Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Determination of Qualitative Composition of Glycyrrhizic Acid Derivatives Using LC-MS/MS
Development of a Method for the Screening of Glycyrrhyzinic Acid Derivatives Using LC-MS/MS
2.3. Biological Activity
2.3.1. Cytotoxicity
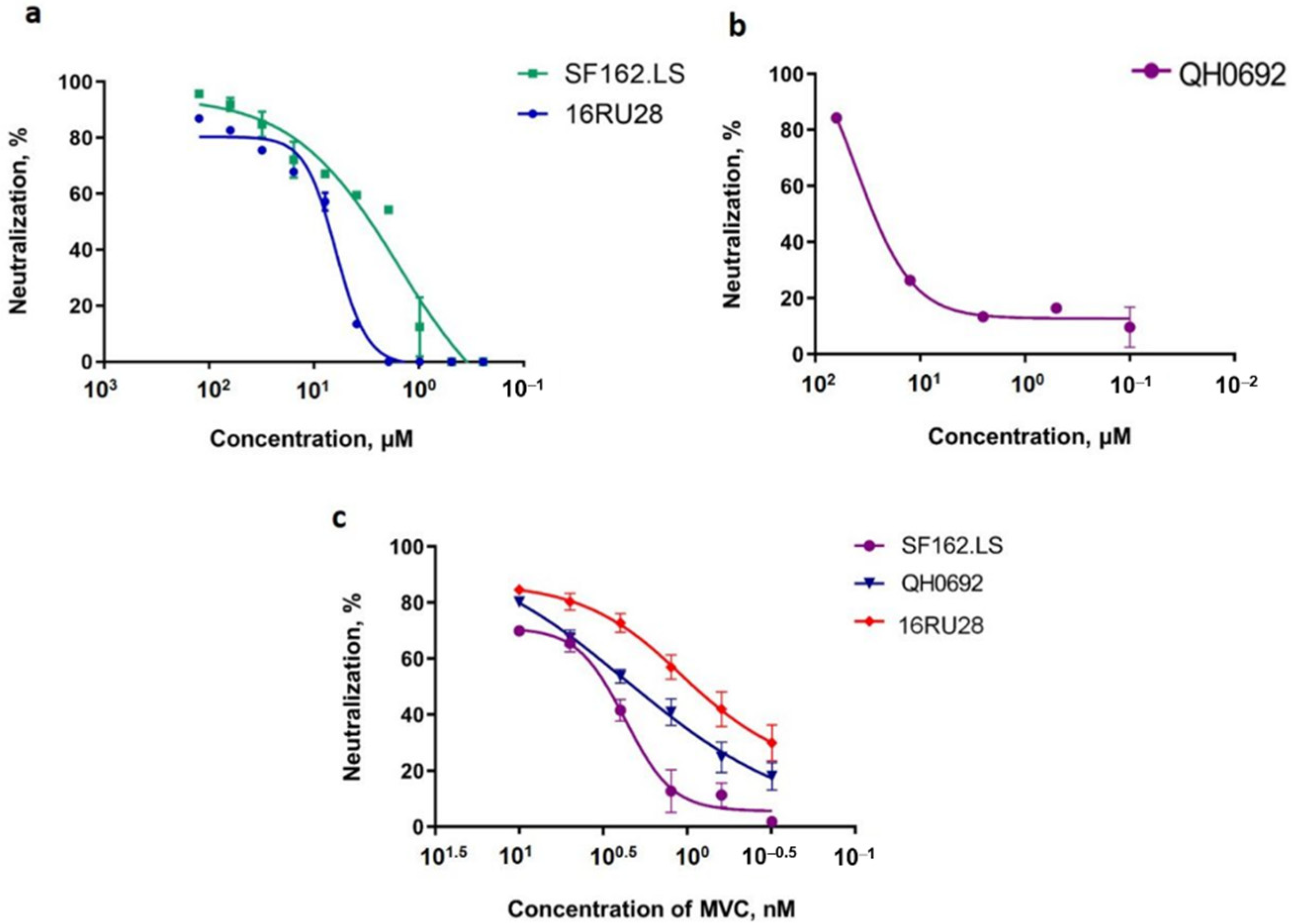
2.3.2. Antiviral Activity against Infection by HIV-1 Pseudotyped Viruses
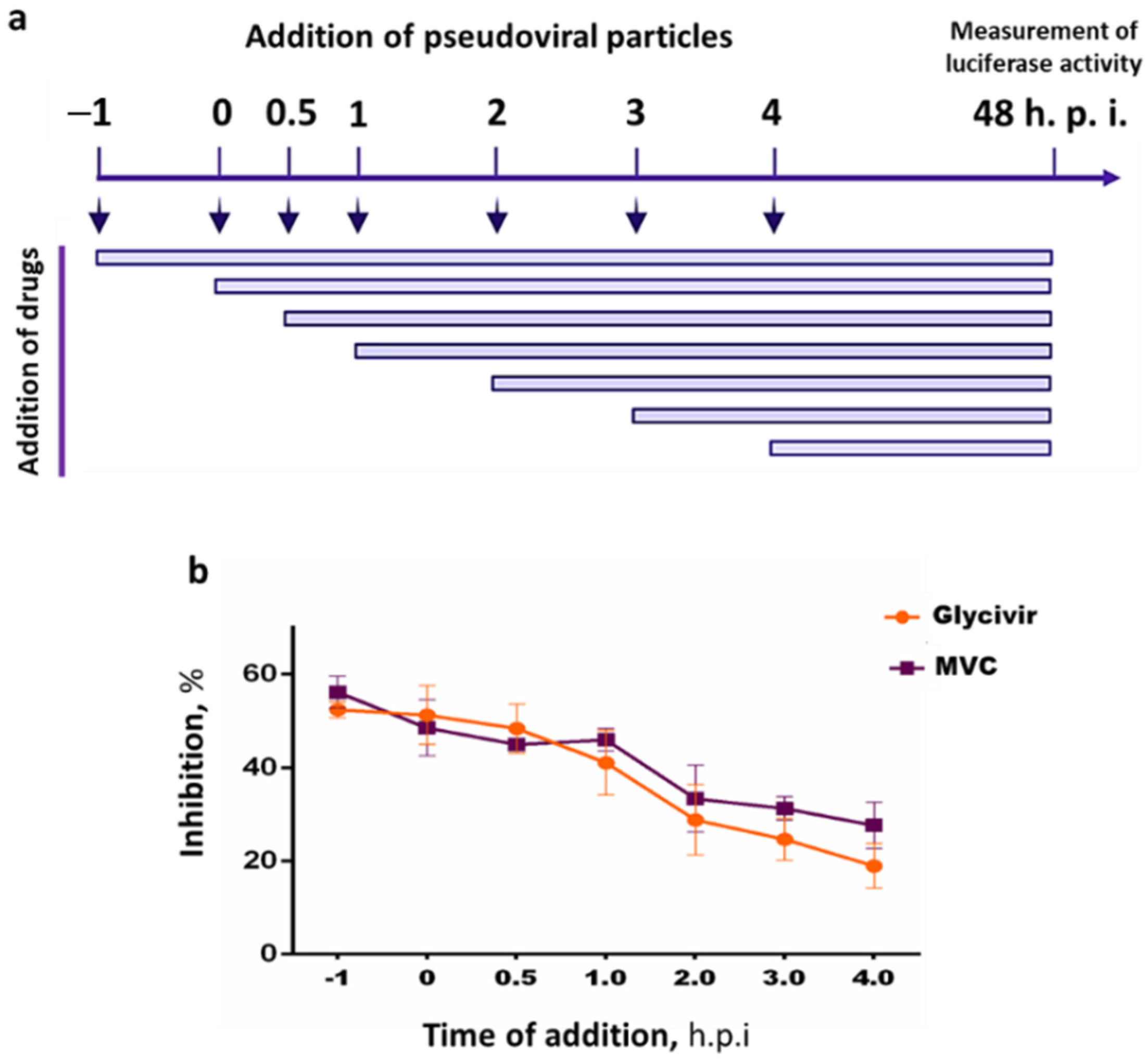
2.3.3. Time Dependency of the Inhibitory Effects of Glycivir
2.4. Antiviral Activity against SARS-CoV-2 Viruses
3. Materials and Methods
3.1. Synthesis of Glycivir Compounds
3.1.1. NMR Spectral Analyses
3.1.2. Synthesis of Glycyvir
3.1.3. Synthesis of Nic-GA
3.1.4. LC-MS/MS Analysis
3.2. Cell Lines and Viral Strains
3.2.1. MTT Cytotoxicity Assays
3.2.2. Evaluation of the Inhibitory Activity of Drugs Using HIV-1 Pseudoviruses
3.2.3. Time-of-Addition Assay
3.2.4. Evaluation of the Antiviral Activities against SARS-CoV-2 Viruses
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 29 December 2021).
- The Effects of the COVID-19 Pandemic on the HIV Response. Available online: https://www.unaids.org/en/resources/documents/2021/effects-of-covid19-pandemic-on-hiv-response (accessed on 29 December 2021).
