Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Agronomic Traits
2.2. Total Phenolics Content (TPC)
2.3. Total Anthocyanins Content (TAC)
2.4. Antioxidant Activities
2.5. Correlation between Antioxidant Activity and Total Phenolics and Total Anthocyanins Contents
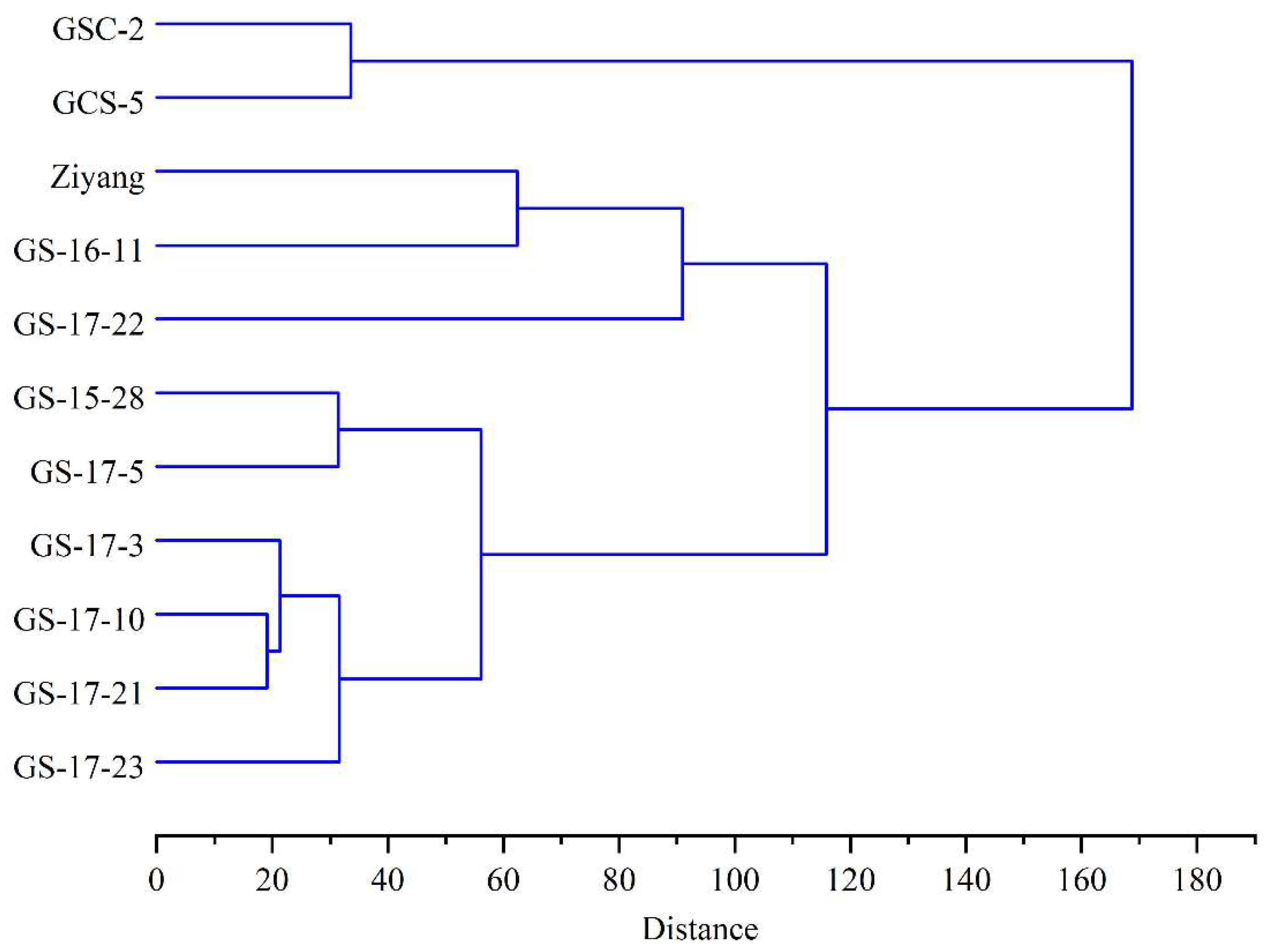
2.6. Cluster Analysis
3. Materials and Methods
3.1. Reagents
3.2. Plant Materials
3.3. Agronomic Traits Investigation
3.4. Sample Extraction
3.5. Total Phenolics and Anthocyanins Determination
3.6. DPPH Radical Scavenging Activity
3.7. ABTS Radical Scavenging Activity
3.8. Ferric Reducing Antioxidant Power (FRAP) Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- CIP. Discovery to Impact: Science-Based Solutions for Global Challenges; CIP Annual Report 2019; Lima, P., Ed.; CIP: Pyrmont, Australia, 2020; p. 37. [Google Scholar]
- Dong, J.U.; Tai-Hua, M.U.; Sun, H.N. Sweet potato and potato residual flours as potential nutritional and healthy food material. J. Integr. Agric. 2017, 16, 2632–2645. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, C.; Li, D.; Song, J. Effect of processing on taste quality and health-relevant functionality of sweet potato tips. Agric. Sci. China 2011, 10, 456–462. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Xiao, H.; Li, Z.; Zhang, Q.; Wang, H.; Hu, Y.; Zhang, L. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of sweet potato (Ipomoea batatas L.) leaves flavonoid. Food Bioprod. Process. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Fu, Z.; Tu, Z.; Zhang, L.; Wang, H.; Wen, Q.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Mau, J.; Lee, C.; Yang, C.; Chen, R.; Zhang, Q.; Lin, S. Physicochemical, antioxidant and sensory characteristics of bread partially substituted with aerial parts of sweet potato. LWT 2020, 117, 108602. [Google Scholar] [CrossRef]
- Islam, S. Sweetpotato (Ipomoea batatas L.) leaf: Its potential effect on human health and nutrition. J. Food Sci. 2006, 71, R13–R121. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-Activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Lako, J.; Trenerry, V.C.; Wahlqvist, M.; Wattanapenpaiboon, N.; Sotheeswaran, S.; Premier, R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007, 101, 1727–1741. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Furuta, S.; Nishiba, Y.; Suda, I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. J. Food Sci. 2010, 67, 1752–1756. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. 2016, 56, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Taira, K.; Ohmine, W.; Nagata, J. Mineral determination and anti-LDL oxidation activity of sweet potato (Ipomoea batatas L.) leaves. J. Food Compos. Anal. 2013, 29, 117–125. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Karna, P.; Gundala, S.R.; Gupta, M.V.; Shamsi, S.A.; Pace, R.D.; Yates, C.; Narayan, S.; Aneja, R. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis 2011, 32, 1872–1880. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Clifford, M.N. Profiling the chlorogenic acids of sweet potato (Ipomoea batatas) from China. Food Chem. 2008, 106, 147–152. [Google Scholar] [CrossRef]
