Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles
Abstract
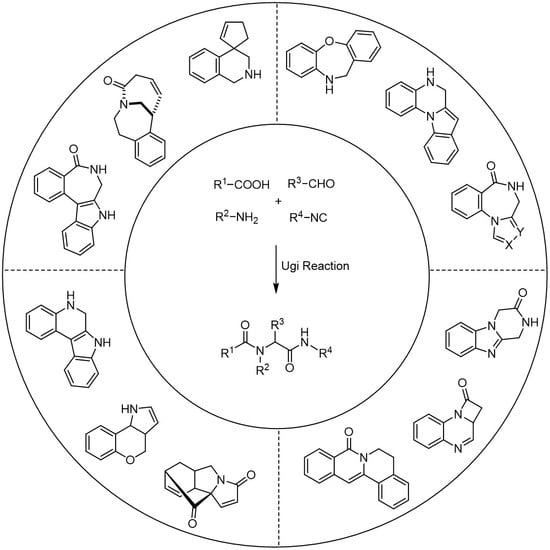
1. Introduction
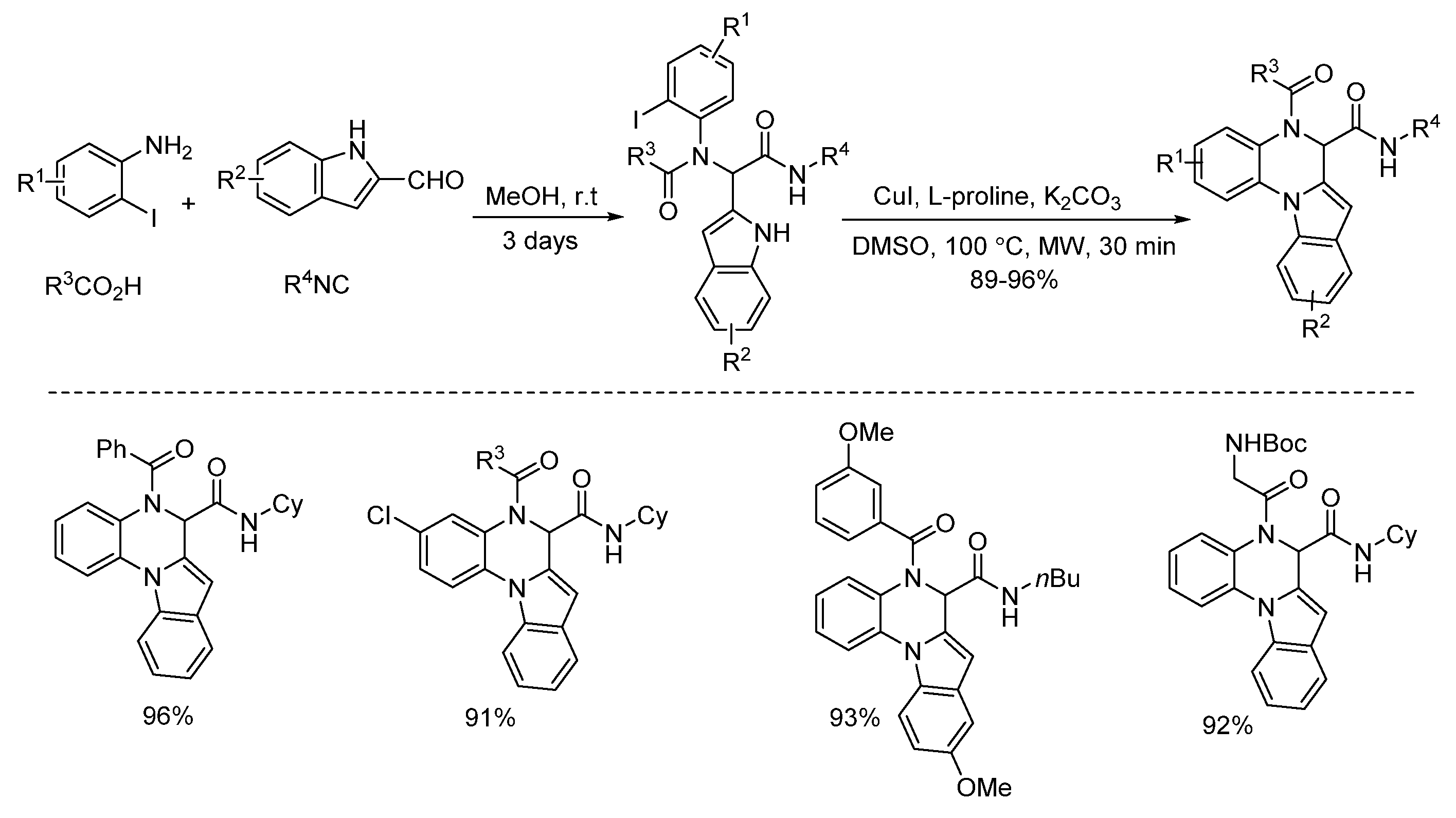
2. Copper Catalysis
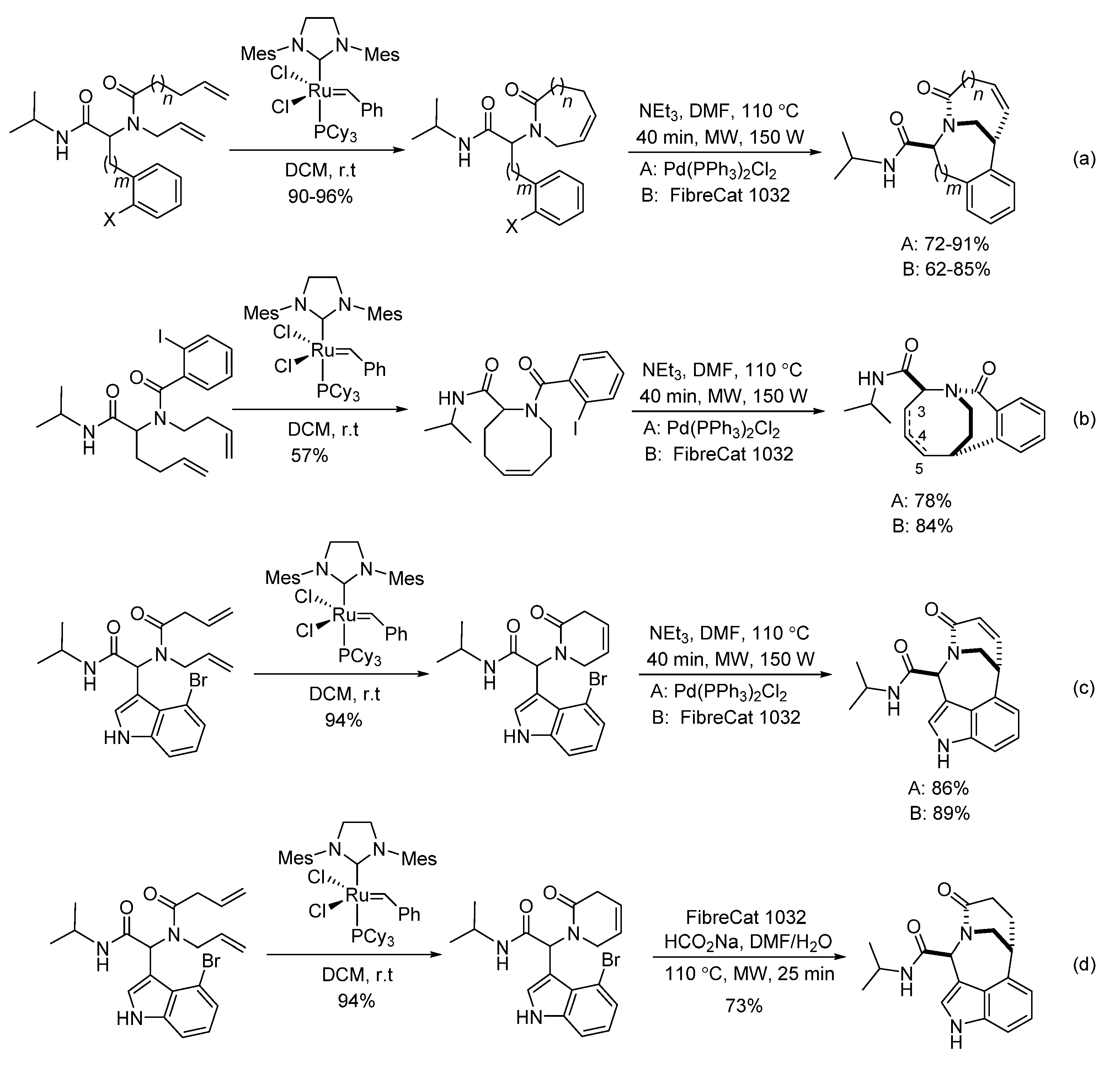
3. Palladium Catalysis
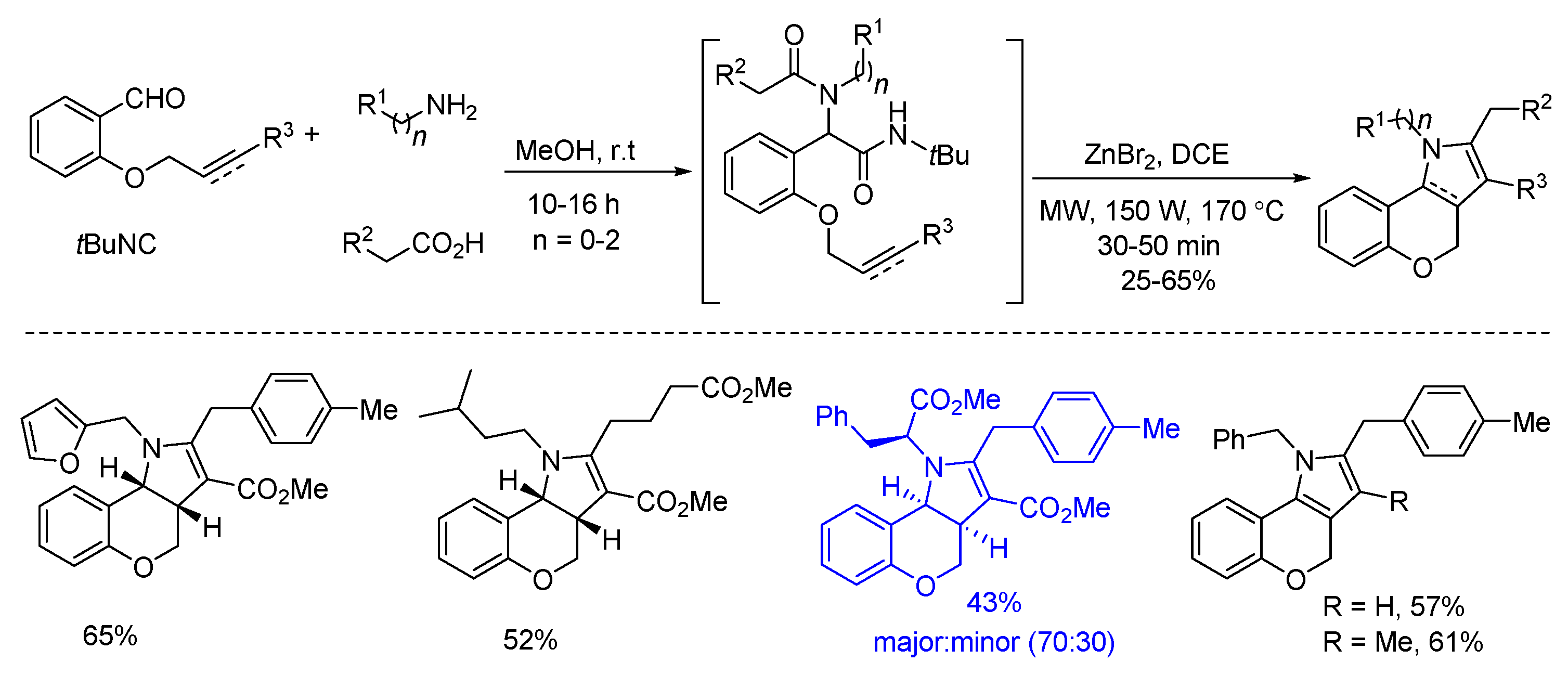
4. Other Transition Metal Catalysis
5. Transition Metal-Free Catalysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuhnert, N. Microwave-assisted reactions in organic synthesis—Are there any nonthermal microwave effects? Angew. Chem. Int. Ed. 2002, 41, 1863–1866. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz, A.; Loupy, A. Microwaves in Organic Synthesis, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sharma, N.; Sharma, U.K.; Li, Z.; Song, G.; Van der Eycken, E.V. Microwave-assisted copper-catalyzed oxidative cyclization of acrylamides with non-activated ketones. Chem. Eur. J. 2016, 22, 5878–5882. [Google Scholar] [CrossRef]