- Cattaneo, D.; Cattaneo, D.; Gervasoni, C.; Corbellino, M.; Galli, M.; Riva, A.; Gervasoni, C.; Clementi, E.; Clementi, E. Does lopinavir really inhibit SARS-CoV-2? Pharmacol. Res. 2020, 158, 104898. [Google Scholar] [CrossRef]
- Patel, T.K.; Patel, P.B.; Barvaliya, M.; Saurabh, M.K.; Bhalla, H.L.; Khosla, P.P. Efficacy and safety of lopinavir-ritonavir in COVID-19: A systematic review of randomized controlled trials. J. Infect. Public Health 2021, 14, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Vallish, B.; Dang, S. Hydroxychloroquine and Remdesivir in COVID-19: A critical analysis of recent events. Indian J. Med. Ethics 2020, 5, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ayerdi, O.; Puerta, T.; Clavo, P.; Vera, M.; Ballesteros, J.; Fuentes, M.E.; Estrada, V.; Rodríguez, C.; Del Romero, J.; Del Romero, J.; et al. Preventive Efficacy of Tenofovir/Emtricitabine Against Severe Acute Respiratory Syndrome Coronavirus 2 Among Pre-Exposure Prophylaxis Users. Open Forum Infect. Dis. 2020, 7, ofaa455. [Google Scholar] [CrossRef] [PubMed]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Martinez, M.A. Lack of Effectiveness of Repurposed Drugs for COVID-19 Treatment. Front. Immunol. 2021, 12, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019. Curr. Opin. HIV AIDS 2020, 15, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Arts, E.J.; Hazuda, D.J. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161. [Google Scholar] [CrossRef]
- Lobritz, M.A.; Ratcliff, A.N.; Arts, E.J. HIV-1 Entry, Inhibitors, and Resistance. Viruses 2010, 2, 1069–1105. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Cai, Y.; Chen, B. HIV-1 Entry and Membrane Fusion Inhibitors. Viruses 2021, 13, 735. [Google Scholar] [CrossRef]
