Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage
Abstract
:1. Introduction
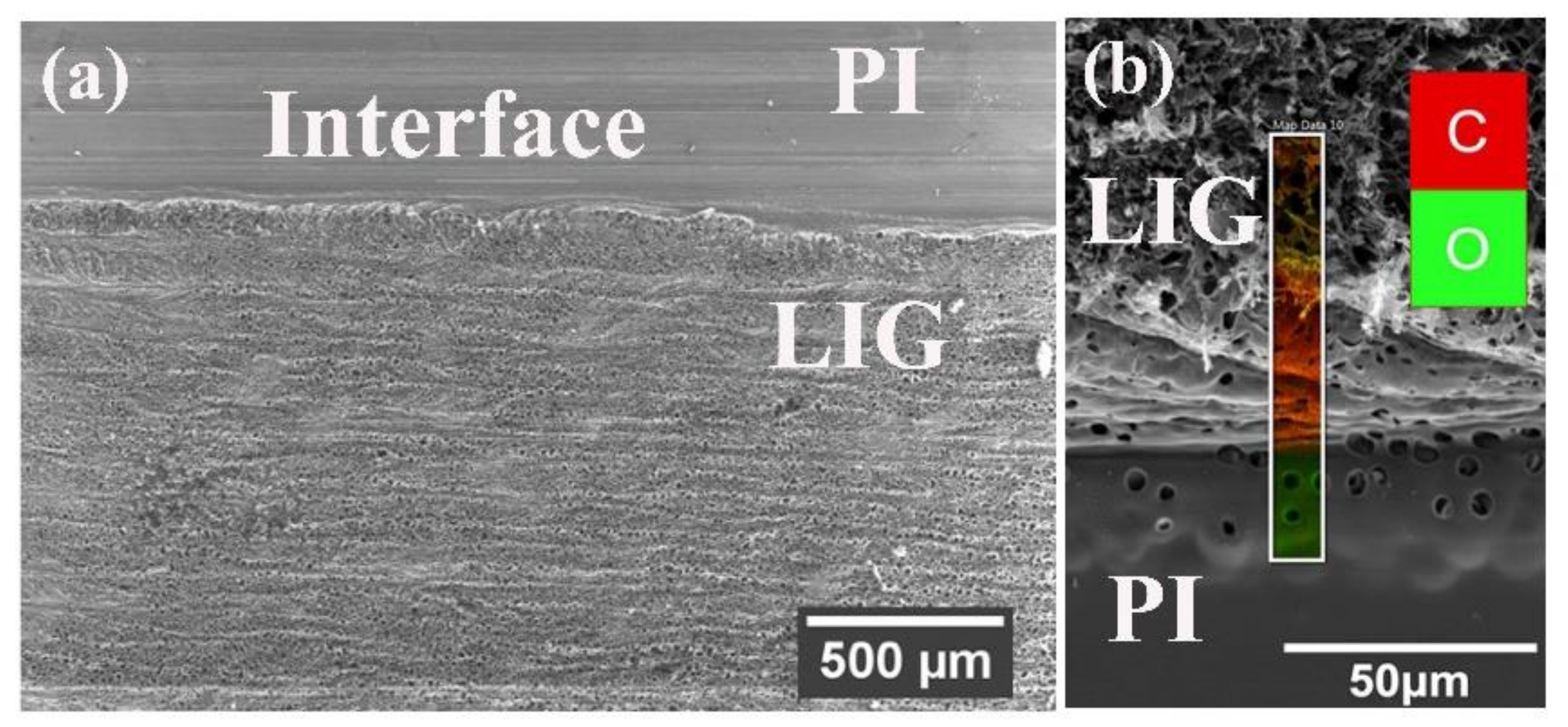
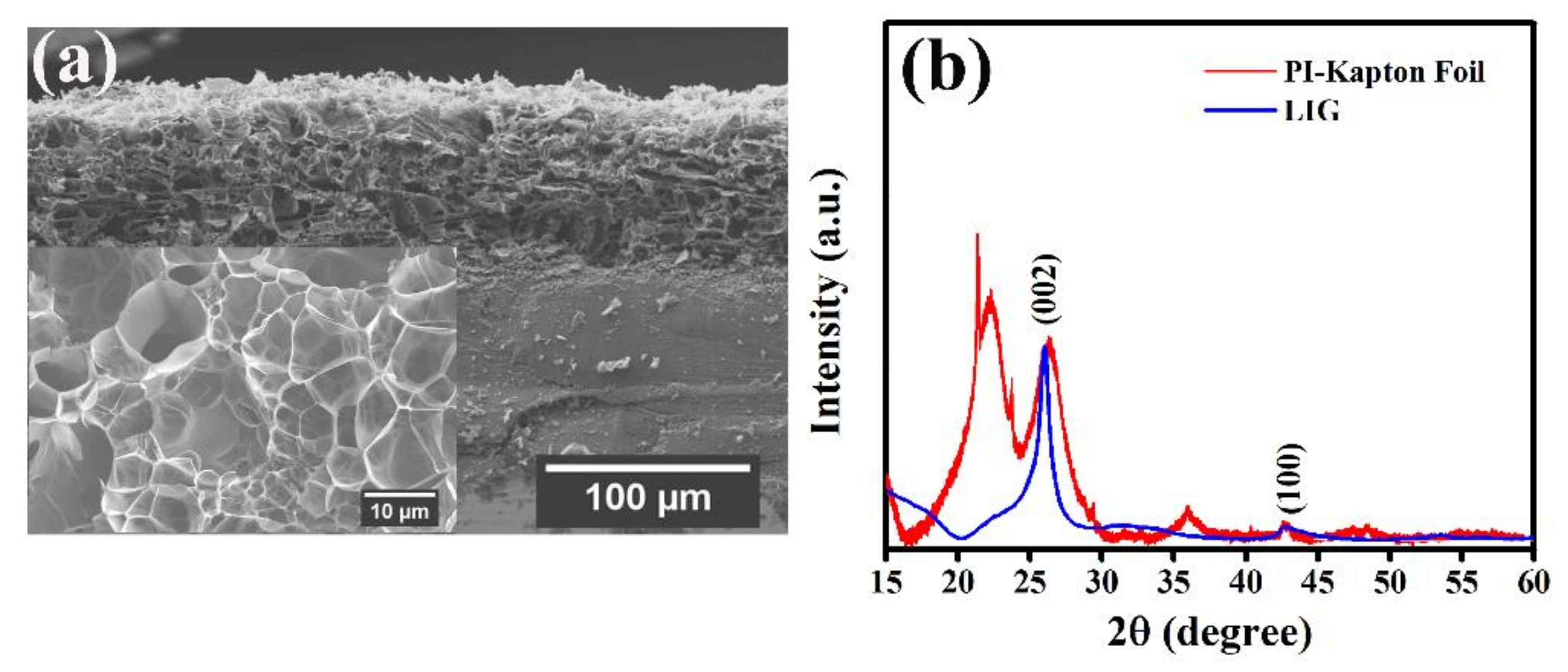
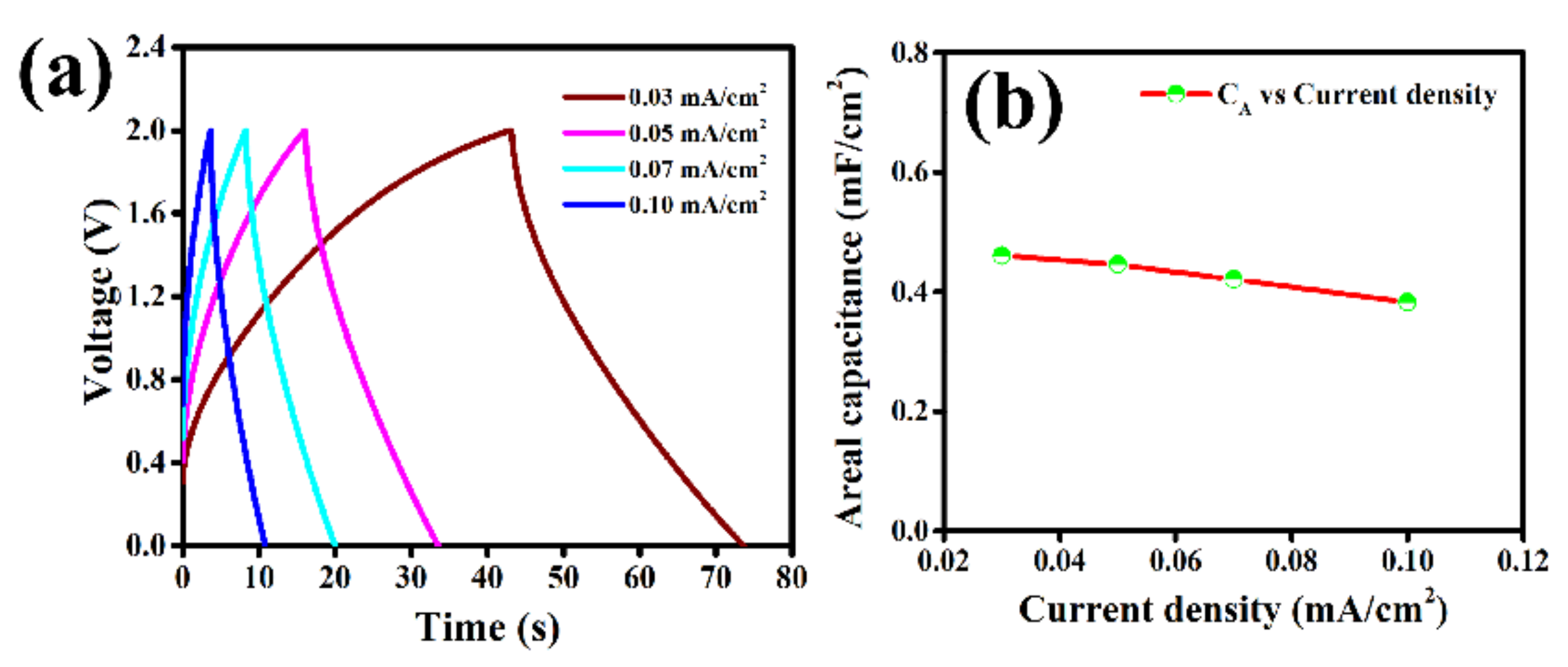
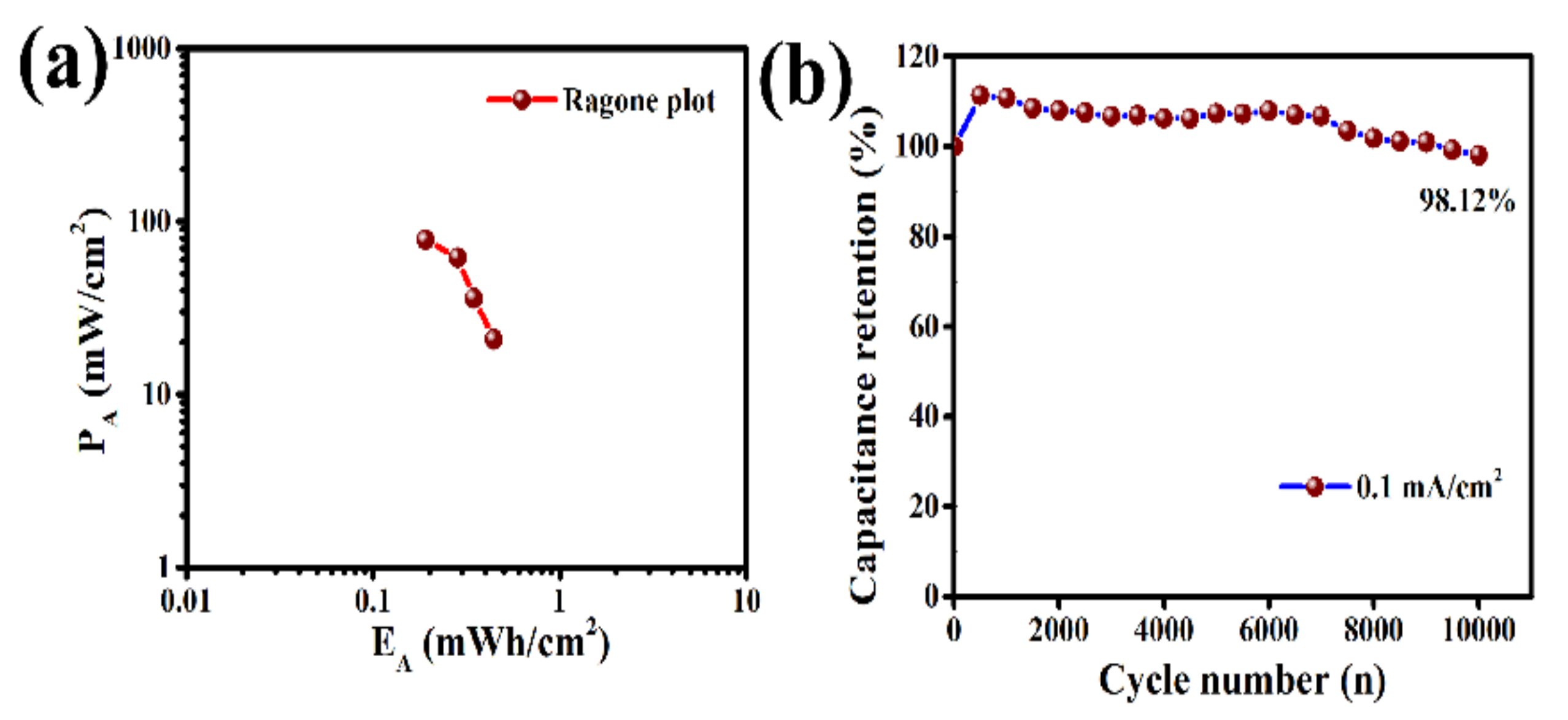
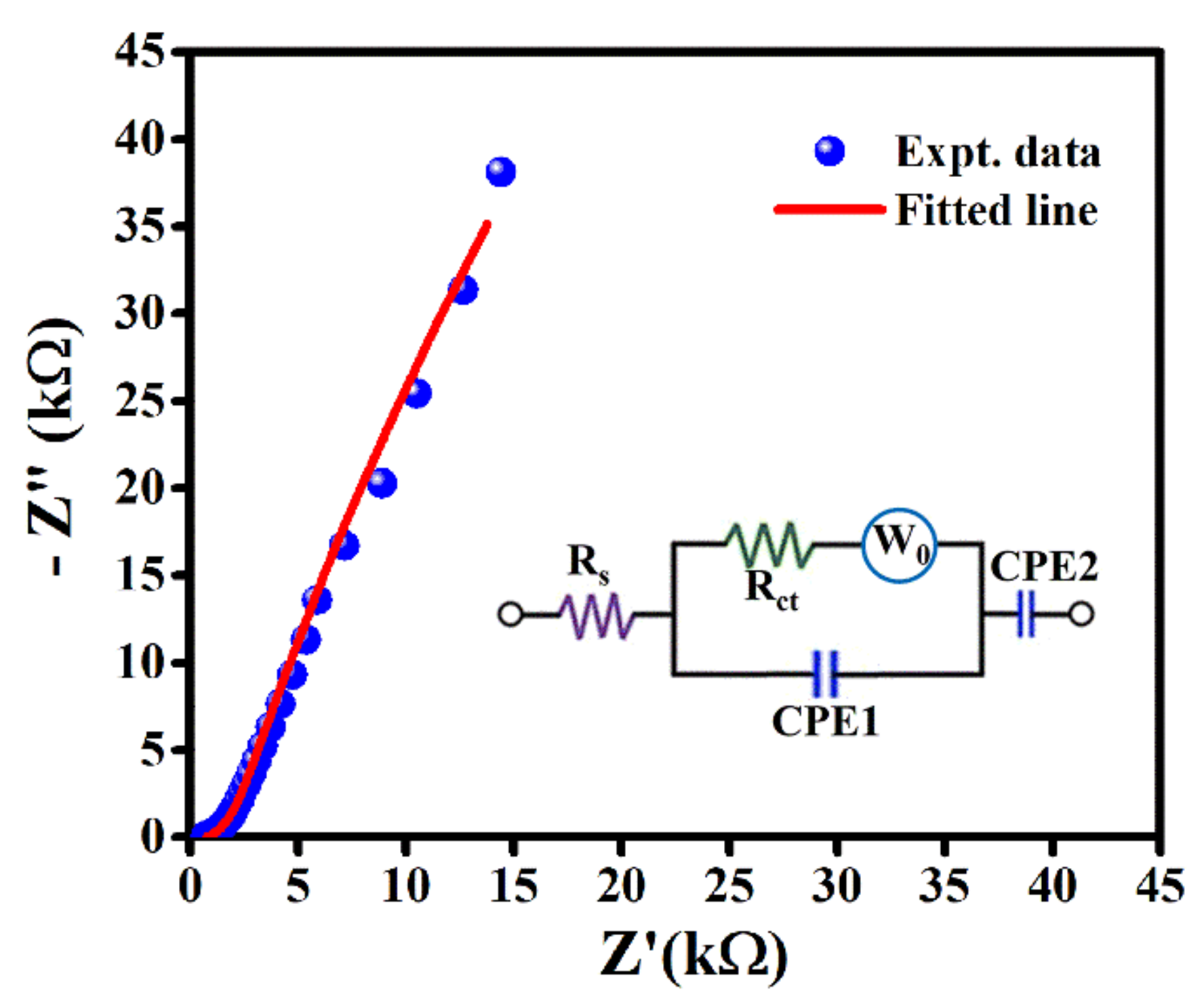
2. Results and Discussion
3. Materials and Methods
3.1. Materials
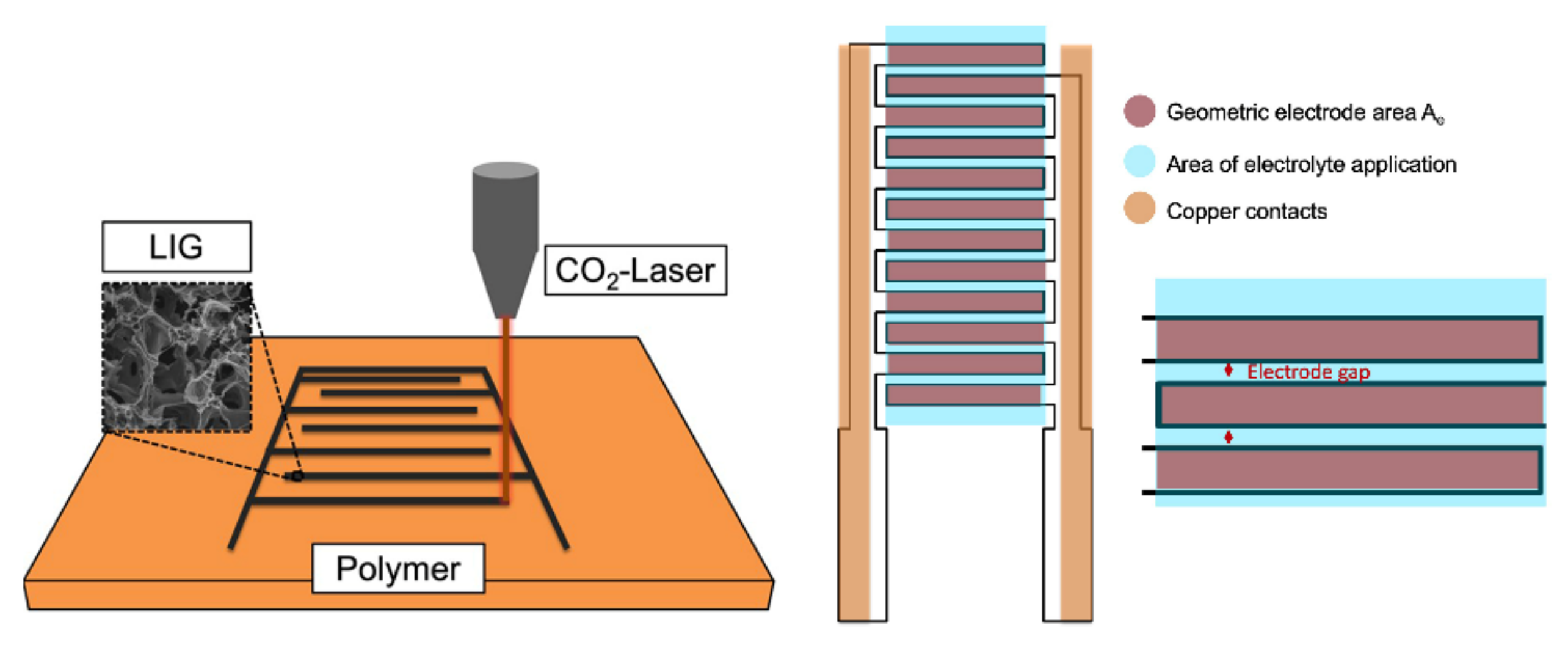
3.2. Fabrication of CO2-Laser-Induced Interdigital Structured Graphene Electrodes
3.3. Preparation of Polymer Gel Electrolyte (PGE)
3.4. Fabrication of In-Plane Micro-Supercapacitor (IMSC) Devices
3.5. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, X.; Li, H.; Fan, X.; Shi, X.; Liang, J. 3D-Printed Stretchable Micro-Supercapacitor with Remarkable Areal Performance. Adv. Energy Mater. 2020, 10, 1903794. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ahmed, M.M.M.; Du, W.T.; Kuo, S.W. Meso/microporous carbons from conjugated hyper-crosslinked polymers based on tetraphenylethene for high-performance CO2 capture and supercapacitor. Molecules 2021, 26, 738. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kim, Y.S.; Kwac, L.K.; Shin, H.K. Characterization of Activated Carbon Paper Electrodes Prepared by Rice Husk-Isolated Cellulose Fibers for Supercapacitor Applications. Molecules 2020, 25, 3951. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.; Jung, J.; Cho, A.R.; Jeong, J.R.; Dang Van, C.; Nah, J.; Lee, M.H. Enhanced Electrochemical Performance of Micro-Supercapacitors Via Laser-Scribed Cobalt/Reduced Graphene Oxide Hybrids. ACS Appl. Mater. Interfaces 2021, 13, 18821–18828. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Satpati, B.; Nandi, M.; Das, S. NiO-CNT composite for high performance supercapacitor electrode and oxygen evolution reaction. Electrochim. Acta 2018, 283, 327–337. [Google Scholar] [CrossRef]