- Kappe, C.O. My twenty years in microwave chemistry: From kitchen ovens to microwaves that aren’t microwaves. Chem. Rec. 2019, 19, 15–39. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versuche mit isonitrilen. Angew. Chem. 1959, 71, 386. [Google Scholar]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent reaction design in the quest for molecular complexity and diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- Reguera, L.; Méndez, Y.; Humpierre, A.R.; Valdés, O.; Rivera, D.G. Multicomponent reactions in ligation and bioconjugation chemistry. Acc. Chem. Res. 2018, 51, 1475–1486. [Google Scholar] [CrossRef]
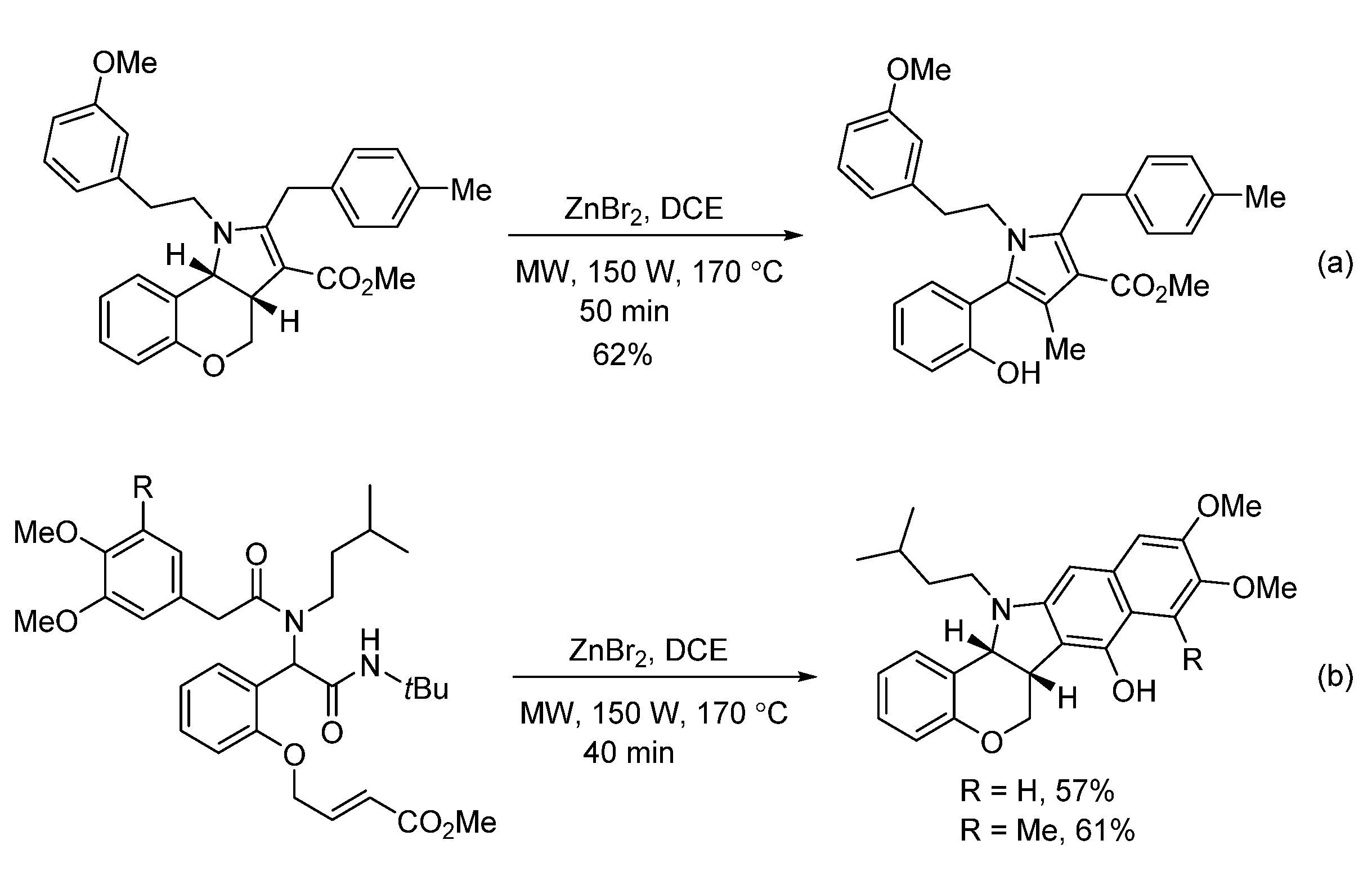
- Sharma, U.K.; Sharma, N.; Vachhani, D.D.; Van der Eycken, E.V. Metal-mediated post-Ugi transformations for the construction of diverse heterocyclic scaffolds. Chem. Soc. Rev. 2015, 44, 1836–1860. [Google Scholar] [CrossRef]