- Yarovaya, O.I.; Salakhutdinov, N.F. Mono- and sesquiterpenes as a starting platform for the development of antiviral drugs. Russ. Chem. Rev. 2021, 90, 488–510. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Farooq, S.; Ngaini, Z. Natural and Synthetic Drugs as Potential Treatment for Coronavirus Disease 2019 (COVID-2019). Chem. Afr. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Perez-Castillo, Y.; Elshabrawy, H.A.; Filho, C.d.S.M.B.; de Sousa, D.P. Bioactive Terpenes and Their Derivatives as Potential SARS-CoV-2 Proteases Inhibitors from Molecular Modeling Studies. Biomolecules 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Anywar, G.; Akram, M.; Chishti, M.A. African and Asian Medicinal Plants as a Repository for Prospective Antiviral Metabolites Against HIV-1 and SARS CoV-2: A Mini Review. Front. Pharmacol. 2021, 12, 703837. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Iwata, S.; Matsumoto, H.; Mori, T.; Shibata, S.; Baba, M.; Ito, M.; Shigeta, S.; Nakashima, H.; Yamamoto, N. Antiviral activities of glycyrrhizin and its modified compounds against human immunodeficiency virus type 1(HIV-1) and herpes simplex virus type 1(HSV-1) in vitro. Chem. Pharm. Bull. 1991, 39, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of Glycyrrhizin, an Active Component of Licorice Roots, on HIV Replication in Cultures of Peripheral Blood Mononuclear Cells from HIV-Seropositive Patients. Pathobiology 2002, 70, 229–236. [Google Scholar] [CrossRef]
