Design of Fluorescent Hybrid Materials Based on POSS for Sensing Applications
Abstract
:1. Introduction
2. Synthesis and Design of POSS-Based Fluorescent Materials
2.1. POSS-Based Organic-Molecule Fluorescent Materials
2.2. POSS-Based Polymer Fluorescent Materials
2.3. POSS-Based QDs Fluorescent Materials
2.4. POSS-Based Lanthanide Fluorescent Materials
3. Sensing Application of POSS-Based Fluorescent Materials
3.1. Metal Ion Sensing
3.2. Anion Sensing
4. Conclusions and Perspectives
- (1)
- Facile and controllable synthesis of POSS-based fluorescent hybrid materials. The “click” chemistry reaction, especially metal-free “thiol-ene” click chemistry, will brilliantly shine in this respect.
- (2)
- Sensing mechanism. Theoretical calculation may be a very effective and practical method to study the sensing mechanism of POSS-based fluorescent hybrid materials by using the density functional theory (DFT) and time-dependent density functional theory (TDDFT).
- (3)
- Sensing application and the others. The detection of explosives and organic small molecular pollutants and the application of fluorescent materials in photodynamic therapy and biological imaging are attracting more and more researchers’ attention. POSS-based fluorescent hybrid materials will make great progress in these areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saraf, M.; Tavakkoli Yaraki, M.; Prateek; Tan, Y.N.; Gupta, R.K. Insights and perspectives regarding nanostructured fluorescent materials toward tackling COVID-19 and future pandemics. ACS Appl. Nano Mater. 2021, 4, 911–948. [Google Scholar] [CrossRef]
- Kundu, A.; Layek, R.K.; Kuila, A.; Nandi, A.K. Highly fluorescent graphene oxide-poly(vinyl alcohol) hybrid: An effective material for specific Au3+ ion sensors. ACS Appl. Mater. Interfaces 2012, 4, 5576–5582. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Kaur, R.; Singh, N.; Kuwar, A.S.; Kaur, N. High performance fluorescent turn-on probe for amitriptyline based on hybrid nanoassembly of organic–inorganic nanoparticles. ACS Appl. Bio Mater. 2019, 2, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Soares, M.C.P.; Cabral, T.D.; Fujiwara, E.; de Barros Cordeiro, C.M.; Criado, A.; Prato, M.; Bartoli, J.R. Agarose-based fluorescent waveguide with embedded silica nanoparticle–carbon nanodot hybrids for pH sensing. ACS Appl. Nano Mater. 2021, 4, 9738–9751. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Liu, H.-B.; Zhang, D.; Nong, H.; Wu, P.; Chen, P.; Li, D. Aggregation induced emission active fluorescent sensor for the sensitive detection of Hg2+ based on organic–inorganic hybrid mesoporous material. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117585. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, J.; Zhao, L.; Yan, B. Eu3+-β-diketone functionalized covalent organic framework hybrid material as a sensitive and rapid response fluorescent sensor for glutaraldehyde. Talanta 2022, 236, 122877. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, H.; Tang, X.; Ren, S.; He, X.; Li, J.; Du, C.; Feng, Z.; Lu, P. Novel blue fluorescent materials for high-performance nondoped blue OLEDs and hybrid pure white OLEDs with ultrahigh color rendering index. Nano Energy 2020, 68, 104325. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Chen, L.; Deng, Y.; Peng, Y.; Lu, F.; Zhu, W. High-performance hybrid white organic light-emitting diodes with bipolar host material and thermally activated delayed fluorescent emitter. Opt. Mater. 2020, 100, 109673. [Google Scholar] [CrossRef]