- Li, Z.; Song, L.; Meervelt, L.V.; Tian, G.; Van der Eycken, E.V. Cationic gold (I)-catalyzed cascade bicyclizations for divergent synthesis of (spiro) polyheterocycles. ACS Catal. 2018, 8, 6388–6393. [Google Scholar] [CrossRef]
- Liu, C.; Song, L.; Van Meervelt, L.; Peshkov, V.A.; Li, Z.; Van der Eycken, E.V. Palladium-catalyzed arylative dearomatization and subsequent aromatization/dearomatization/aza-Michael addition: Access to Zephycarinatine and Zephygranditine skeletons. Org. Lett. 2021, 23, 5065–5070. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, F.; Zheng, M.; Zhai, Y.; Liu, H. Rapid and selective access to three distinct sets of indole-based heterocycles from a single set of Ugi-adducts under microwave heating. Chem. Commun. 2013, 49, 2894–2896. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, F.; Zheng, M.; Zhai, Y.; Wang, J.; Liu, H. Selective synthesis of 5,6-dihydroindolo[1,2-a]quinoxalines and 6,7-dihydroindolo[2,3-c]quinolines by orthogonal copper and palladium catalysis. Eur. J. Org. Chem. 2013, 5710–5715. [Google Scholar] [CrossRef]
- Li, Z.; Legras, L.; Kumar, A.; Vachhani, D.D.; Sharma, S.K.; Parmar, V.S.; Van der Eycken, E.V. Microwave-assisted synthesis of 4H-benzo[f]imidazo[1,4]diazepin-6-ones via a post-Ugi copper-catalyzed intramolecular Ullmann coupling. Tetrahedron Lett. 2014, 55, 2070–2074. [Google Scholar] [CrossRef]
- Shi, J.; Wu, J.; Cui, C.; Dai, W. Microwave-assisted intramolecular Ullmann diaryl etherification as the post-Ugi annulation for generation of dibenz[b,f][1,4]oxazepine scaffold. J. Org. Chem. 2016, 81, 10392–10403. [Google Scholar] [CrossRef]
- Feng, G.; Wu, J.; Dai, W. Isolation and characterization of 2-alkylaminobenzo[b]furans. Evidence for competing O-arylation in Cu-catalyzed intramolecular amidation. Tetrahedron Lett. 2007, 48, 401–404. [Google Scholar] [CrossRef]