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e1964. [Google Scholar] [CrossRef] [Green Version]
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; Cádiz-Gurrea, M.D.L.L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Antioxidant capacity of 44 cultivars of fruits and vegetables grown in Andalusia (Spain). Food Res. Int. 2014, 58, 35–46. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Song, Z. Effects of domestic cooking methods on polyphenols and antioxidant activity of sweet potato leaves. J. Agric. Food Chem. 2014, 62, 8982–8989. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Hu, B.; Sun, Y.; Ye, H.; Ma, D.; Zeng, X. TPC in the leaves of 116 sweet potato (Ipomoea batatas L.) varieties and Pushu 53 leaf extracts. J. Food Compos. Anal. 2010, 23, 599–604. [Google Scholar] [CrossRef]
- Li, M.; Jang, G.Y.; Lee, S.H.; Kim, M.Y.; Hwang, S.G.; Sin, H.M.; Kim, H.S.; Lee, J.; Jeong, H.S. Comparison of functional components in various sweet potato leaves and stalks. Food Sci. Biotechnol. 2017, 26, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koh, E. Antioxidant content and activity in leaves and petioles of six sweet potato (Ipomoea batatas L.) and antioxidant properties of blanched leaves. Food Sci. Biotechnol. 2019, 28, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Park, S.J.; Lee, C.S.; Ren, C.; Kim, S.S.; Shin, M. Functional properties of different Korean sweet potato varieties. Food Sci. Biotechnol. 2011, 20, 1501–1507. [Google Scholar] [CrossRef]
- Steed, L.E.; Truong, V.D. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J. Food Sci. 2008, 73, S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Truong, V.; Yencho, G.C.; Lila, M.A. Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2010, 120, 993–1003. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
- Padda, M.S.; Picha, D.H. Antioxidant activity and phenolic composition in ‘Beauregard’ sweetpotato are affected by root size and leaf age. J. Am. Soc. Hortic. Sci. 2007, 132, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Z.; Hassan, S.E.; Fatima, M.; Mahdi, C.; Chaqroune, A.; Taleb, M. Effects of extraction technique and solvent on phytochemicals, antioxidant, and antimicrobial activities of cultivated and wild rosemary (Rosmarinus officinalis L.) from Taounate Region (Northern Morocco). Biointerface Res. Appl. Chem. 2021, 12, 8441–8452. [Google Scholar] [CrossRef]
