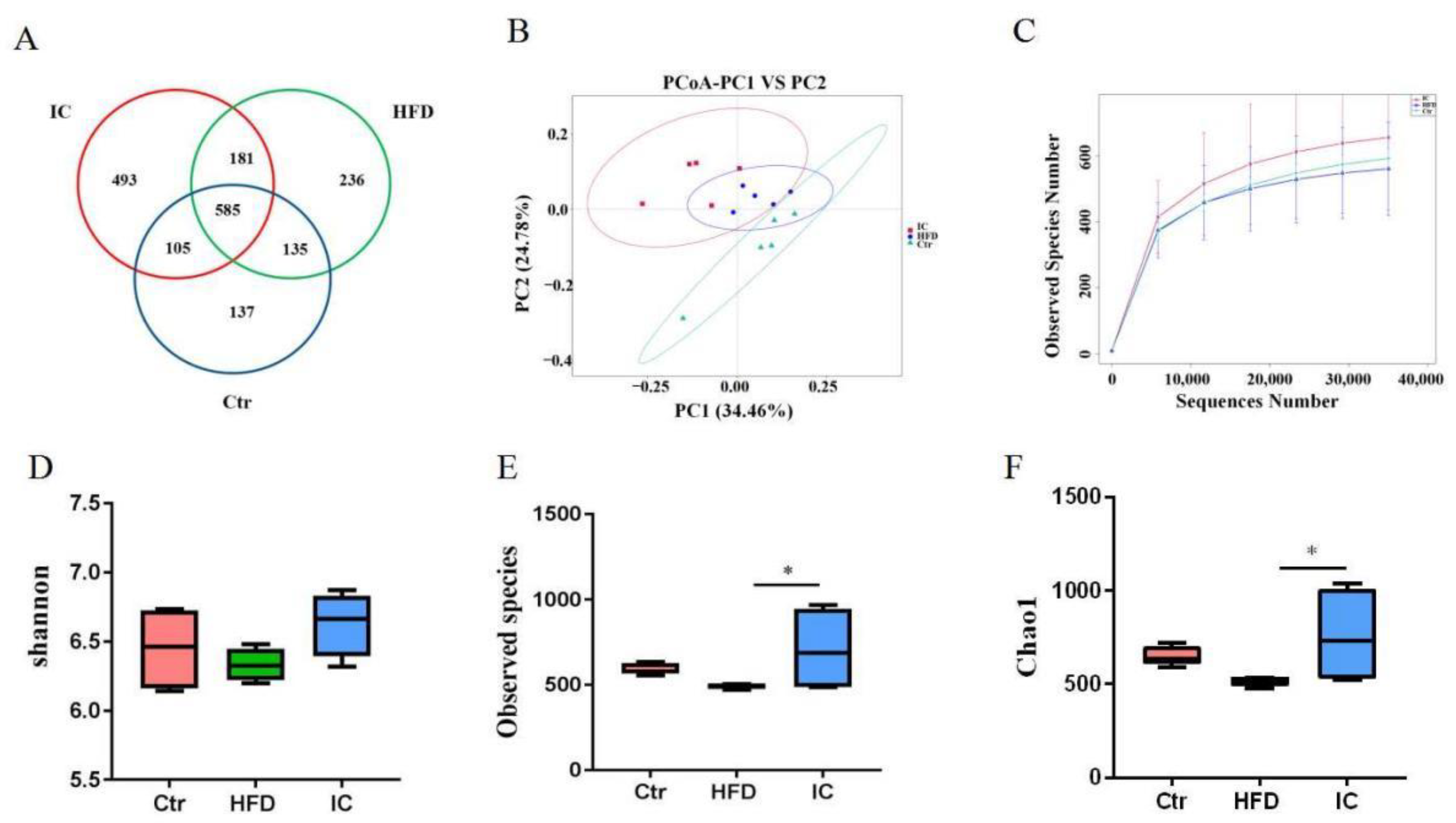
2.5. Gut Microbiota Analysis
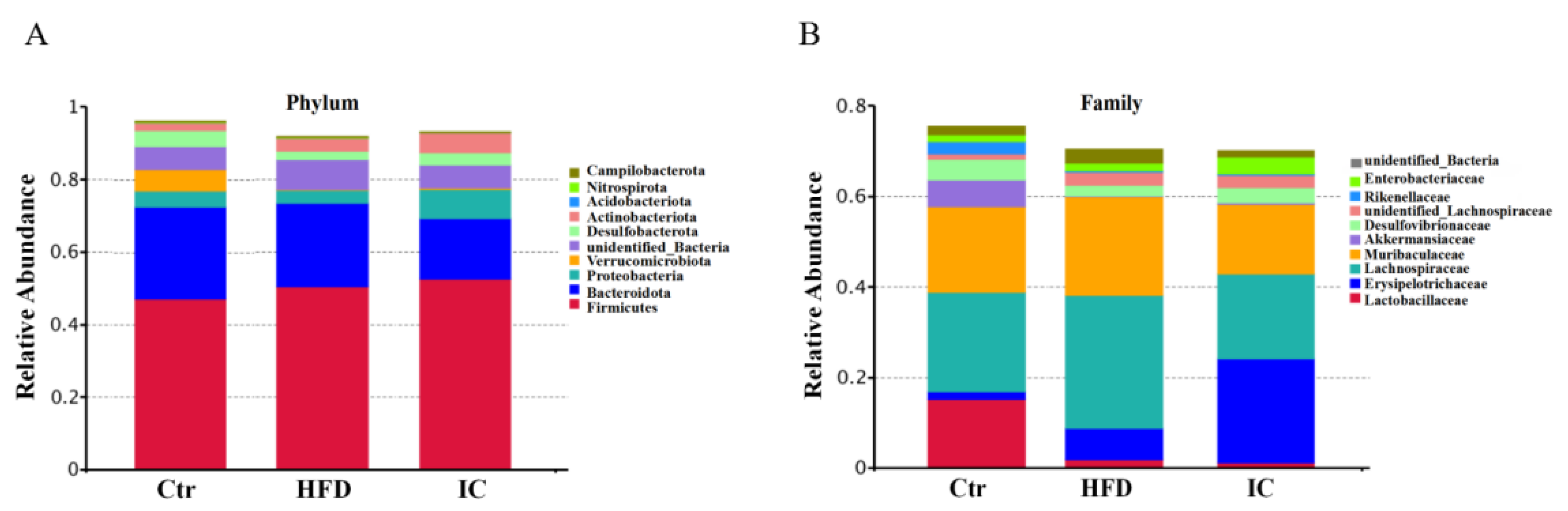
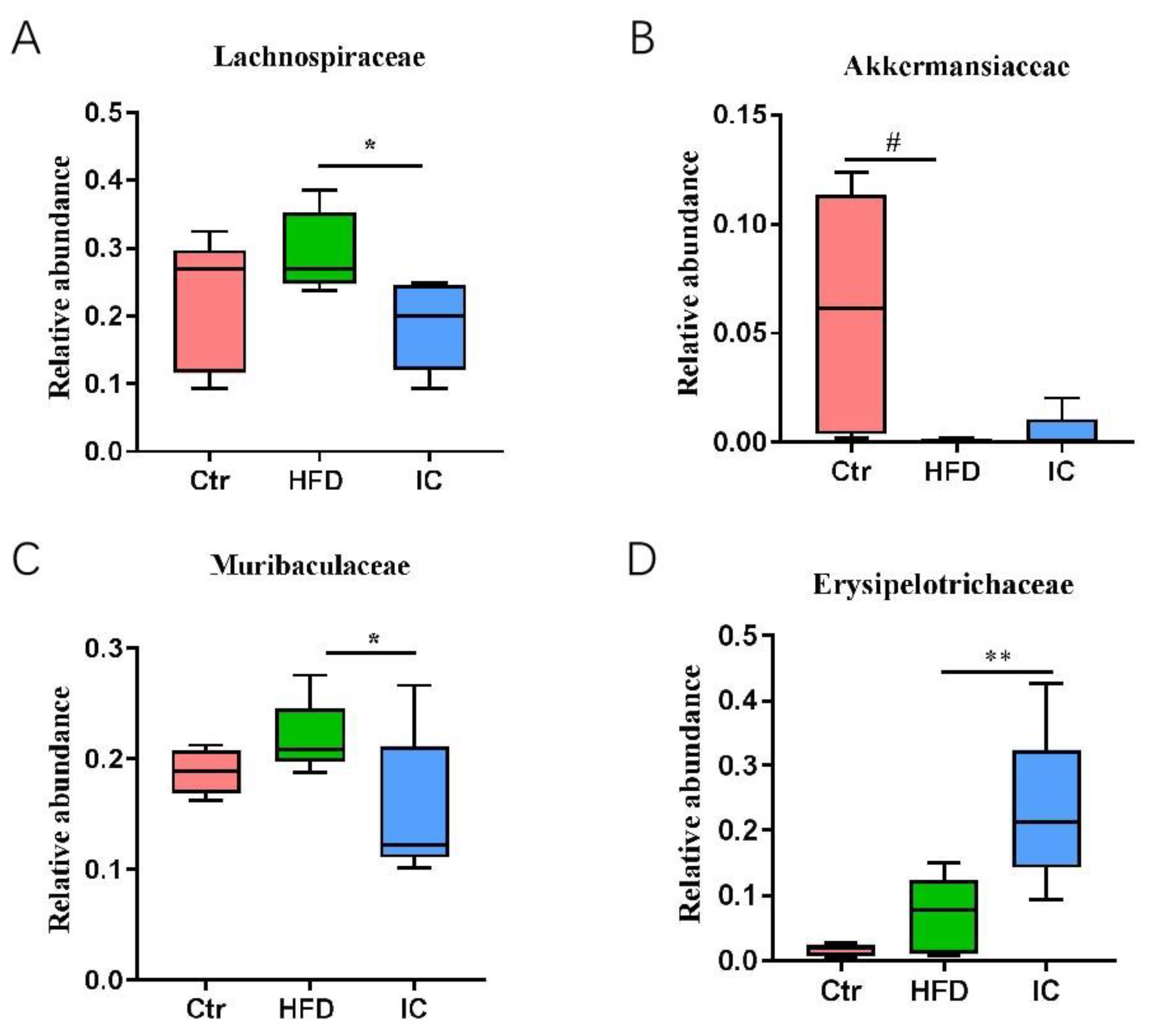
2.6. Analysis of Species Abundance
2.7. Untargeted Metabolomic Study
2.7.1. Data Quality and Identification of Metabolites
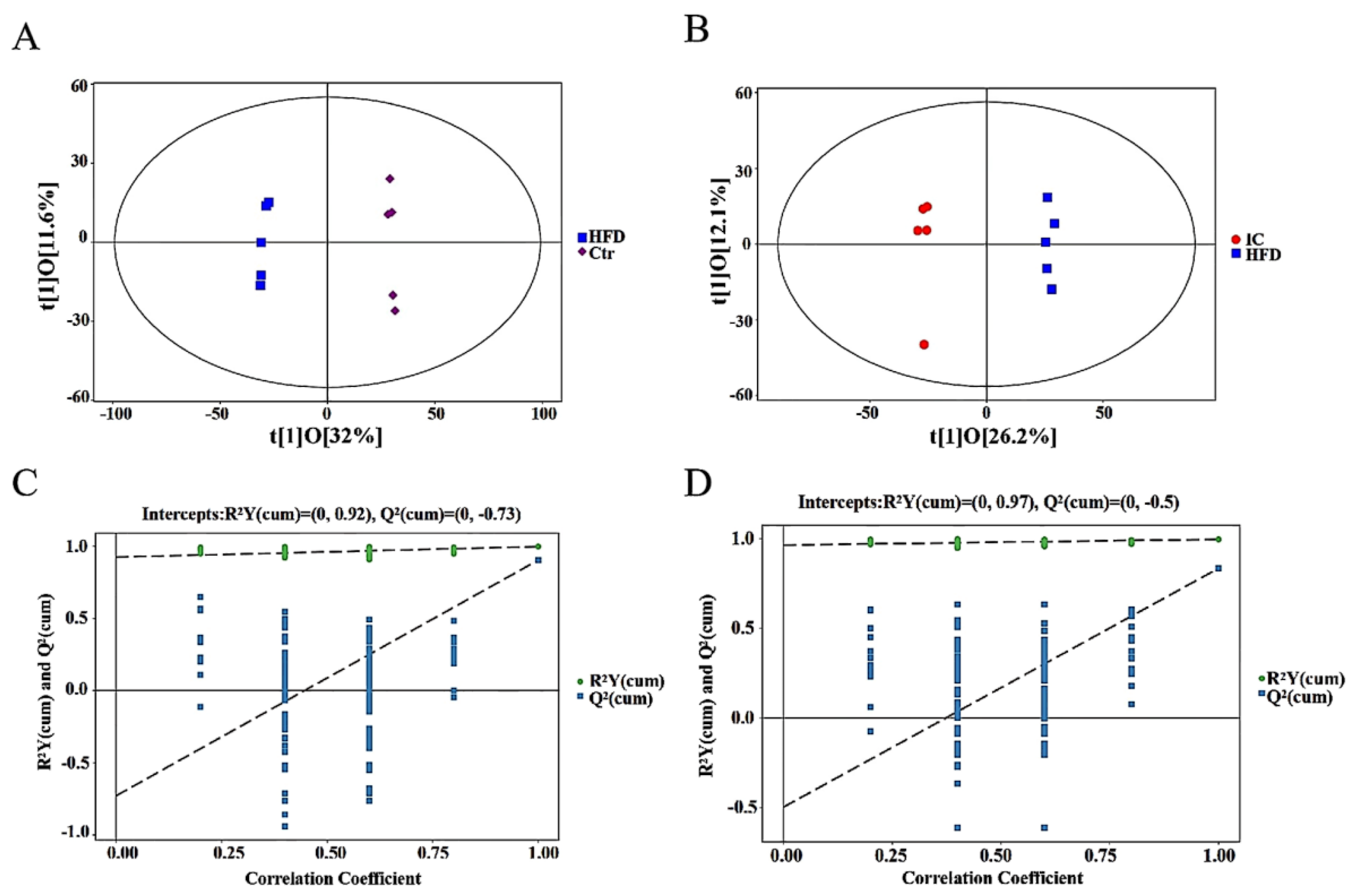
2.7.2. OPLS-DA Analysis
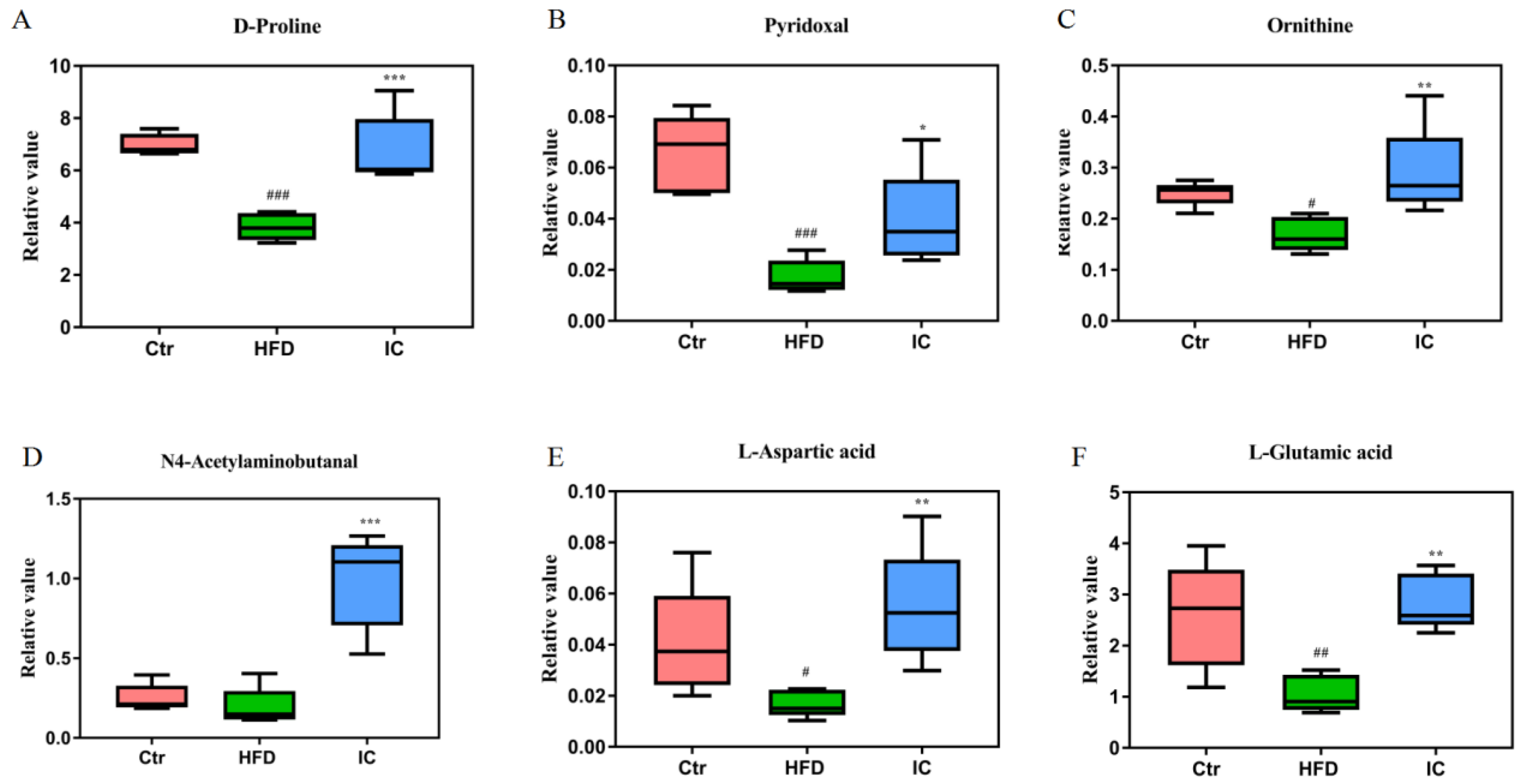
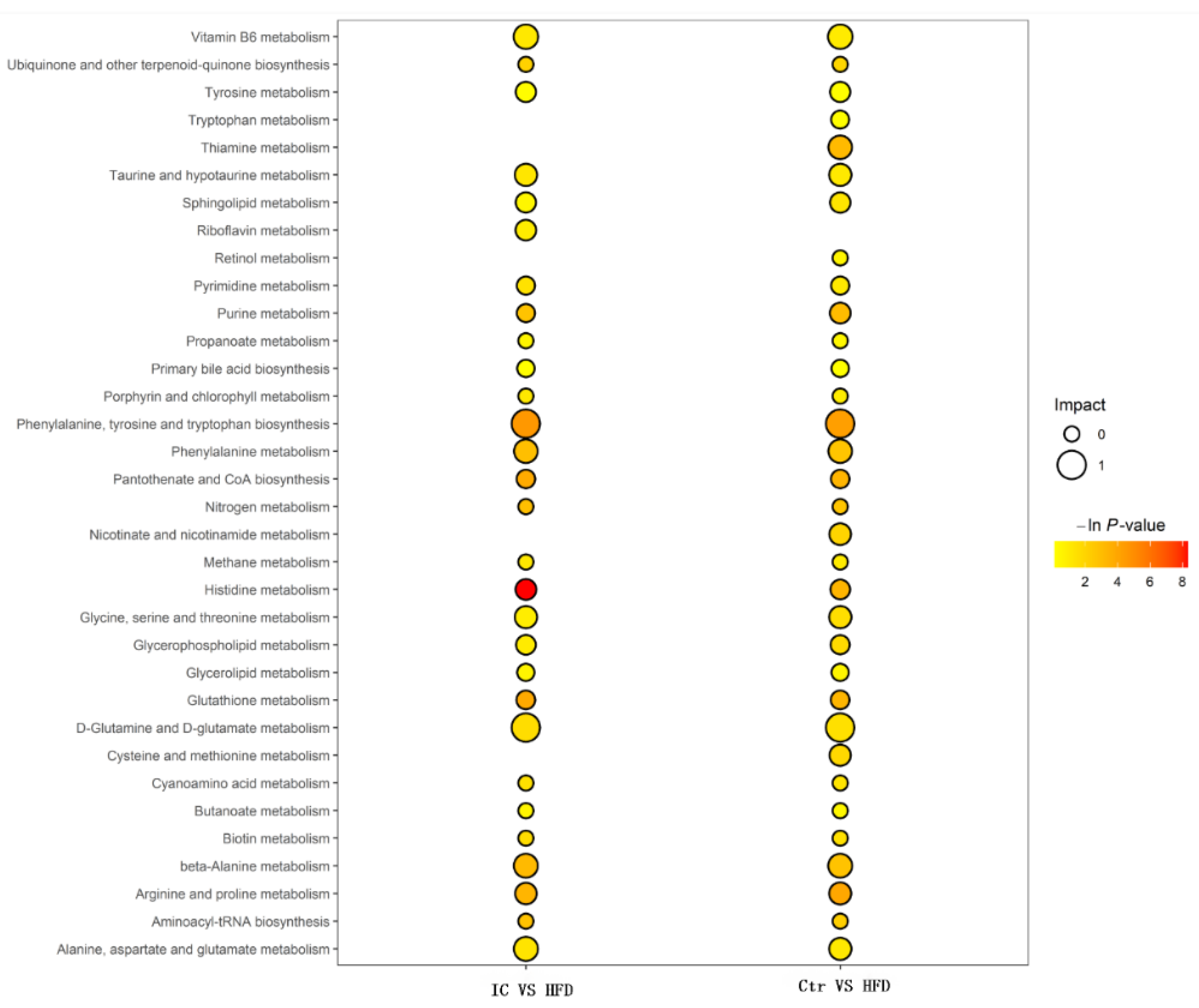
2.7.3. Changes in Metabolites and Biological Metabolic Pathways
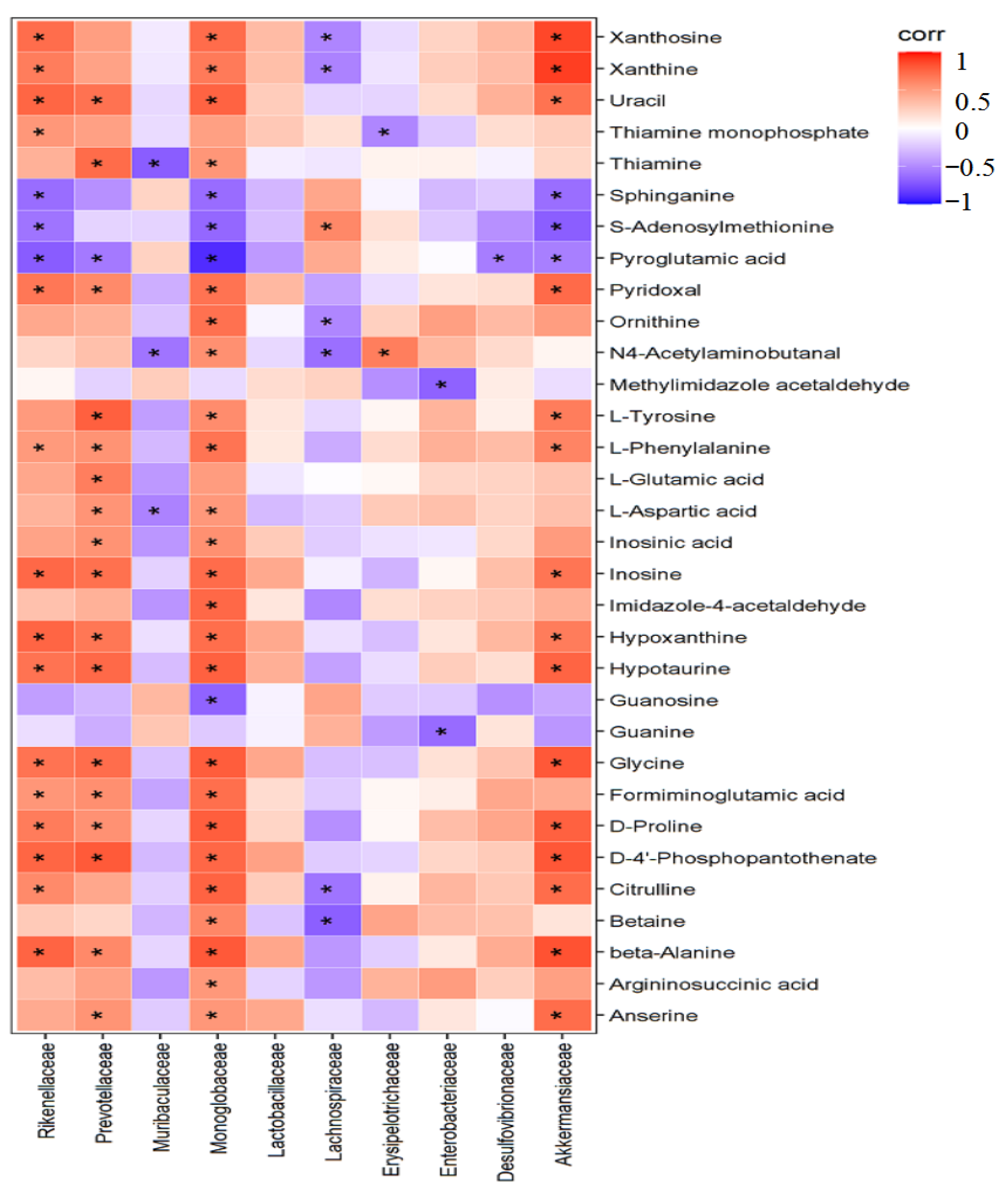
2.8. Correlation Analysis between Differential Bacteria and Liver Metabolism
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Serum Biochemical Markers Assay
4.4. H&E Staining
4.5. Liver IL-6, IL-1β, and TNF-α Analysis
4.6. Gut Microbiota Analysis
4.7. Metabolomic Analysis of Liver Samples
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease-A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Pett, S.; Dalle Grave, R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: Pathophysiology, evidence, and practice. Hepatology 2016, 63, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Mulders, R.; de Git, K.; Schele, E.; Dickson, S.; Sanz, Y.; Adan, R. Microbiota