- Gracias, V.; Moore, J.D.; Djuric, S.W. Sequential Ugi/Heck cyclization strategies for the facile construction of highly functionalized N-heterocyclic scaffolds. Tetrahedron Lett. 2004, 45, 417–420. [Google Scholar] [CrossRef]
- Ribelin, T.P.; Judd, A.S.; Akritopoulou-Zanze, I.; Henry, R.F.; Cross, J.L.; Whittern, D.N.; Djuric, S.W. Concise construction of novel bridged bicyclic lactams by sequenced Ugi/RCM/Heck reactions. Org. Lett. 2007, 9, 5119–5122. [Google Scholar] [CrossRef]
- Srinivasulu, V.; McN Sieburth, S.; El-Awady, R.; Kariem, N.M.; Tarazi, H.; O’Connor, M.J.; Al-Tel, T.H. Post-Ugi cascade transformations for accessing diverse chromenopyrrole collections. Org. Lett. 2018, 20, 836–839. [Google Scholar] [CrossRef]
- Song, L.; Tian, G.; Blanpain, A.; Van Meervelt, L.; Van der Eycken, E.V. Diversification of peptidomimetics and oligopeptides through microwave-assisted rhodium (III)-catalyzed intramolecular annulation. Adv. Synth. Catal. 2019, 361, 4442–4447. [Google Scholar] [CrossRef]