- Yang, H.; Peng, X.; Cao, C.; Wu, L.; Chen, N.; Zhang, X.; Xie, W.; Tong, Q.; Wu, Z. A deep blue fluorescent emitter functioning as host material in highly efficient phosphorescent and hybrid white organic light-emitting devices. Org. Electron. 2020, 85, 105848. [Google Scholar] [CrossRef]
- Guan, H.-M.; Hu, Y.-X.; Xie, D.-D.; Chi, H.-J.; Xiao, G.-Y.; Lv, Y.-L.; Li, X.; Zhang, D.-Y.; Hu, Z.-Z. Novel multifunctional fluorene-phenanthroimidazole hybrid materials: Non-doped near-ultraviolet fluorescent emitter and host for green phospho-rescent OLEDs. Dyes Pigments 2021, 186, 109019. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Isoppo, V.G.; Moro, A.V.; Rodembusch, F.S. Photoactive organic-inorganic hybrid materials: From silylated compounds to optical applications. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100474. [Google Scholar] [CrossRef]
- Tunstall-Garcia, H.; Charles, B.L.; Evans, R.C. The role of polyhedral oligomeric silsesquioxanes in optical applications. Adv. Photon Res. 2021, 2, 2000196. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhou, M.; Chen, J.; Zhao, T.; Tang, L.; Bi, H. Fluorescent CDs@PCL hybrids via tartaric acid, CDs-cocatalyzed polymerization. Mater. Sci. Eng. C -Mater. 2017, 79, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Ma, H.; Lv, J.; Wang, T.; Yang, Y.; Yin, P.; Lei, Z.; Qin, Y.; Ma, Y.; Yao, W. Fluorescent hybrid materials: A versatile platform for gas sensing, pH sensing, and cell imaging. React. Funct. Polym. 2018, 125, 84–92. [Google Scholar] [CrossRef]
- Alhmoud, H.; Brodoceanu, D.; Elnathan, R.; Kraus, T.; Voelcker, N.H. Reprint of: A MACEing silicon: Towards single-step etching of defined porous nanostructures for biomedicine. Prog. Mater. Sci. 2021, 120, 100817. [Google Scholar] [CrossRef]
- Jin, H.; Yang, M.; Sun, Z.; Gui, R. Ratiometric two-photon fluorescence probes for sensing, imaging and biomedicine applications at living cell and small animal levels. Coordin. Chem. Rev. 2021, 446, 214114. [Google Scholar] [CrossRef]
- Stone, V.; Nowack, B.; Baun, A.; van den Brink, N.; von der Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellöv, M.; Joner, E.; et al. Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physicochemical characterisation. Sci. Total Environ. 2010, 408, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sharma, A.; Ma, Q.; Zhu, Y.; Li, D.; Liu, Z.; Yang, Y. A developed hybrid fixed-bed bioreactor with Fe-modified zeolite to enhance and sustain biohydrogen production. Sci. Total Environ. 2021, 758, 143658. [Google Scholar] [CrossRef]
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal. Chem. 2014, 54, 83–102. [Google Scholar] [CrossRef]
- Mahyad, B.; Janfaza, S.; Hosseini, E.S. Bio-nano hybrid materials based on bacteriorhodopsin: Potential applications and future strategies. Adv. Colloid Interface Sci. 2015, 225, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Hanprasit, S.; Tungkijanansin, N.; Prompawilai, A.; Eangpayung, S.; Ervithayasuporn, V. Synthesis and isolation of non-chromophore cage-rearranged silsesquioxanes from base-catalyzed reactions. Dalton Trans. 2016, 45, 16117–16120. [Google Scholar] [CrossRef] [PubMed]