in obesity: Interactions with enteroendocrine, immune and central nervous systems. Obes. Rev. 2018, 19, 435–451. [Google Scholar] [CrossRef]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Non-alcoholic fatty liver disease in adults: Current concepts in etiology, outcomes and management. Endocr. Rev. 2020, 41, bnz009. [Google Scholar] [CrossRef]
- Luo, B.S. Mongolian Pharmacy; Inner Mongolia Nationalities Publishing House Press: Huhhot, China, 2006; pp. 99–100. [Google Scholar]
- Li, H.; Xie, W.; Qiao, X.; Cui, H.; Yang, X.; Xue, C. Structural characterization of arabinogalactan extracted from Ixeris chinensis (Thunb.) Nakai and its immunomodulatory effect on RAW264.7 macrophages. Int. J. Biol. Macromol. 2020, 143, 977–983. [Google Scholar] [CrossRef]
- Ba, G.N. Mongolian Medicine Prescription; Inner Mongolia People’s Publishing House Press: Huhhot, China, 2007; pp. 95–96. [Google Scholar]
- Xi, G.; Haliga; Bai, S.Z.; Raxinamujila. Research progress of chemical compound and pharmacology of Ixeris chinensis (Thunb.) Nakai. Nat. Prod. Res. Dev. 2020, 32, 1259–1267. [Google Scholar]