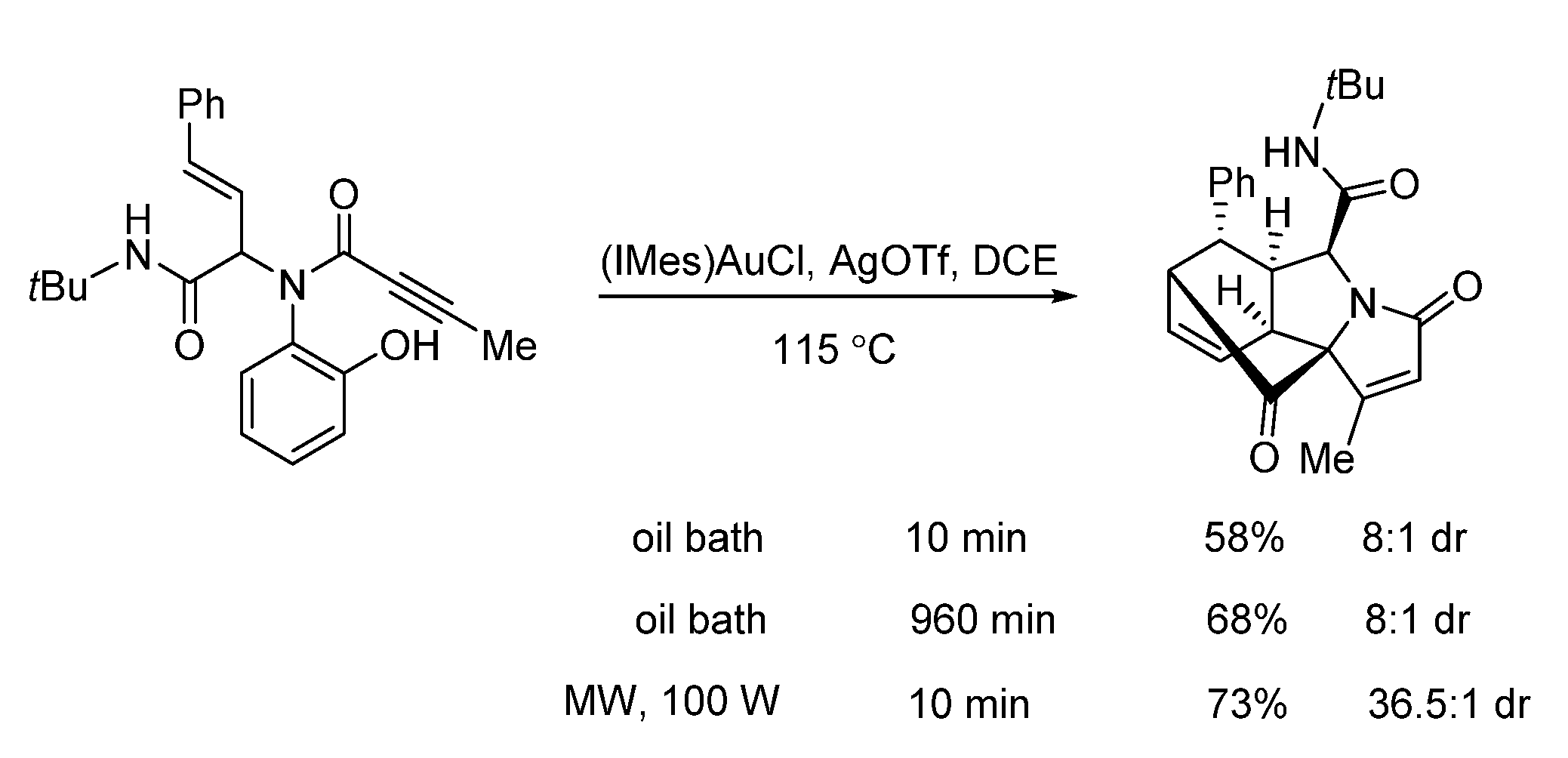
- He, Y.; Narmon, T.; Wu, D.; Li, Z.; Van Meerveltc, L.; Van der Eycken, E.V. A gold-triggered dearomative spirocarbocyclization/Diels-Alder reaction cascade towards diverse bridged N-heterocycles. Org. Biomol. Chem. 2019, 17, 9529–9536. [Google Scholar] [CrossRef]
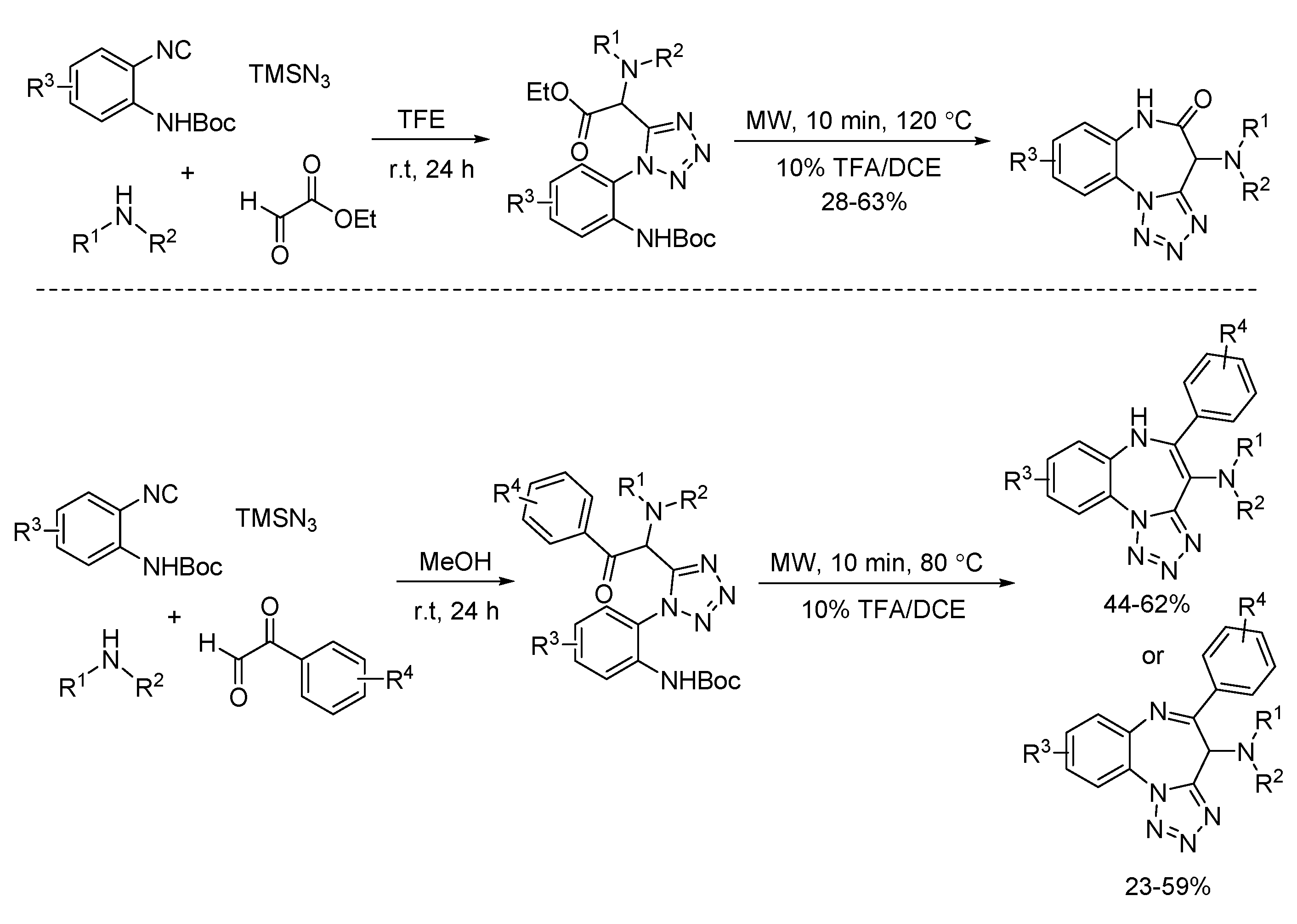
- Gunawan, S.; Ayaz, M.; De Moliner, F.; Frett, B.; Kaiser, C.; Patrick, N.; Xu, Z.; Hulme, C. Synthesis of tetrazolo-fused benzodiazepines and benzodiazepinones by a two-step protocol using an Ugi-azide reaction for initial diversity generation. Tetrahedron 2012, 68, 5606–5611. [Google Scholar] [CrossRef]
- Gordillo-Cruz, R.E.; Rentería-Gómez, A.; Islas-Jácome, A.; Cortes-García, C.J.; Díaz-Cervantes, E.; Robles, J.; Gámez-Montaño, R. Synthesis