- Li, J.; Xu, D.; Wang, L.; Zhang, M.; Zhang, G.; Li, E.; He, S. Glycyrrhizic Acid Inhibits SARS-CoV-2 Infection by Blocking Spike Protein-Mediated Cell Attachment. Molecules 2021, 26, 6090. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O.; et al. Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 2021, 13, 609. [Google Scholar] [CrossRef]
- Elebeedy, D.; Elkhatib, W.F.; Kandeil, A.; Ghanem, A.; Kutkat, O.; Alnajjar, R.; Saleh, M.A.; Abd El Maksoud, A.I.; Badawy, I.; Al-Karmalawy, A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021, 11, 29267–29286. [Google Scholar] [CrossRef]
- Kondratenko, R.M.; Balina, L.A.; Mustafina, S.R.; Pljasunova, O.A.; Pokrovskij, A.G.; Tolstikov, G.A. Glycyrrhizic Acid Amide with 5-Aminouracil Eliciting Anti-Hiv-Activity. Patent RU2199547C2, 27 February 2003. [Google Scholar]
- Pokrovskij, A.; Salakhutdinov, N.; Tolstikov, G. Method for Preparation of Pentanicotinate of Glycyrrhizic Acid Being Reproduction Inhibitor of Human Immunodeficiency Virus. Patent RU2363703C2, 10 August 2009. [Google Scholar]
- Tolstikov, G.; Baltina, L.; Volcho, K.; Pljasunova, O.; Pokrovskij, A.; Salakhutdinov, N. Di- and Trinicotinates of Glycyrrhizic Acid and Inhibitor of Human Immunodeficiency Virus Reproduction. Patent RU2304145C1, 10 August 2007. [Google Scholar]
- Suzuki, T.; Tsukahara, M.; Akasaka, Y.; Inoue, H. A highly sensitive LC-MS/MS method for simultaneous determination of glycyrrhizin and its active metabolite glycyrrhetinic acid: Application to a human pharmacokinetic study after oral administration. Biomed. Chromatogr. 2017, 31, e4032. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhao, Y.; Yu, P.; Yang, B.; Zhou, C.; Yu, Z. LC-ESI-MS/MS method for simultaneous determination of eleven bioactive compounds in rat plasma after oral administration of Ling-Gui-Zhu-Gan Decoction and its application to a pharmacokinetics study. Talanta 2018, 190, 450–459. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Putilova, V.P.; Yarovaya, O.I.; Zybkina, A.V.; Mordvinova, E.D.; Zaykovskaya, A.V.; Shcherbakov, D.N.; Orshanskaya, I.R.; Sinegubova, E.O.; Esaulkova, I.L.; et al. Synthesis and Antiviral Activity of Camphene Derivatives against Different Types of Viruses. Molecules 2021, 26, 2235. [Google Scholar] [CrossRef]
- Volobueva, A.S.; Yarovaya, O.I.; Kireeva, M.V.; Borisevich, S.S.; Kovaleva, K.S.; Mainagashev, I.Y.; Gatilov, Y.V.; Ilyina, M.G.; Zarubaev, V.V.; Salakhutdinov, N.F. Discovery of New Ginsenol-Like Compounds with High Antiviral Activity. Molecules 2021, 26, 6794. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; de Benito, N. Alpha variant SARS-CoV-2 infection: How it all starts. EBioMedicine 2021, 74, 103703. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2021, 22, 35–42. [Google Scholar] [CrossRef]

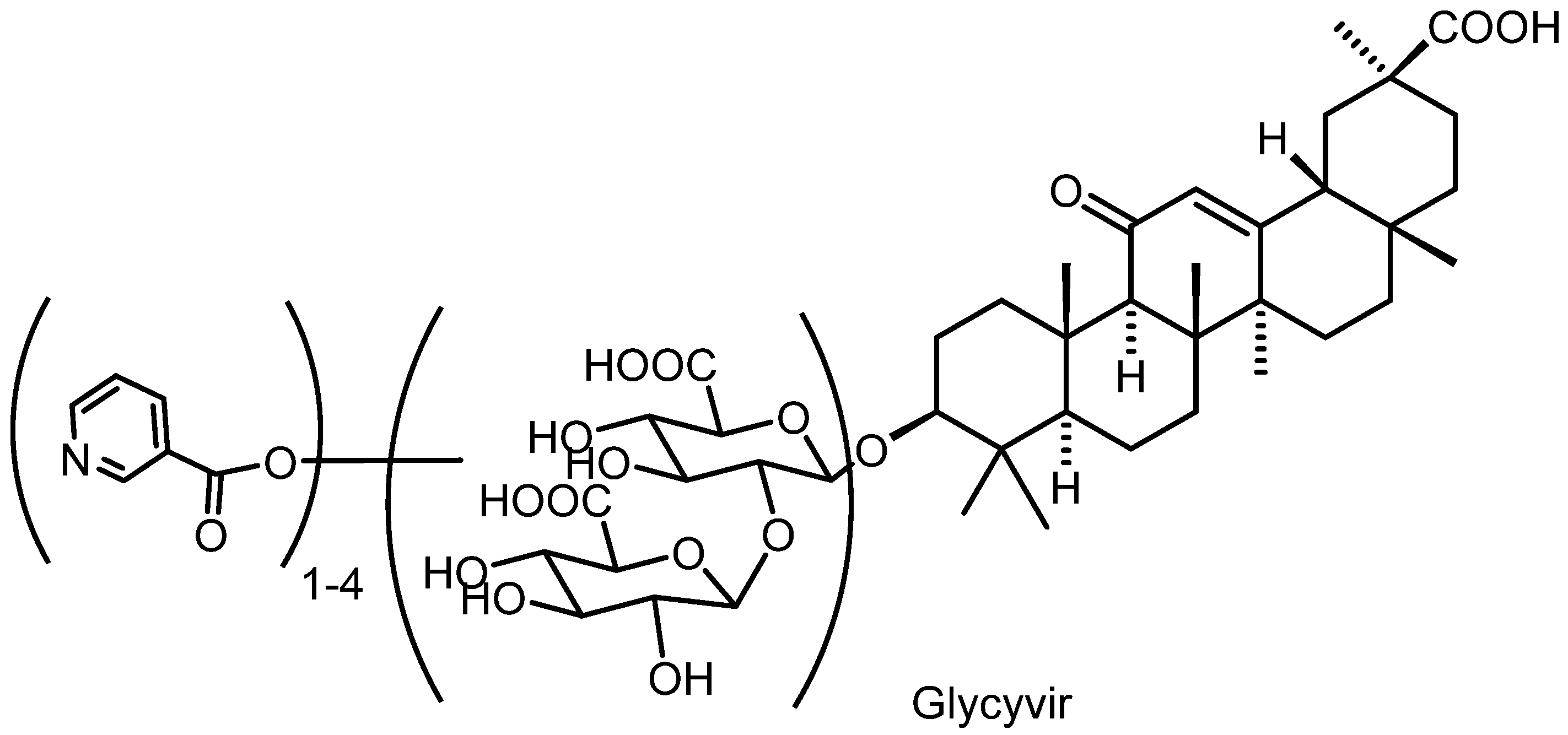
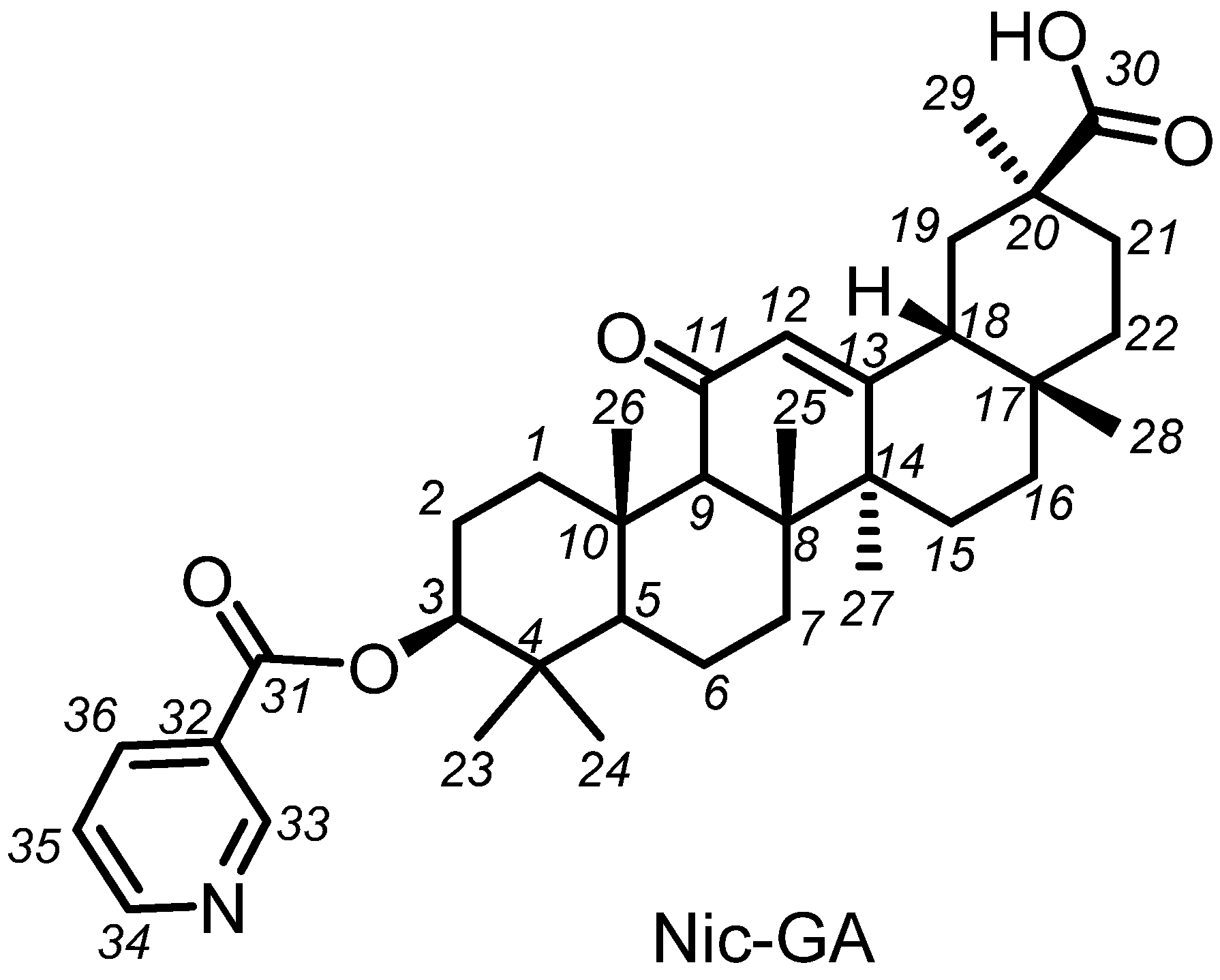
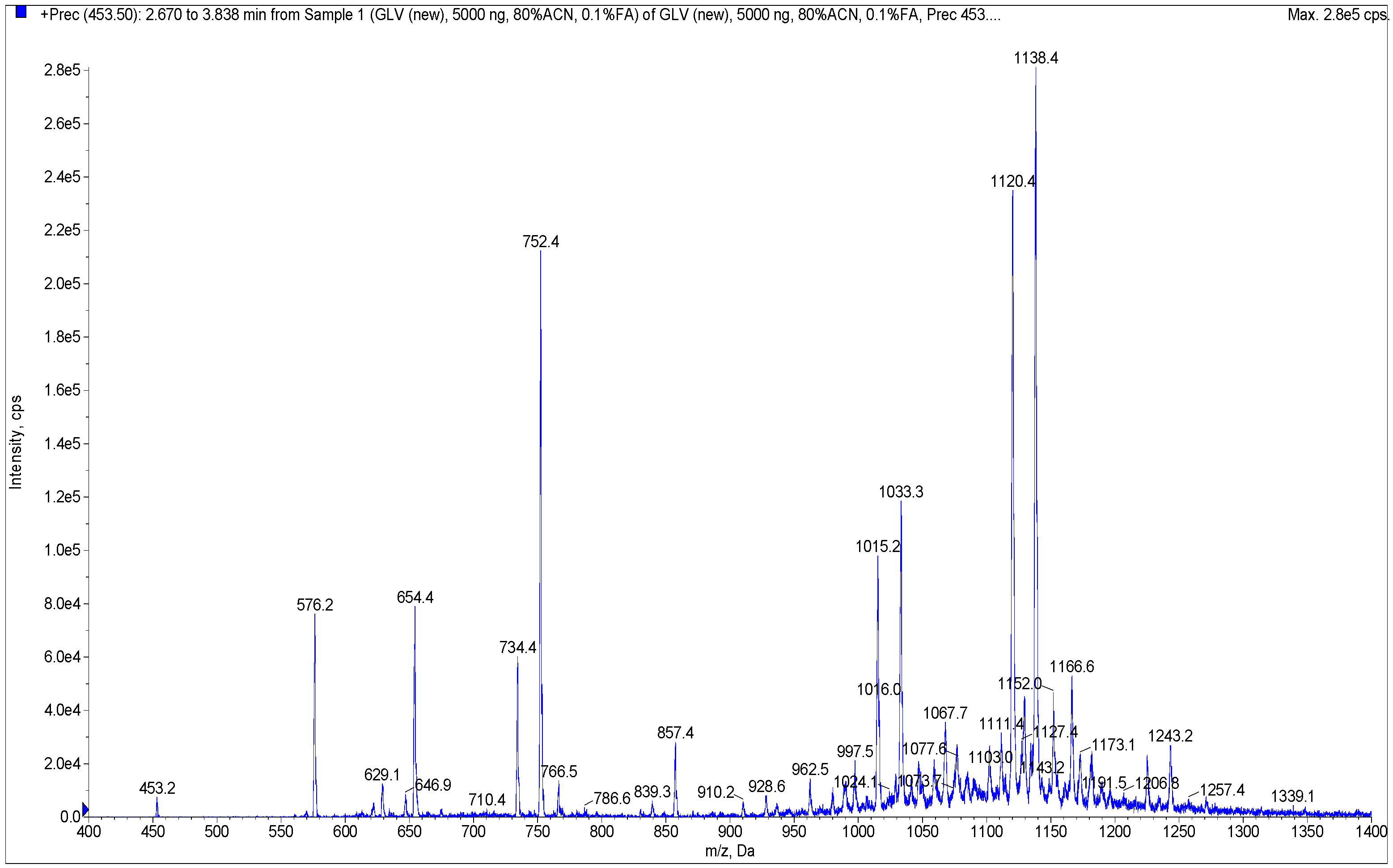
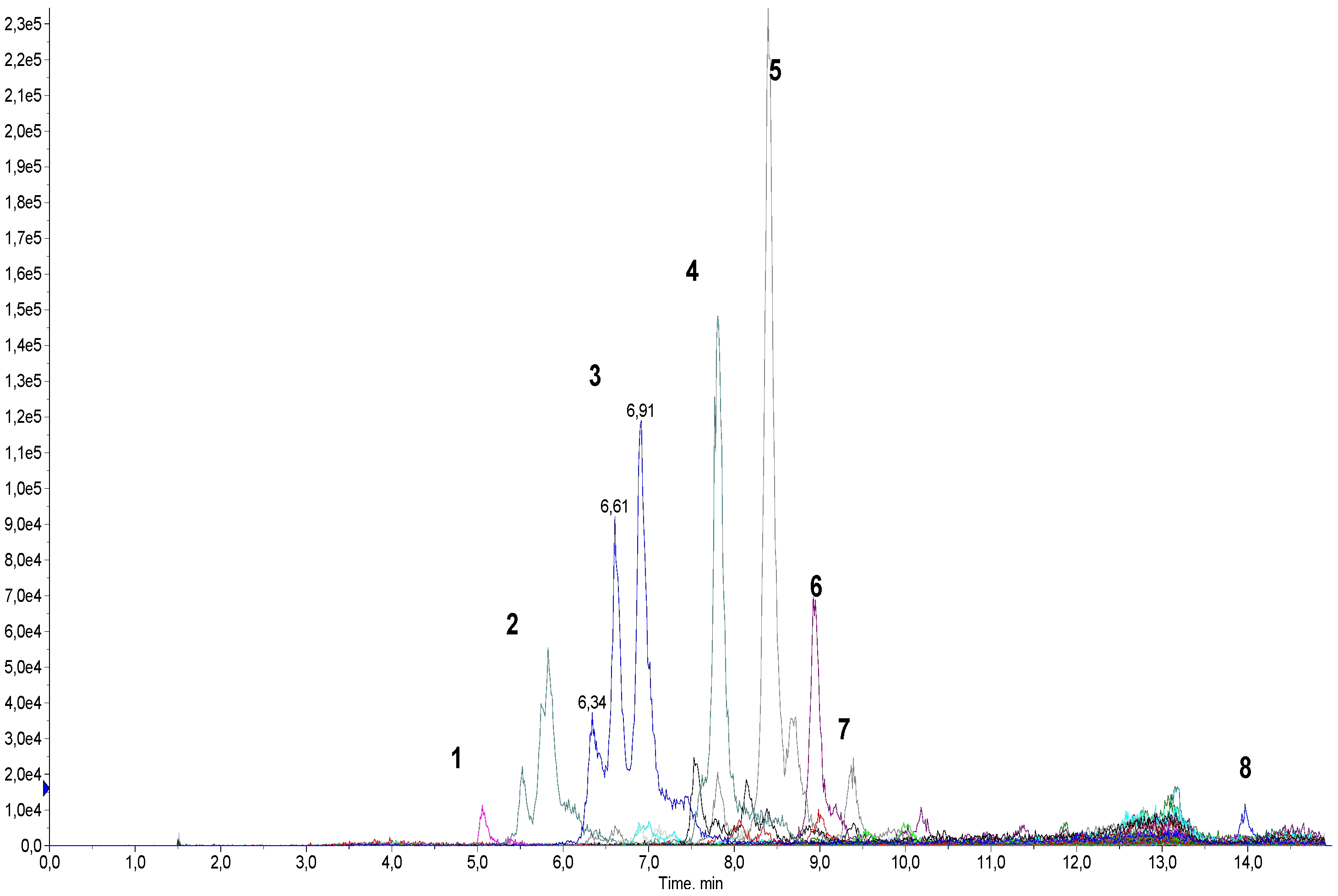
| Component Name and/or Molecular Weight, Da a | M + H+ | Amount of the Component in Sample, % b | |
|---|---|---|---|
| Glycyvir | Nic-GA | ||
| glycyrrhetinic acid nicotinate (575.2, 8) | 576.2 | 0.69 | 99.87 |
| 653.4 | 654.4 | 0.04 | 0.02 |
| Mononicotinate of glycyrrhetinic acid lactouronide (733.4) | 734.4 | 0.49 | 0.01 |
| Mononicotinate of glycyrrhetinic acid glucuronide (751.4, 5) | 752.4 | 26.14 d | N.D. c |
| 765.5 | 766.5 | N.D. | N.D. |
| Glycyrrhizinic acid (822.5, 1) | 823.5 | 0.79 | N.D. |
| 838.3 | 839.3 | 0.02 | N.D. |
| 856.5 | 857.5 | 0.34 | N.D. |