- Zeroual, A.; Sakar, E.H.; Eloutassi, N.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A. Wild chamomile [Cladanthus mixtus (L.) Chevall.] collected from central-northern Morocco: Phytochemical profiling, antioxidant, and antimicrobial activities. Biointerface Res. Appl. Chem. 2021, 11, 11440–11457. [Google Scholar] [CrossRef]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Ainane, A.; Ainane, T.; Gharby, S.; Rharrabti, Y. Variability of oil content and its physicochemical traits from the main almond [Prunus dulcis Mill. Webb, D.A.] cultivars grown under contrasting environments in north-eastern Morocco. Biocatal. Agric. Biotechnol. 2021, 32, 101952. [Google Scholar] [CrossRef]
- Andarwulan, N.; Batari, R.; Sandrasari, D.A.; Bolling, B.; Wijaya, H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chem. 2010, 121, 1231–1235. [Google Scholar] [CrossRef] [Green Version]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Kongkachuichai, R.; Charoensiri, R.; Yakoh, K.; Kringkasemsee, A.; Insung, P. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 2015, 173, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Wang, S.Y.; Huang, M.Y.; Lin, K.H.; Hua, S.M.; Lu, H.H.; Lai, Y.C.; Yang, C.M. Physiological and molecular analyses of chlorophyllase in sweet potatoes with different-colored leaves. S. Afr. J. Bot. 2018, 114, 272–279. [Google Scholar] [CrossRef]
- Ciž, M.; Cižova, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Truong, V.D.; Mcfeeters, R.F.; Thompson, R.T.; Dean, L.L.; Shofran, B. Phenolic acid content and composition in leaves and roots of common commercial sweetpotato (Ipomea batatas L.) cultivars in the United States. J. Food Sci. 2007, 72, C343–C349. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Deng, G.; Lin, X.; Xu, X.; Gao, L.; Xie, J.; Li, H. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Antioxidative properties of 34 green leafy vegetables. J. Funct. Foods 2016, 26, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.; Yang, X.; Ke, J.; Corke, H. Anthocyanins, hydroxycinnamic acid derivatives, and antioxidant activity in roots of different chinese purple-fleshed sweetpotato genotypes. J. Agric. Food Chem. 2010, 58, 7588–7596. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.J.; Yang, D.; Shin, J.A.; Kim, S.J.; Hong, S.T.; Lee, J.H.; Sung, C.K.; Lee, K.T. Oxidative comparison of emulsion systems from fish oil-based structured lipid versus physically blended lipid with purple-fleshed sweet potato (Ipomoea batatas L.) extracts. J. Agric. Food Chem. 2012, 60, 467–475. [Google Scholar] [CrossRef]
- Anastácio, A.; Carvalho, I.S. Spotlight on PGI sweet potato from Europe: Study of plant part, time and solvent effects on antioxidant activity. J. Food Biochem. 2013, 37, 628–637. [Google Scholar] [CrossRef]
- Kuan, L.; Thoo, Y.; Siow, L. Bioactive components, ABTS radical scavenging capacity and physical stability of orange, yellow and purple sweet potato (Ipomoea batatas) powder processed by convection- or vacuum-drying methods. Int. J. Food Sci. Technol. 2016, 51, 700–709. [Google Scholar] [CrossRef]