- Chimjarn, S.; Kunthom, R.; Chancharone, P.; Sodkhomkhum, R.; Sangtrirutnugul, P.; Ervithayasuporn, V. Synthesis of aromatic functionalized cage-rearranged silsesquioxanes (T8, T10, and T12) via nucleophilic substitution reactions. Dalton Trans. 2015, 44, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Ervithayasuporn, V.; Chimjarn, S. Synthesis and isolation of methacrylate-and acrylate-functionalized polyhedral oligomeric silsesquioxanes (T8, T10, and T12) and characterization of the relationship between their chemical structures and physical properties. Inorg. Chem. 2013, 52, 13108–13112. [Google Scholar] [CrossRef]
- Kaneko, Y.; Shoiriki, M.; Mizumo, T. Preparation of cage-like octa(3-aminopropyl)silsesquioxane trifluoromethanesulfonate in higher yield with a shorter reaction time. J. Mater. Chem. 2012, 22, 14475–14478. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Hanprasit, S.; Masik, M.; Prigyai, N.; Kiatkamjornwong, S. Silsesquioxane cages as fluoride sensors. Chem. Commun. 2017, 53, 12108–12111. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Gnanasekaran, D.; Madhavan, K.; Reddy, B. Developments of polyhedral oligomeric silsesquioxanes (POSS), possnanocom-posites and their applications: A review. J. Sci. Ind. Res. India 2009, 68, 437–464. [Google Scholar]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Lu, F.; Qu, J.; Chen, H.; Dai, L. Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J. Phys. Chem. Lett. 2012, 3, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Marcolli, C.; Calzaferri, G. Monosubstituted octasilasesquioxanes. Appl. Organomet. Chem. 1999, 13, 213–226. [Google Scholar] [CrossRef]
- Ro, H.W.; Soles, C.L. Silsesquioxanes in nanoscale patterning applications. Mater. Today 2011, 14, 20–33. [Google Scholar] [CrossRef]
- Wang, M.; Chi, H.; Joshy, K.S.; Wang, F. Progress in the synthesis of bifunctionalized polyhedral oligomeric silsesquioxane. Polymers 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. POSS hybrid hydrogels: A brief review of synthesis, properties and applications. Eur. Polym. J. 2021, 143, 110180. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Seifalian, A.M. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Acc. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, L.C.; Ni, H.L.; Pittman, C.U., Jr. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. Mater. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Loman-Cortes, P.; Binte Huq, T.; Vivero-Escoto, J.L. Use of polyhedral oligomeric silsesquioxane (POSS) in drug delivery, photodynamic therapy and bioimaging. Molecules 2021, 26, 6453. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, J.; Pielichowski, K. Recent advances in polyurethane/POSS hybrids for biomedical applications. Molecules 2022, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, G.S.; Shah, S.M.; Hussain, Z.; Muhammad, S.; Siddiq, M.; Hussain, H. Water soluble polyhedral oligomeric silsesquioxane based amphiphilic hybrid polymers: Synthesis, self-assembly, and applications. Eur. Polym. J. 2016, 75, 67–92. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; He, C. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral oligomeric silsesquioxane hybrid polymers: Well-defined architectural design and potential functional applications. Macromol. Rapid Comm. 2019, 40, 1900101. [Google Scholar] [CrossRef]