- Tabassum, H.; Guo, W.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q.; Zou, R. Metal–Organic Frameworks Derived Cobalt Phosphide Architecture Encapsulated into B/N Co-Doped Graphene Nanotubes for All pH Value Electrochemical Hydrogen Evolution. Adv. Energy Mater. 2017, 7, 1601671. [Google Scholar] [CrossRef]
- Lethien, C.; Le Bideau, J.; Brousse, T. Challenges and prospects of 3D micro-supercapacitors for powering the internet of things. Energy Environ. Sci. 2019, 12, 96–115. [Google Scholar] [CrossRef]
- Hong, X.; He, L.; Ma, X.; Yang, W.; Chen, Y.; Zhang, L.; Yan, H.; Li, Z.; Mai, L. Microstructuring of carbon/tin quantum dots via a novel photolithography and pyrolysis-reduction process. Nano Res. 2017, 10, 3743–3753. [Google Scholar] [CrossRef]
- Létiche, M.; Brousse, K.; Demortière, A.; Huang, P.; Daffos, B.; Pinaud, S.; Respaud, M.; Chaudret, B.; Roussel, P.; Buchaillot, L.; et al. Sputtered Titanium Carbide Thick Film for High Areal Energy on Chip Carbon-Based Micro-Supercapacitors. Adv. Funct. Mater. 2017, 27, 1606813. [Google Scholar] [CrossRef]
- Huang, P.; Lethien, C.; Pinaud, S.; Brousse, K.; Laloo, R.; Turq, V.; Respaud, M.; Demortière, A.; Daffos, B.; Taberna, P.L.; et al. On-chip and freestanding elastic carbon films for micro-supercapacitors. Science 2016, 351, 691–695. [Google Scholar] [CrossRef] [Green Version]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Xiao, D.; Du, H.; Yang, Z.; Wang, X.; Tu, L.; Zhao, C.; Hu, F.; Lu, B. Silicon-based 3D all-solid-state micro-supercapacitor with superior performance. ACS Appl. Mater. Interfaces 2020, 12, 43864–43875. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, B.; Lu, M.; Li, Z.; Qiang, H. Fabrication and performance of self-supported flexible cellulose nanofibrils/reduced graphene oxide supercapacitor electrode materials. Molecules 2020, 25, 2793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jiang, M.; Wang, W.; Liu, S.; Huang, W.; Zhao, Q. Flexible Transparent Supercapacitors: Materials and Devices. Adv. Funct. Mater. 2021, 31, 2009136. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Zhao, Y.; Qiu, F.; Jiang, K.; Huang, S.; Ke, C.; Zhu, J.; Tranca, D.; Zhuang, X. B/N-Enriched Semi-Conductive Polymer Film for Micro-Supercapacitors with AC Line-Filtering Performance. Langmuir 2021, 37, 2523–2531. [Google Scholar] [CrossRef]
- Schubert, M.; Hanft, D.; Nazarenus, T.; Exner, J.; Nieke, P.; Glosse, P.; Leupold, N.; Kita, J.; Moos, R. Powder aerosol deposition method—Novel applications in the field of sensing and energy technology. Funct. Mater. Lett. 2019, 12, 1930005. [Google Scholar] [CrossRef] [Green Version]
- Asbani, B.; Robert, K.; Roussel, P.; Brousse, T.; Lethien, C. Asymmetric micro-supercapacitors based on electrodeposited Ruo2 and sputtered VN films. Energy Storage Mater. 2021, 37, 207–214. [Google Scholar] [CrossRef]
- Saha, S.; Maji, P.; Pethsangave, D.A.; Roy, A.; Ray, A.; Some, S.; Das, S. Effect of morphological ordering on the electrochemical performance of MnO2-Graphene oxide composite. Electrochim. Acta 2019, 317, 199–210. [Google Scholar] [CrossRef]