- Chen, J.; Fang, B.; Li, Y.; Zhang, X.; Wang, Z.; Huang, L.; Luo, Z.; Chen, X. Breeding of a new variety Guang Cai Shu No.3 for the tips of sweet potato vine as vegetables. Guangdong Agric. Sci. 2013, 40, 19–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, B. Descriptors and Data Standard for Sweetpotato [Ipomoea batatas (L.) Lam.], 1st ed.; China Agriculture Press: Beijing, China, 2006; pp. 38–48. [Google Scholar]
- Yang, Z.; Chen, Z.; Yuan, S.; Zhai, W.; Piao, X.; Piao, X. Extraction and identification of anthocyanin from purple corn (Zea mays L.). Int. J. Food Sci. Technol. 2009, 44, 2485–2492. [Google Scholar] [CrossRef]
- Song, J.; Li, D.; Liu, C.; Zhang, Y. Optimized microwave-assisted extraction of total phenolics (TP) from Ipomoea batatas leaves and its antioxidant activity. Innov. Food Sci. Emerg. 2011, 12, 282–287. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Y.; Wu, J.; Xiao, G.; Fu, M.; Zhang, Y. Effect of ultra-high pressure homogenisation processing on phenolic compounds, antioxidant capacity and anti-glucosidase of mulberry juice. Food Chem. 2014, 153, 114–120. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 1968; Volume 33, pp. 266–274. [Google Scholar] [CrossRef]
- Sokół-Betowska, A.; Kucharska, A.Z.; Winska, K.; Szumny, A.; Nawirska-Olszanska, A.; Mizgier, P.; Wyspianska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157C, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Liao, M.; Zou, B.; Chen, J.; Yao, Z.; Huang, L.; Luo, Z.; Wang, Z. Effect of domestic cooking methods on the anthocyanins and antioxidant activity of deeply purple-fleshed sweetpotato GZ9. Heliyon 2019, 5, e1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.; Li, M.; Ma, F.; Dong, L. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]

| Item | Total Phenolics Content (mg GAE/100 g fw) | Total Anthocyanins Content (mg/100 g fw) | ||||||
|---|---|---|---|---|---|---|---|---|
| Bud | Leaf | Petiole | Stem | Bud | Leaf | Petiole | Stem | |
| Variety | ||||||||
| GSC-2 | 231.87 ± 13.57b | 125.76 ± 2.36b | 32.35 ± 3.17b | 17.38 ± 2.36c | 1.75 ± 0.08d | 3.73 ± 0.06f | 0.88 ± 0.01f | 0.36 ± 0.11g |
| GCS-5 | 248.22 ± 14.85a | 148.36 ± 1.36a | 36.39 ± 1.29a | 32.69 ± 1.12a | 1.72 ± 0.45d | 3.22 ± 0.06f | 0.79 ± 0.07f | 0.45 ± 0.07g |
| Ziyang | 193.20 ± 2.58c | 123.97 ± 4.46b | 21.64 ± 1.01c | 23.03 ± 0.52b | 25.14 ± 0.85c | 82.91 ± 2.68b | 27.35 ± 0.92a | 13.88 ± 0.99a |
| GS-15-28 | 132.98 ± 11.23d | 53.19 ± 4.49fg | 10.70 ± 0.78e | 10.24 ± 1.18de | 11.40 ± 0.93d | 42.43 ± 3.31c | 10.99 ± 0.37d | 6.87 ± 0.11cd |
| GS-16-11 | 143.39 ± 5.15d | 100.78 ± 5.57c | 15.93 ± 1.27d | 15.59 ± 1.18c | 47.69 ± 0.53b | 80.02 ± 4.73b | 18.43 ± 1.58b | 8.94 ± 0.31b |
| GS-17-3 | 65.32 ± 3.41f | 56.17 ± 2.36fg | 7.37 ± 0.45g | 9.35 ± 0.45e | 2.04 ± 0.12d | 35.56 ± 2.80d | 6.41 ± 0.92e | 4.03 ± 0.26f |