- Dudziec, B.; Zak, P.; Marciniec, B. Synthetic routes to silsesquioxane-based systems as photoactive materials and their precursors. Polymers 2019, 11, 504. [Google Scholar] [CrossRef] [Green Version]
- Xiang, K.; Li, Y.; Xu, C.; Li, S. POSS-based organic–inorganic hybrid nanomaterials: Aggregation-enhanced emission, and highly sensitive and selective detection of nitroaromatic explosives in aqueous media. J. Mater. Chem. C 2016, 4, 5578–5583. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, H.Y.; Nie, W.Y.; Ying, J.Y. Synthesis and characterization of luminescent organic-inorganic hybrid nanocomposite from polyhedral oligomeric silsesquioxane. Chin. Chem. Lett. 2010, 21, 753–757. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, T.; Zhao, Y.; Zhang, H.; Zhao, G.; Li, J.; Chai, L. A new star-shaped carbazole derivative with polyhedral oligomeric silsesquioxane core: Crystal structure and unique photoluminescence property. J. Fluoresc. 2016, 26, 149–154. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, H.; Xu, J. Cubic polyhedral oligomeric silsesquioxane based functional materials: Synthesis, assembly, and applications. Chem. Asian J. 2016, 11, 1322–1337. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Li, L.; Zhao, X.; Feng, S.; Liu, H. Hybrid networks constructed from tetrahedral silicon-centered precursors and cubic POSS-based building blocks via Heck reaction: Porosity, gas sorption, and luminescence. J. Mater. Chem. A 2013, 1, 13549–13558. [Google Scholar] [CrossRef]
- Ke, F.; Wang, S.; Guang, S.; Liu, Q.; Xu, H. Synthesis and properties of broad-band absorption POSS-based hybrids. Dyes Pigments 2015, 121, 199–203. [Google Scholar] [CrossRef]
- Wang, D.; Feng, S.; Liu, H. Fluorescence-tuned polyhedral oligomeric silsesquioxane-based porous polymers. Chem. Eur. J. 2016, 22, 14319–14327. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-González, J.; Ferrer-Ugalde, A.; Bhattacharyya, S.; Chaari, M.; Teixidor, F.; Gierschner, J.; Núñez, R. Fluorescent carborane–vinylstilbene functionalised octasilsesquioxanes: Synthesis, structural, thermal and photophysical properties. J. Mater. Chem. C 2017, 5, 10211–10219. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Song, X.; Zeng, L.; Xing, J. Synthesis and assembly of polyhedral oligomeric silsesquioxane end-capped amphiphilic polymer to enhance the fluorescent intensity of tetraphenylethene. Colloid Polym. Sci. 2016, 294, 1315–1324. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Wang, X.; Hu, Y.; Hu, H.; Wu, D.-C.; Xu, F.-J. Bioreducible POSS-cored star-shaped polycation for efficient gene delivery. ACS Appl. Mater. Interfaces 2014, 6, 1044–1052. [Google Scholar] [CrossRef]
- Li, M.; Song, X.; Zhang, T.; Zeng, L.; Xing, J. Aggregation induced emission controlled by a temperature-sensitive organic–inorganic hybrid polymer with a particular LCST. RSC Adv. 2016, 6, 86012–86018. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, Y.; Zhang, Z.; Zhang, W. Enhancing the efficacy of photodynamic therapy through a porphyrin/POSS alternating copolymer. Angew. Chem. Int. Ed. 2018, 57, 16354–16358. [Google Scholar] [CrossRef]
- Zhang, W.; Müller, A.H. Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsequioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Y.; Zheng, S.; Li, S.; Hu, T.; Tang, A.; Gao, C. Water soluble octa-functionalized POSS: All-click chemistry synthesis and efficient host–guest encapsulation. Chem. Commun. 2014, 50, 8712–8714. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Wang, W.; Yang, Y.; Li, D.; Ma, Y. One-pot synthesis of bio-functionally water-soluble POSS derivatives via efficient click chemistry methodology. React. Funct. Polym. 2019, 140, 103–110. [Google Scholar] [CrossRef]