- Ghosh, M.; Ray, A.; Rao, G.M. Performance dependence of electrochemical capacitor on surface morphology for vertically aligned graphene nanosheets. Ionics 2020, 26, 981–990. [Google Scholar] [CrossRef]
- Shinde, N.M.; Shinde, P.V.; Mane, R.S.; Ho Kim, K. Solution-method processed Bi-type nanoelectrode materials for supercapacitor applications: A review. Renew. Sustain. Energy Rev. 2021, 135, 110084. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Lumban Raja, S.; Supeno, M.; Simanjuntak, C. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Gong, Y.; Wang, M.; Pan, C. Preparation of sandwich-like NiCo2O4/rGO/NiO heterostructure on nickel foam for high-performance supercapacitor electrodes. Nano Micro Lett. 2017, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Wang, C. Free-standing Reduced Graphene Oxide/Carbon Nanotube Paper for Flexible Sodium-ion Battery Applications. Molecules 2020, 25, 1014. [Google Scholar]
- Chen, G.; Zhang, X.; Ma, Y.; Song, H.; Pi, C.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K. In-situ synthesis of heterostructured carbon-coated Co/MnO nanowire arrays for high-performance anodes in asymmetric supercapacitors. Molecules 2020, 25, 3218. [Google Scholar] [CrossRef]
- Tian, W.; Li, Y.; Zhou, J.; Wang, T.; Zhang, R.; Cao, J.; Luo, M.; Li, N.; Zhang, N.; Gong, H.; et al. Implantable and biodegradable micro-supercapacitor based on a superassembled three-dimensional network Zn@ppy hybrid electrode. ACS Appl. Mater. Interfaces 2021, 13, 8285–8293. [Google Scholar] [CrossRef]