| mono-nic, mono-lact (909.5) | 910.5 | 1.08 | N.D. |
| mono-nic (927.0, 2) | 928 | 8.80 | N.D. |
| 955.3 | 956.3 | 0.30 | N.D. |
| 961.5 | 962.5 | 0.15 | N.D. |
| 979.2 | 980.2 | 0.02 | N.D. |
| di-nic, di-lact (996.5) | 997.5 | N.D. | N.D. |
| 998 | 999 | N.D. | N.D. |
| di-nic, mono-lact (1014.4) | 1015.4 | 5.21 | N.D. |
| di-nic (1032.4, 3) | 1033.4 | 23.91 | N.D. |
| 1060.5 | 1061.5 | N.D. | N.D. |
| 1067 | 1068 | 1.81 | 0.01 |
| 1075.9 | 1076.9 | 0.42 | N.D. |
| 1090 | 1091 | 0.90 | 0.01 |
| tri-nic, di-lact (1101.2) | 1102.2 | 1.17 | 0.01 |
| 1110.3 | 1111.3 | 1.69 | 0.01 |
| tri-nic, mono-lact (1119.3, 6) | 1120.3 | 6.10 | N.D. |
| 1128.4 | 1129.4 | 1.27 | 0.01 |
| tri-nic (1137.4, 4) | 1138.4 | 15.48 | N.D. |
| 1151 | 1152 | 0.12 | 0.03 |
| 1165.2 | 1166.2 | 1.02 | N.D. |
| 1171.6 | 1172.6 | 0.27 | N.D. |
| 1185.9 | 1186.9 | 0.58 | N.D. |
| 1189.1 | 1190.1 | N.D. | N.D. |
| 1194.6 | 1195.6 | 0.73 | 0.01 |
| 1223 | 1224 | 0.36 | N.D. |
| tetra-nic, mono-lact (1224.3) | 1225.3 | N.D. | N.D. |
| tetra-nic (1242.6) | 1243.6 | 0.09 | N.D. |
| 1341.9 | 1342.9 | 0.02 | N.D. |
| Agent | CC50 | SF162.LS | OH0692 | 16RU28 | |||
|---|---|---|---|---|---|---|---|
| µM | IC50. µM | SI | IC50. µM | SI | IC50. µM | SI | |
| Glycyrrhizin | >1000 | NA | - | NA | - | NA | - |
| Glycyvir | >1000 | 2.88 ± 0.12 | 347 | 27.5 ± 2.8 | 36 | 6.91 ± 0.23 | 138 |
| Nic-GA | <7.8 | NA | - | NA | - | NA | - |
| MVC | >1000 | 0.002 | 5 × 103 | 0.002 | 5 × 103 | 0.0016 | 6 × 103 |
| Agent | CC50 | hCoV-19/Aust/VIC01/2020 a | hCoV-19/Russ/MOS/2020 b | hCoV-19/Russ/PSK/2021 c | |||
|---|---|---|---|---|---|---|---|
| µM | IC50, µM | SI | IC50, µM | SI | IC50, µM | SI | |