- Xi, W.; Scott, T.F.; Kloxin, C.J.; Bowman, C.N. Click chemistry in materials science. Adv. Funct. Mater. 2014, 24, 2572–2590. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Rizvi, S.B.; Yang, S.Y.; Green, M.; Keshtgar, M.; Seifalian, A.M. Novel POSS–PCU nanocomposite material as a biocompatible coating for quantum dots. Bioconjug. Chem. 2015, 26, 2384–2396. [Google Scholar] [CrossRef]
- Zhao, X.; Du, J.; Wu, Y.; Liu, H.; Hao, X. Synthesis of highly luminescent POSS-coated CdTe quantum dots and their application in trace Cu2+ detection. J. Mater. Chem. A 2013, 1, 11748–11753. [Google Scholar] [CrossRef]
- Yi, S.S.; Jung, J.Y. Rare earth doped organic–inorganic hybrid polyhedral oligomeric silsesquioxane phosphors applied for flexible sheet and anti-counterfeiting. Mater. Express 2021, 11, 1732–1738. [Google Scholar] [CrossRef]
- Yan, B. Photophysical applications of photofunctional rare-earth hybrid materials. In Photofunctional Rare Earth Hybrid Materials; Springer: Berlin, Germany, 2017; pp. 199–255. [Google Scholar]
- Imae, I.; Kawakami, Y. Unique photoluminescence property of a novel perfectly carbazole-substituted POSS. J. Mater. Chem. 2005, 15, 4581–4583. [Google Scholar] [CrossRef]
- Cho, H.-J.; Hwang, D.-H.; Lee, J.-I.; Jung, Y.-K.; Park, J.-H.; Lee, J.; Lee, A.S.-K.; Shim, H.-K. Electroluminescent polyhedral oligomeric silsesquioxane-based nanoparticle. Chem. Mater. 2006, 18, 3780–3787. [Google Scholar] [CrossRef]
- Clarke, D.; Mathew, S.; Matisons, J.; Simon, G.; Skelton, B.W. Synthesis and characterization of a range of POSS imides. Dyes Pigments 2012, 92, 659–667. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, H.; Sun, D.; Li, X. Synthesis and aggregation properties of a series of dumbbell polyhedral oli-gosilsesquioxane-perylene diimide triads. CrystEngComm 2015, 17, 1453–1463. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, Y.; Guang, S.; Ke, F.; Xu, H. Facile preparation and investigation of the properties of single molecular POSS-based white-light-emitting hybrid materials using click chemistry. N. J. Chem. 2018, 42, 555–563. [Google Scholar] [CrossRef]
- Du, F.; Wang, H.; Bao, Y.; Liu, B.; Zheng, H.; Bai, R. Conjugated coordination polymers based on 8-hydroxyquinoline ligands: Impact of polyhedral oligomeric silsesquioxanes on solubility and luminescence. J. Mater. Chem. 2011, 21, 10859–10864. [Google Scholar] [CrossRef]
- Du, F.F.; Tian, J.; Wang, H.; Liu, B.; Jin, B.K.; Bai, R.K. Synthesis and luminescence of POSS-containing perylene bisimide-bridged amphiphilic polymers. Macromolecules 2012, 45, 3086–3093. [Google Scholar] [CrossRef]
- Ertan, S.; Kaya, M.; Cihaner, A. Polyhedral oligomeric silsesquioxane cage integrated soluble and fluorescent poly(3,4-propylenedioxythiophene) dye. Polymer 2021, 212, 123127. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef] [PubMed]