| GS-17-5 | 109.93 ± 6.81e | 69.25 ± 6.93de | 9.21 ± 0.37fg | 12.62 ± 0.93d | 4.79 ± 0.11d | 33.60 ± 2.33d | 7.27 ± 0.06e | 4.40 ± 0.04ef |
| GS-17-10 | 80.93 ± 3.41f | 60.63 ± 3.09ef | 8.62 ± 0.21fg | 9.05 ± 1.12e | 5.04 ± 0.03d | 30.63 ± 0.14d | 7.39 ± 0.21e | 4.75 ± 0.07ef |
| GS-17-21 | 77.21 ± 4.64f | 48.43 ± 1.36g | 8.97 ± 1.62fg | 11.43 ± 2.58de | 2.65 ± 0.60d | 20.99 ± 0.53e | 6.81 ± 1.45e | 5.99 ± 1.72de |
| GS-17-22 | 112.16 ± 10.54e | 71.33 ± 7.93d | 9.33 ± 0.10fg | 11.58 ± 0.45de | 74.17 ± 18.48a | 109.60 ± 1.62a | 16.68 ± 0.39c | 9.04 ± 1.68b |
| GS-17-23 | 81.30 ± 1.58f | 62.11 ± 8.94ef | 9.92 ± 0.90f | 9.35 ± 0.77e | 4.64 ± 0.49d | 44.04 ± 9.15c | 10.01 ± 0.75d | 7.79 ± 0.11bc |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Color | ||||||||
| Green | 240.04 ± 15.56A | 137.06 ± 12.50A | 34.37 ± 3.10A | 25.03 ± 8.55A | 1.74 ± 0.29B | 3.48 ± 0.30B | 0.82 ± 0.07B | 0.41 ± 0.09B |
| Green-purple | 111.84 ± 39.87B | 71.76 ± 24.28B | 11.30 ± 4.45B | 12.47 ± 4.40B | 19.52 ± 25.30A | 51.15 ± 27.32A | 12.63 ± 7.07A | 7.40 ± 3.13A |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Item | DPPH (μM TE/g fw) | |||
|---|---|---|---|---|
| Bud | Leaf | Petiole | Stem | |
| Variety | ||||
| GSC-2 | 79.00 ± 1.23a | 69.96 ± 1.64bc | 70.31 ± 1.30ab | 69.68 ± 1.35b |
| GCS-5 | 78.72 ± 0.38a | 73.14 ± 4.78abc | 70.29 ± 3.41ab | 69.89 ± 3.69b |
| Ziyang | 65.30 ± 2.58def | 59.22 ± 0.95d | 57.64 ± 2.57c | 55.55 ± 2.89c |
| GS-15-28 | 59.45 ± 3.29f | 60.36 ± 3.49d | 57.98 ± 1.12c | 59.49 ± 1.34c |
| GS-16-11 | 62.47 ± 4.84f | 58.16 ± 0.82d | 58.49 ± 1.73c | 55.60 ± 9.54c |
| GS-17-3 | 76.25 ± 4.19ab | 78.47 ± 1.68a | 75.34 ± 0.54a | 74.90 ± 3.06ab |
| GS-17-5 | 63.55 ± 2.73ef | 59.51 ± 4.45d | 59.63 ± 3.48c | 58.53 ± 3.49c |
| GS-17-10 | 74.78 ± 2.20abc | 74.18 ± 2.04abc | 77.09 ± 10.66a | 71.89 ± 4.64ab |
| GS-17-21 | 73.04 ± 4.30abc | 73.34 ± 2.25abc | 71.20 ± 3.82ab | 77.84 ± 1.17a |
| GS-17-22 | 71.22 ± 8.19bcd | 75.84 ± 3.84ab | 65.10 ± 2.39bc | 62.26 ± 1.48c |
| GS-17-23 | 69.11 ± 1.81cde | 68.47 ± 5.82c | 61.81 ± 0.93c | 60.39 ± 5.40c |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Color | ||||
| Green | 78.86 ± 0.83A | 71.55 ± 3.64 | 70.30 ± 2.31 | 69.79 ± 2.49 |
| Green-purple | 68.24 ± 6.59B | 67.51 ± 8.34 | 64.91 ± 8.34 | 64.05 ± 8.98 |
| p value | <0.001 | >0.05 | >0.05 | >0.05 |
| Item | ABTS (μM TE/g fw) | |||
|---|---|---|---|---|
| Bud | Leaf | Petiole | Stem | |
| Variety | ||||
| GSC-2 | 33.49 ± 1.31a | 30.98 ± 0.59ab | 14.69 ± 1.55b | 9.61 ± 2.12b |
| GCS-5 | 36.33 ± 0.22a | 34.44 ± 1.50a | 18.68 ± 0.89a | 16.42 ± 2.55a |
| Ziyang | 29.15 ± 0.04b | 28.28 ± 0.60b | 5.78 ± 0.68d | 3.71 ± 0.48cd |
| GS-15-28 | 27.37 ± 1.55bc | 21.33 ± 1.30c | 3.48 ± 1.32e | 4.73 ± 0.04cd |
| GS-16-11 | 28.75 ± 1.03b | 27.53 ± 1.72b | 5.95 ± 0.87d | 5.93 ± 1.60cd |
| GS-17-3 | 24.13 ± 1.34cd | 22.77 ± 0.69c | 8.46 ± 2.23c | 4.45 ± 1.18cd |
| GS-17-5 | 25.17 ± 0.19cd | 19.59 ± 1.06c | 4.18 ± 0.32de | 3.19 ± 1.59d |