- Munuera, J.M.; Paredes, J.I.; Enterría, M.; Villar-Rodil, S.; Kelly, A.G.; Nalawade, Y.; Coleman, J.N.; Rojo, T.; Ortiz-Vitoriano, N.; Martínez-Alonso, A.; et al. High Performance Na-O2 Batteries and Printed Microsupercapacitors Based on Water-Processable, Biomolecule-Assisted Anodic Graphene. ACS Appl. Mater. Interfaces 2020, 12, 494–506. [Google Scholar] [CrossRef] [Green Version]
- Bhagwan, J.; Rani, S.; Sivasankaran, V.; Yadav, K.L.; Sharma, Y. Improved energy storage, magnetic and electrical properties of aligned, mesoporous and high aspect ratio nanofibers of spinel-NiMn2O4. Appl. Surf. Sci. 2017, 426, 913–923. [Google Scholar] [CrossRef]
- Pitkänen, O.; Eraslan, T.; Sebok, D.; Szenti, I.; Kukovecz, Á.; Vajtai, R.; Kordas, K. Flexible planar supercapacitors by straightforward filtration and laser processing steps. Nanotechnology 2020, 31, 495403. [Google Scholar] [CrossRef]
- Karbhal, I.; Basu, A.; Patrike, A.; Shelke, M.V. Laser patterning of boron carbon nitride electrodes for flexible micro-supercapacitor with remarkable electrochemical stability/capacity. Carbon 2021, 171, 750–757. [Google Scholar] [CrossRef]
- Yadav, P.; Basu, A.; Suryawanshi, A.; Game, O.; Ogale, S. Highly Stable Laser-Scribed Flexible Planar Microsupercapacitor Using Mushroom Derived Carbon Electrodes. Adv. Mater. Interfaces 2016, 3, 1600057. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, L.; Li, X.; Li, C.; Shao, C.; Zuo, P.; Liang, M.; Qu, L.; Cui, T. Miniaturized high-performance metallic 1T-Phase MoS2 micro-supercapacitors fabricated by temporally shaped femtosecond pulses. Nano Energy 2020, 67, 104260. [Google Scholar] [CrossRef]