- Liu, X.; Sun, R.; Li, Z.; Xiao, R.; Lv, P.; Sun, X.; Olson, M.A.; Gong, Y. Luteolin alleviates non-alcoholic fatty liver disease in rats via restoration of intestinal mucosal barrier damage and microbiota imbalance involving in gut-liver axis. Arch. Biochem. Biophys. 2021, 711, 109019. [Google Scholar] [CrossRef]
- Shih, K.N.; Huang, W.T.; Chang, C.L.; Feng, C.C. Effects of Ixeris Chinensis (Thunb.) Nakai boiling water extract on hepatitis B viral activity and hepatocellular carcinoma. Afr. J. Tradit. Complement. Altern. Med. 2013, 11, 187–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 70–715. [Google Scholar] [CrossRef]
- Gérard, P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158. [Google Scholar] [CrossRef]
- Adolph, T.E.; Grander, C.; Moschen, A.R.; Tilg, H. Liver-Microbiome Axis in Health and Disease. Trends Immunol. 2018, 39, 712–723. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Ni, Y.; Ni, L.; Zhuge, F.; Fu, Z. The Gut Microbiota and Its Metabolites, Novel Targets for Treating and Preventing Non-Alcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2020, 64, e2000375. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shan, W.; Gao, L.; Gao, D.; Hu, Y.; Wang, G.; Zhang, N.; Li, Z.; Tian, X.; Xu, W.; et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 16013. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Mazzotti, A. NAFLD incidence and remission: Only a matter of weight gain and weight loss? J. Hepatol. 2015, 62, 15–17. [Google Scholar] [CrossRef]
- Patel, N.S.; Doycheva, I.; Peterson, M.R.; Hooker, J.; Kisselva, T.; Schnabl, B.; Seki, E.; Sirlin, C.B.; Loomba, R. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2015, 1, 561–568.e1. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Cheung, O.; Sanyal, A.J. Recent advances in nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2010, 26, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.S.; Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Alanine and aspartate aminotransferase and glutamine-cycling pathway: Their roles in pathogenesis of metabolic syndrome. World J. Gastroenterol. 2012, 18, 3775–3781. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Bonsembiante, L.; Targher, G.; Maffeis, C. Non-alcoholic fatty liver disease in obese children and adolescents: A role for nutrition? Eur. J. Clin. Nutr. 2021, 76, 28–39. [Google Scholar] [CrossRef]
- Haukeland, J.W.; Damas, J.K.; Konopski, Z.; Loberg, E.M.; Haaland, T.; Goverud, I.; Torjesen, P.A.; Birkeland, K.; Bjøro, K.; Aukrust, P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 2006, 44, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- van der Poorten, D.; Milner, K.L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Muelle, O.; Barret, M.; Machado, M.; Fizanne, L.; Araujo-Perez, F.; Guy, C.D.; Seed, P.C.; Rawls, J.F.; David, L.A.; et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016, 63, 764–775. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef]
- Le, R.T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed. Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, F.; Lin, N.; Ye, J.; Zheng, Q.; Ding, G. Effects of glutamine on oxidative stress and nuclear factor-κB expression in the livers of rats with nonalcoholic fatty liver disease. Exp. Ther. Med. 2014, 7, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Jin, C.J.; Degen, C.; De Bandt, J.P.; Bergheim, I. Oral Glutamine Supplementation Protects Female Mice from Nonalcoholic Steatohepatitis. J. Nutr. 2015, 145, 2280–2286. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Carè, I.; Mazza, E.; Provenzano, F.; Colica, C.; Torti, C.; Romeo, S.; Pujia, A.; Montalcini, T. Protein and vitamin B6 intake are associated with liver steatosis assessed by transient elastography, especially in obese individuals. Clin. Mol. Hepatol. 2017, 23, 249–259. [Google Scholar] [CrossRef]
- Liu, Z.; Li, P.; Zhao, Z.H.; Zhang, Y.; Ma, Z.M.; Wang, S.X. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation in Apoe (−/−) Mice Fed with High-Fat Diet. J. Diabetes Res. 2016, 2016, 1748065. [Google Scholar] [CrossRef]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef]
- Wu, Z.; Hou, Y.; Hu, S.; Bazer, F.W.; Meininger, C.J.; McNeal, C.J.; Wu, G. Catabolism and safety of supplemental l-arginine in animals. Amino Acids 2016, 48, 1541–1552. [Google Scholar] [CrossRef]
- McKnight, J.R.; Satterfeld, M.C.; Jobgen, W.S.; Smith, S.B.; Spencer, T.E.; Meininger, C.J.; McNeal, C.J.; Wu, G. Benefcial efects of l-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids 2010, 39, 349–357. [Google Scholar] [CrossRef]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: The interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]



| Groups | ALT (U/L) | AST (U/L) | TG (mg/dL) | TC (mg/dL) | HDL-C (mmoL/L) | LDL-C (mmoL/L) |