- Filali, S.; Pirot, F.; Miossec, P. Biological applications and toxicity minimization of semiconductor quantum dots. Trends Biotechnol. 2020, 38, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Tandale, P.; Choudhary, N.; Singh, J.; Sharma, A.; Shukla, A.; Sriram, P.; Soni, U.; Singla, N.; Barnwal, R.P.; Singh, G.; et al. Fluorescent quantum dots: An insight on synthesis and potential biological application as drug carrier in cancer. Biochem. Biophys. Rep. 2021, 26, 100962. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal nanocrystals with molecular metal chalcogenide surface ligands. Science 2009, 324, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vaneski, A.; Yang, H.; Gupta, S.; Hetsch, F.; Kershaw, S.V.; Teoh, W.Y.; Li, H.; Rogach, A.L. Polyhedral oligomeric silsesquioxane as a ligand for CdSe quantum dots. J. Phys. Chem. C 2013, 117, 1857–1862. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Wu, Y.; Liu, H.; Hao, X. Facile fabrication of OA-POSS modified near-infrared-emitting CdSeTe alloyed quantum dots and their bioapplications. N. J. Chem. 2014, 38, 3242–3249. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and graphene quantum dots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Park, M.; Jeong, Y.; Kim, H.S.; Lee, W.; Nam, S.-H.; Lee, S.; Yoon, H.; Kim, J.; Yoo, S.; Jeon, S. Quenching-resistant solid-state photoluminescence of graphene quantum dots: Reduction of π−π stacking by surface functionalization with POSS, PEG, and HDA. Adv. Funct. Mater. 2021, 31, 2102741. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H. Recent progress in the lanthanide-complexes based luminescent hybrid materials. Coord. Chem. Rev. 2021, 441, 213988. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, P.; Wang, T.; Li, H. The first europium(III) β-diketonate complex functionalized polyhedral oligomeric silsesquioxane. Chem. Eur. J. 2014, 20, 2551–2556. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Deng, R.; Zhou, L.; Yu, Y.; Li, Y. Synthesis and near infrared luminescence properties of a series of lanthanide complexes with POSS modified ligands. Molecules 2019, 24, 1253. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, S.; Carniato, F.; Boccaleri, E. Synthesis and characterisation of a novel europium(III)-containing heptaisobutyl-POSS. N. J. Chem. 2014, 38, 2480–2485. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Li, H. Water-soluble luminescent hybrid composites consisting of oligosilsesquioxanes and lanthanide complexes and their sensing ability for Cu2+. Chem. Eur. J. 2016, 22, 3037–3043. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Ling, H.; Zhou, L.; Wang, F.; Li, Y. Construction and photoluminescence properties of octaimidazolium-based poly-hedral oligomeric silsesquioxanes hybrids through the “bridge” of ionic liquids: Chemical sensing for Cu2+. Dyes Pigments 2021, 184, 108840. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Tian, M.; Gou, Z.; Zuo, Y. The diversity of the coordination bond generated a POSS-based fluorescent probe for the reversible detection of Cu(II), Fe(III) and amino acids. J. Mater. Chem. B 2021, 9, 9744–9753. [Google Scholar] [CrossRef]
- Kunthom, R.; Piyanuch, P.; Wanichacheva, N.; Ervithayasuporn, V. Cage-like silsesequioxanes bearing rhodamines as fluorescence Hg2+ sensors. J. Photochem. Photobiol. A Chem. 2018, 356, 248–255. [Google Scholar] [CrossRef]
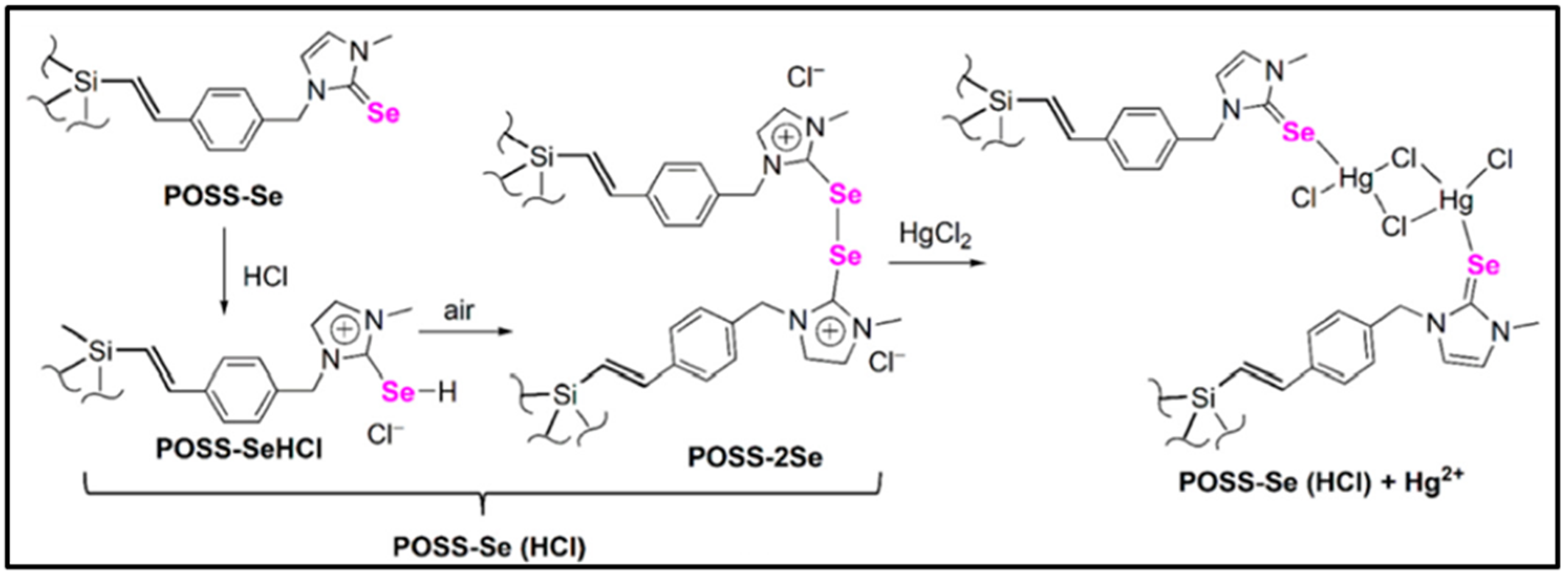