- Tahir, M.; He, L.; Yang, W.; Hong, X.; Haider, W.A.; Tang, H.; Zhu, Z.; Owusu, K.A.; Mai, L. Boosting the electrochemical performance and reliability of conducting polymer microelectrode via intermediate graphene for on-chip asymmetric micro-supercapacitor. J. Energy Chem. 2020, 49, 224–232. [Google Scholar] [CrossRef]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R.; et al. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef]
- Saha, S.; Sadhukhan, P.; Roy Chowdhury, S.; Das, S. Rotary-Jet spin assisted fabrication of MnO2 microfiber for supercapacitor electrode application. Mater. Lett. 2020, 277, 128342. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Ghosh, M.; Ramos-ramón, J.A.; Saha, S. Applied Surface Science Study on charge storage mechanism in working electrodes fabricated by sol- gel derived spinel NiMn2O4 nanoparticles for supercapacitor application. Appl. Surf. Sci. 2019, 463, 513–525. [Google Scholar] [CrossRef]
- Bhattacharya, G.; Kandasamy, G.; Soin, N.; Upadhyay, R.K.; Deshmukh, S.; Maity, D.; McLaughlin, J.; Roy, S.S. Novel π-conjugated iron oxide/reduced graphene oxide nanocomposites for high performance electrochemical supercapacitors. RSC Adv. 2017, 7, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Koo, H.J.; Lee, A.; Braun, P.V. Selective wetting-induced micro-electrode patterning for flexible micro-supercapacitors. Adv. Mater. 2014, 26, 5108–5112. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wu, G.; Wu, X.; Cheng, H.; Xu, Z.; Chen, S. Microfluidic-Architected Nanoarrays/Porous Core–Shell Fibers toward Robust Micro-Energy-Storage. Adv. Sci. 2020, 7, 1901931. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Saruhan, B. Application of ionic liquids for batteries and supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Saha, S.; Ghosh, M.; Chowdhury, S.R.; Maiyalagan, T.; Bhattacharya, S.K.; Das, S. Electrochemical Energy Storage Properties of Ni-Mn-Oxide Electrodes for Advance Asymmetric Supercapacitor Application. Langmuir 2019, 35, 8257–8267. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Guo, H.; Wang, M.; Liu, J.; Wang, G.; Hu, C.; Xi, Y. A Flexible micro-supercapacitor based on a pen ink-carbon fiber thread. J. Mater. Chem. A 2014, 2, 19665–19669. [Google Scholar] [CrossRef]
- Ferris, A.; Garbarino, S.; Guay, D.; Pech, D. 3D RuO2 microsupercapacitors with remarkable areal energy. Adv. Mater. 2015, 27, 6625–6629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, R.; Zheng, H.; Bao, J.; Tang, Y.; Zhou, K. Laser-Assisted Printing of Electrodes Using Metal–Organic Frameworks for Micro-Supercapacitors. Adv. Funct. Mater. 2021, 31, 2009057. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-Dimensional Graphene Carbon Nanotube Carpet-Based Microsupercapacitors With High Electrochemical Performance. Nano Lett. 2013, 13, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.X.; Han, Y.; Zhao, Y.T.; Zeng, Y.; Yu, M.H.; Liu, Y.J.; Tang, H.L.; Tong, Y.X.; Lu, X.H. Advanced Ti-Doped Fe2O3@PEDOT Core/Shell Anode for High-Energy Asymmetric Supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Ray, A.; Korkut, D.; Saruhan, B. Efficient flexible all-solid supercapacitors with direct sputter-grown needle-like mn/mnox @graphite-foil electrodes and ppc-embedded ionic electrolytes. Nanomaterials 2020, 10, 1768. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on carbon/polyaniline hybrids: Design and synthesis for supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef] [Green Version]