| GS-17-10 | 24.01 ± 0.59cd | 20.32 ± 1.13c | 2.91 ± 0.17e | 5.15 ± 1.05cd |
| GS-17-21 | 23.29 ± 3.96d | 19.56 ± 3.10c | 3.57 ± 0.27e | 6.86 ± 2.63bc |
| GS-17-22 | 24.17 ± 1.91cd | 19.75 ± 2.82c | 2.11 ± 0.07e | 14.18 ± 2.82a |
| GS-17-23 | 19.23 ± 2.56e | 22.88 ± 4.86c | 2.29 ± 0.86e | 5.51 ± 0.39cd |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Color | ||||
| Green | 34.62 ± 1.81A | 32.36 ± 2.08A | 17.08 ± 2.40A | 12.33 ± 4.22A |
| Green-purple | 24.87 ± 3.30B | 22.45 ± 3.76B | 4.19 ± 2.02B | 6.10 ± 3.51B |
| p value | <0.001 | <0.001 | <0.001 | <0.01 |
| Item | FRAP (μM TE/g fw) | |||
|---|---|---|---|---|
| Bud | Leaf | Petiole | Stem | |
| Variety | ||||
| GSC-2 | 21.94 ± 2.20a | 9.73 ± 0.99b | 3.17 ± 0.07a | 1.68 ± 0.13c |
| GCS-5 | 21.65 ± 3.14a | 10.31 ± 0.69b | 2.80 ± 0.16b | 2.25 ± 0.03b |
| Ziyang | 23.16 ± 3.45a | 12.51 ± 1.58a | 2.76 ± 0.39b | 2.57 ± 0.05a |
| GS-15-28 | 10.25 ± 0.86bcd | 4.17 ± 0.22de | 0.30 ± 0.00f | 0.47 ± 0.04d |
| GS-16-11 | 12.11 ± 0.63b | 9.18 ± 0.40b | 1.18 ± 0.35c | 0.92 ± 0.17d |
| GS-17-3 | 8.46 ± 0.60cd | 4.61 ± 0.39cde | 0.65 ± 0.21def | 0.70 ± 0.05e |
| GS-17-5 | 11.24 ± 1.56bc | 5.76 ± 0.75c | 0.90 ± 0.28cd | 0.85 ± 0.08d |
| GS-17-10 | 10.83 ± 0.27bc | 5.27 ± 0.09cd | 0.62 ± 0.03def | 0.69 ± 0.06e |
| GS-17-21 | 7.59 ± 0.76d | 3.54 ± 0.09e | 0.38 ± 0.10ef | 0.96 ± 0.00d |
| GS-17-22 | 9.91 ± 0.10bcd | 5.21 ± 0.27cd | 0.77 ± 0.07de | 0.90 ± 0.02d |
| GS-17-23 | 3.37 ± 0.44e | 3.91 ± 0.28e | 0.36 ± 0.12f | 0.43 ± 0.05d |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Color | ||||
| Green | 21.80 ± 2.43A | 10.02 ± 0.82A | 2.99 ± 0.23A | 1.96 ± 0.32A |
| Green-purple | 10.77 ± 5.24B | 6.02 ± 2.88B | 0.89 ± 0.77B | 0.94 ± 0.63B |
| p value | <0.001 | <0.01 | <0.001 | <0.001 |
| Item | Total Phenolics Content | Total Anthocyanins Content | ||||||
|---|---|---|---|---|---|---|---|---|
| Bud | Leaf | Petiole | Stem | Bud | Leaf | Petiole | Stem | |
| DPPH | 0.191 | −0.190 | 0.044 | −0.069 | −0.335 | −0.394 * | −0.589 ** | −0.556 * |
| ABTS | 0.885 ** | 0.853 ** | 0.909 ** | 0.429 * | −0.016 | −0.212 | −0.513 ** | −0.340 |
| FRAP | 0.892 ** | 0.918 ** | 0.912 ** | 0.876 ** | −0.066 | 0.105 | 0.062 | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. https://doi.org/10.3390/molecules27103117
Jia R, Tang C, Chen J, Zhang X, Wang Z. Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.). Molecules. 2022; 27(10):3117. https://doi.org/10.3390/molecules27103117
Chicago/Turabian StyleJia, Ruixue, Chaochen Tang, Jingyi Chen, Xiongjian Zhang, and Zhangying Wang. 2022. "Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.)" Molecules 27, no. 10: 3117. https://doi.org/10.3390/molecules27103117
APA StyleJia, R., Tang, C., Chen, J., Zhang, X., & Wang, Z. (2022). Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.). Molecules, 27(10), 3117. https://doi.org/10.3390/molecules27103117