of 3-tetrazolylmethyl-azepino[4,5-b]indol-4-ones in two reaction steps: (Ugi-azide/N-acylation/SN2)/free radical cyclization and docking studies to a 5-Ht6 model. Org. Biomol. Chem. 2013, 11, 6470–6476. [Google Scholar] [CrossRef]
- Cárdenas-Galindo, L.E.; Islas-Jácome, A.; Alvarez-Rodríguez, N.V.; El Kaim, L.; Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a One-Pot Ugi-Azide/Pictet–Spengler Process. Synthesis 2014, 46, 0049–0056. [Google Scholar] [CrossRef]
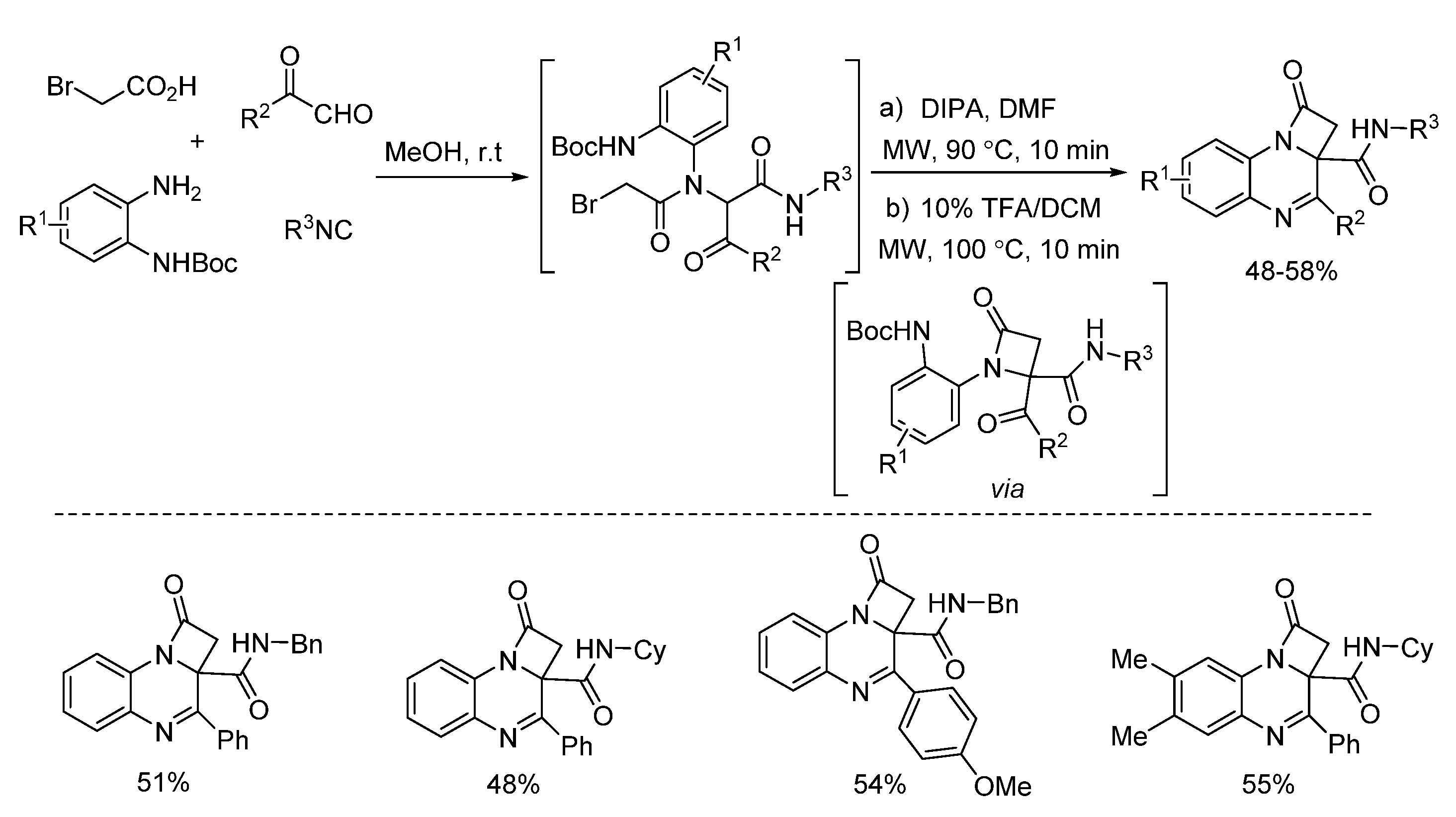
- Li, Y.; He, L.; Xu, J.; Ding, Y.; Xu, Z.; Lei, J.; Meng, J.; Zhu, J. Microwave-assisted facile synthesis of 2-azetidinone derivatives and evaluation of their antitumor activities. Sci. Sin. Chim. 2018, 48, 751–758. [Google Scholar] [CrossRef][Green Version]
- Song, G.; Li, Y.; Xu, J.; Xu, Z.; Ding, Y.; Lei, J.; McConnell, N.; Zhu, J.; Chen, Z. Microwave-assisted facile construction of quinoxalinone and benzimidazopyrazinone derivatives via two paths of post-Ugi cascade reaction. Mol. Divers. 2019, 23, 137–145. [Google Scholar] [CrossRef]

















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Cai, L.; Van der Eycken, E.V. Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. Molecules 2022, 27, 3105. https://doi.org/10.3390/molecules27103105
Song L, Cai L, Van der Eycken EV. Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. Molecules. 2022; 27(10):3105. https://doi.org/10.3390/molecules27103105
Chicago/Turabian StyleSong, Liangliang, Lingchao Cai, and Erik V. Van der Eycken. 2022. "Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles" Molecules 27, no. 10: 3105. https://doi.org/10.3390/molecules27103105
APA StyleSong, L., Cai, L., & Van der Eycken, E. V. (2022). Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. Molecules, 27(10), 3105. https://doi.org/10.3390/molecules27103105