|---|---|---|---|---|---|---|
| Control | 31.26 ± 8.65 | 76.00 ± 13.71 | 0.32 ± 0.01 | 2.43 ± 0.13 | 2.26 ± 0.07 | 1.18 ± 0.18 |
| HFD | 57.13 ± 28.60 # | 87.00 ± 16.35 | 0.38 ± 0.02 # | 4.93 ± 1.15 ## | 3.79 ± 0.12 | 3.64 ± 0.68 ## |
| IC 0.5 g/kg | 35.67 ± 7.45 * | 88.67 ± 3.67 | 0.27 ± 0.01 ** | 3.50 ± 0.50 | 3.98 ± 0.12 | 2.56 ± 0.15 * |
| IC 1.5 g/kg | 20.50 ± 10.10 ** | 82.50 ± 11.29 | 0.31 ± 0.01 * | 3.43 ± 0.29 | 3.75 ± 0.10 | 2.46 ± 0.08 * |
| IC 3.0 g/kg | 30.00 ± 19.49 ** | 75.71 ± 9.69 * | 0.30 ± 0.02 * | 3.65 ± 0.42 | 3.80 ± 0.11 | 2.55 ± 0.10 * |
| Groups | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) |
|---|---|---|---|
| Control | 18.95 ± 0.26 | 17.91 ± 0.28 | 106.99 ± 2.91 |
| HFD | 19.38 ± 0.34 | 18.74 ± 0.50 | 115.60 ± 2.59 |
| IC 0.5 g/kg | 17.32 ± 0.97 ** | 15.80 ± 0.62 ** | 107.27 ± 2.32 |
| IC 1.5 g/kg | 18.33 ± 0.25 * | 16.79 ± 0.40 ** | 111.68 ± 2.59 |
| IC 3.0 g/kg | 18.57 ± 0.20 | 17.42 ± 0.28 * | 99.70 ± 6.22 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Cho, S.; Laxi, N.; Bao, T.; Dai, L.; Yu, H.; Qi, R.; Zhang, J.; Ba, G.; Fu, M. Hepatoprotective Effects of Ixeris chinensis on Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice: An Integrated Gut Microbiota and Metabolomic Analysis. Molecules 2022, 27, 3148. https://doi.org/10.3390/molecules27103148
Jin W, Cho S, Laxi N, Bao T, Dai L, Yu H, Qi R, Zhang J, Ba G, Fu M. Hepatoprotective Effects of Ixeris chinensis on Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice: An Integrated Gut Microbiota and Metabolomic Analysis. Molecules. 2022; 27(10):3148. https://doi.org/10.3390/molecules27103148
Chicago/Turabian StyleJin, Wenjie, Sungbo Cho, Namujila Laxi, Terigele Bao, Lili Dai, Hongzhen Yu, Rigeer Qi, Junqing Zhang, Genna Ba, and Minghai Fu. 2022. "Hepatoprotective Effects of Ixeris chinensis on Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice: An Integrated Gut Microbiota and Metabolomic Analysis" Molecules 27, no. 10: 3148. https://doi.org/10.3390/molecules27103148
APA StyleJin, W., Cho, S., Laxi, N., Bao, T., Dai, L., Yu, H., Qi, R., Zhang, J., Ba, G., & Fu, M. (2022). Hepatoprotective Effects of Ixeris chinensis on Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in Mice: An Integrated Gut Microbiota and Metabolomic Analysis. Molecules, 27(10), 3148. https://doi.org/10.3390/molecules27103148