| Glycyrrhizin | >1000 | NA | - | NA | - | NA | - |
| Glycyvir | 334.5 ± 18.5 | 8.3 ± 1.10 | 40 | 2.2 ± 0.14 | 152 | 3.8 ± 0.41 | 88 |
| Nic-GA | 101.3 | NA | - | NA | - | NA | - |
| Remdesivir | 710.9 ± 21.2 | 3.3 ± 0.12 | 215 | 1.5 ± 0.14 | 473 | 1.9 ± 0.22 | 359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, V.V.; Rudometova, N.B.; Yarovaya, O.I.; Rogachev, A.D.; Fando, A.A.; Zaykovskaya, A.V.; Komarova, N.I.; Shcherbakov, D.N.; Pyankov, O.V.; Pokrovsky, A.G.; et al. Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses. Molecules 2022, 27, 295. https://doi.org/10.3390/molecules27010295
Fomenko VV, Rudometova NB, Yarovaya OI, Rogachev AD, Fando AA, Zaykovskaya AV, Komarova NI, Shcherbakov DN, Pyankov OV, Pokrovsky AG, et al. Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses. Molecules. 2022; 27(1):295. https://doi.org/10.3390/molecules27010295
Chicago/Turabian StyleFomenko, Vladislav V., Nadezhda B. Rudometova, Olga I. Yarovaya, Artem D. Rogachev, Anastasia A. Fando, Anna V. Zaykovskaya, Nina I. Komarova, Dmitry N. Shcherbakov, Oleg V. Pyankov, Andrey G. Pokrovsky, and et al. 2022. "Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses" Molecules 27, no. 1: 295. https://doi.org/10.3390/molecules27010295
APA StyleFomenko, V. V., Rudometova, N. B., Yarovaya, O. I., Rogachev, A. D., Fando, A. A., Zaykovskaya, A. V., Komarova, N. I., Shcherbakov, D. N., Pyankov, O. V., Pokrovsky, A. G., Karpenko, L. I., Maksyutov, R. A., & Salakhutdinov, N. F. (2022). Synthesis and In Vitro Study of Antiviral Activity of Glycyrrhizin Nicotinate Derivatives against HIV-1 Pseudoviruses and SARS-CoV-2 Viruses. Molecules, 27(1), 295. https://doi.org/10.3390/molecules27010295