| Materials | Synthesis Method | Type of SC | Electrolyte | Capacitance | Energy Density | Power Density | Potential Window | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ink-carbon fiber | coated by ink | SSC | LiCl-PVA | 4.3 mF/cm2 | 34 µW h/cm2 | 0.38 μW/ cm2 | 0.8 V | [42] |
| rGO | “in-plane” fabrication approach | ASC | PVA | <0.4 mF/cm2 | 0.01 μWh /cm2 | 9 μW /cm2 | 1.0 V | [34] |
| Graphene | electrodeposition | ASC | 0.5 M Na2 SO4 | 0.5 mF/cm2 | 0.07 μW h /cm2 | 7.5 μW /cm2 | 0.9 V | [43] |
| Mushroom derived carbon (MDC) | Scalable laser scribing technique | SSC | PVA-H2 SO4 | 9 mF/cm2 | 1.8 mWh/ cm3 | 720 mW/ cm3 | 1.0 V | [31] |
| Carbon | laser-induced MOF-derived | SSC | 1 M H2SO4 | 5.02 mF/cm2 | - | - | 1.0 V | [44] |
| Graphene/CNTs | in situ | SSC | 1 M Na2 SO4 IL | 2.16 mF/cm2 | 0.16 mWh /cm3 | 115 W/ cm3 | 1.0 V | [45] |
| Laser-Induced Graphene (LIG) | Laser-induced process | SSC | [EMIM][OTf]:PPC = 0.5 | 1.75 mF/cm2 | 0.256 µWh/cm2 | 0.11 mW/cm2 | 2.0 V | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, A.; Roth, J.; Saruhan, B. Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage. Molecules 2022, 27, 329. https://doi.org/10.3390/molecules27010329
Ray A, Roth J, Saruhan B. Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage. Molecules. 2022; 27(1):329. https://doi.org/10.3390/molecules27010329
Chicago/Turabian StyleRay, Apurba, Jenny Roth, and Bilge Saruhan. 2022. "Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage" Molecules 27, no. 1: 329. https://doi.org/10.3390/molecules27010329
APA StyleRay, A., Roth, J., & Saruhan, B. (2022). Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage. Molecules, 27(1), 329. https://doi.org/10.3390/molecules27010329