- Liu, H.; Chen, Z.; Feng, S.; Wang, D.; Liu, H. A Selenone-functionalized polyhedral oligomeric silsesquioxane for selective detection and adsorption of Hg2+ ions in aqueous solutions. Polymers 2019, 11, 2084. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Chen, Z.; Feng, S.; Wang, D.; Liu, H. A sulfur-containing fluorescent hybrid porous polymer for selective detection and adsorption of Hg2+ ions. Polym. Chem. 2022, 13, 2320–2330. [Google Scholar] [CrossRef]
- Omer, N.; Zhang, F.; Zhao, G.; Guang, S.; Xu, H. Highly selective chemosensor for repetitive detection of Fe3+ in pure water and bioimaging. Analyst 2019, 144, 3414–3421. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, S. New functionalized ionic liquids based on POSS for the detection of Fe3+ ion. Polymers 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, H.; Liu, H. Silsesquioxane-based fluorescent nanoporous polymer derived from a novel AIE chromophore for concurrent detection and adsorption of Ru3+. Sens. Actuators B Chem. 2020, 319, 128154. [Google Scholar] [CrossRef]
- Du, F.F.; Bao, Y.Y.; Liu, B.; Tian, J.; Li, Q.B.; Bai, R.K. POSS-containing red fluorescent nanoparticles for rapid detection of aqueous fluoride ions. Chem. Commun. 2013, 49, 4631–4633. [Google Scholar] [CrossRef]
- Wannasiri, C.; Chanmungkalakul, S.; Bunchuay, T.; Chuenchom, L.; Uraisin, K.; Ervithayasuporn, V.; Kiatkamjornwong, S. Cross-linking silsesquioxane cages with polyaromatics as fluorescent porous polymers for fluoride sensing and removal. ACS Appl. Polym. Mater. 2020, 2, 1244–1255. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Tan, H.R.; Lin, T.T.; Tang, B.Z.; Xu, J. Ionofluorochromic nanoparticles derived from octapyrene-modified polyhedral oligomeric silsesquioxane organic frameworks for fluoride-ion detection. ACS Appl. Nano Mater. 2019, 2, 470–478. [Google Scholar] [CrossRef]
- Sun, M.; Liu, H.; Su, Y.; Yang, W.; Lv, Y. Off/on amino-functionalized polyhedral oligomeric silsesquioxane–perylene diimides based hydrophilic luminescent polymer for aqueous fluoride ion detection. Anal. Chem. 2020, 92, 5294–5301. [Google Scholar] [CrossRef]
- Zeng, Y.-T.; Gao, S.-Y.; Traskovskis, K.; Gao, B.; Ren, X.-K. Polyhedral oligosilsesquioxane tethered tetraphenylethylene as turn-on fluorescent sensor for fluoride ions detection. Dyes Pigments 2021, 193, 109491. [Google Scholar] [CrossRef]
- Ding, G.; Zuo, Y.; Gai, F.; Wang, X.; Gou, Z.; Lin, W. A POSS-assisted fluorescent probe for the rapid detection of HClO in mitochondria with a large emission wavelength in dual channels. J. Mater. Chem. B 2021, 9, 6836–6843. [Google Scholar] [CrossRef] [PubMed]




















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.; Zhao, S.; Gan, X.; Sun, A.; Xia, Y.; Liu, Y. Design of Fluorescent Hybrid Materials Based on POSS for Sensing Applications. Molecules 2022, 27, 3137. https://doi.org/10.3390/molecules27103137
Ding S, Zhao S, Gan X, Sun A, Xia Y, Liu Y. Design of Fluorescent Hybrid Materials Based on POSS for Sensing Applications. Molecules. 2022; 27(10):3137. https://doi.org/10.3390/molecules27103137
Chicago/Turabian StyleDing, Sha, Shuai Zhao, Xingyue Gan, Aokui Sun, Yong Xia, and Yuejun Liu. 2022. "Design of Fluorescent Hybrid Materials Based on POSS for Sensing Applications" Molecules 27, no. 10: 3137. https://doi.org/10.3390/molecules27103137
APA StyleDing, S., Zhao, S., Gan, X., Sun, A., Xia, Y., & Liu, Y. (2022). Design of Fluorescent Hybrid Materials Based on POSS for Sensing Applications. Molecules, 27(10), 3137. https://doi.org/10.3390/molecules27103137






