Abstract
Sphingosine-1-phosphate-1 (S1P1) receptor agonists are well-known drugs for treating multiple sclerosis (MS) caused by autoreactive lymphocytes that attack the myelin sheath. Therefore, an effective therapeutic strategy is to reduce the lymphocytes in the blood by inducing S1P1 receptor internalization. We synthesized serinolamide A, a natural product of the sea, and performed S1P1 receptor internalization assay to evaluate functionally antagonistic S1P1 receptor agonist activity. In order to synthesize derivatives with better efficacy than serinolamide A and B, new derivatives were synthesized by introducing the phenyl ring moiety of fingolimod. Among them, compounds 19 and 21 had superior S1P1 agonistic effects to serinolamide. We also confirmed that compound 19 effectively inhibited lymphocyte outflow in peripheral lymphocyte count (PLC) assay.
1. Introduction
Multiple sclerosis (MS) is a neuroinflammatory autoimmune disease [1]. Of the different types, relapsing–remitting multiple sclerosis (RRMS) is the most common disease course [2,3]. Multiple sclerosis is caused when autoreactive T cells migrate across the blood–brain barrier (BBB) and damage the myelin sheath in the central nervous system, leading to neurodegeneration and demyelination [4]. Sphingosine-1-phosphate (S1P) receptors are a class of G protein-coupled receptors (GPCR) with five subtypes, S1P1–5. Among them, sphingosine-1-phosphate-1 (S1P1) plays a role in regulating the egress of lymphocytes from lymphoid tissue to the lymph [5]. Studies have shown that the S1P1 receptor is internalized and degraded by functional antagonists, prompting lymphocyte sequestration in the lymph node and immunosuppression [6,7,8]. Therefore, developing functionally antagonistic S1P1 receptor agonists is an effective strategy for overcoming autoimmune diseases. Natural marine products serinolamide A and serinolamide B have long lipophilic chains and polar substituents such as the well-known S1P1 receptor agonist fingolimod (FTY720, Gilenya®), as shown in Figure 1. Serinolamide A synthesis methods have been reported in multiple papers [9,10,11,12]. In this study, we partially optimized the existing synthesis method for serinolamides A and B. Serinolamide derivatives were also synthesized by introducing the phenyl moiety of FTY720. S1P1 receptor agonists bind to the S1P1 receptor, which induces S1P1 receptor internalization and, consequently, induces receptor degradation [5,6,7,8]. Therefore, synthesized compounds were evaluated as functional antagonists that effectively degrade S1P1 receptors by performing S1P1 receptor internalization analysis.
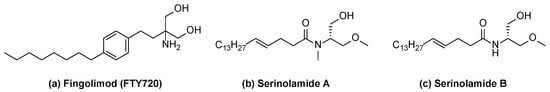
Figure 1.
Structures of fingolimod (a), serinolamide A (b), and serinolamide B (c).
2. Results and Discussion
2.1. Chemical Synthesis of Serinolamide Derivatives
The synthesis of serinolamide A followed the procedures reported by Pandey [10] and Wang [11]. The amine part was synthesized following the amide coupling method reported by Wang. The O-methylation of commercially available methyl (tert-butoxycarbonyl)-L-serinate with commercially available methyl iodide yielded methyl N-(tert-butoxycarbonyl)-O-methyl-L-serinate 22. The reduction of the methyl ester with sodium borohydride yielded an alcohol derivative 23, and TBDMS protection of 23 with butyldimethylsilyl chloride (TBDMSCl) yielded 24. The methylation of 24 with commercially available methyl iodide yielded tert-butyl (S)-(1-((tert-butyldimethylsilyl)oxy)-3-methoxypropan-2-yl)(methyl)carbamate 25. Boc and TBDMS deprotection of 25 with 4 M HCl yielded 26; Boc deprotection of 23 with 4 M HCl yielded 27 (Scheme S1 in the Supporting Information). The carboxylic-acid-containing counterpart was introduced following the metathesis method using the Grubbs catalyst reported by Pandey (Scheme 1) [10].
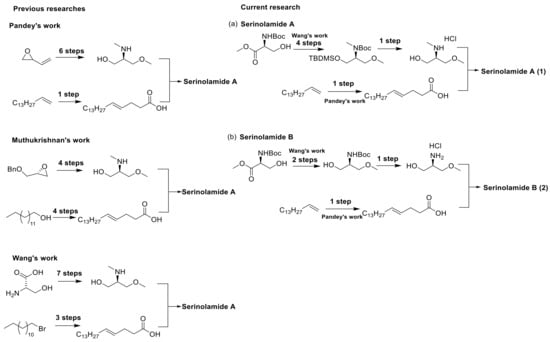
Scheme 1.
Synthesis routes for serinolamides A and B.
The olefin metathesis reaction of commercially available pentadec-1-ene with commercially available pent-4-enoic acid yielded (E)-octadec-4-enoic acid 3. Amide coupling of secondary amine derivative 26 and primary amine derivative 27 with the carboxylic acid derivative 3 yielded serinolamide A and serinolamide B (Scheme 2) [12]. Amide coupling of 3 with commercially available serinol yielded 4. The olefin metathesis reaction of commercially available pentadec-1-ene with commercially available pent-4-enal yielded (E)-octadec-4-enal (5). The reductive amination of 5 with commercially available serinol yielded 6 (Scheme 2).
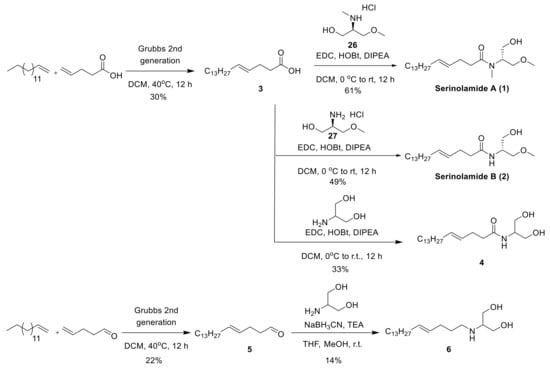
Scheme 2.
Synthesis of 1, 2, 4, 6.
2.2. Chemical Synthesis of Fingolimod Analogues
The nucleophilic substitution reaction of commercially available 1-(bromomethyl)-4-nitrobenzene with commercially available diethyl 2-acetamidomalonate yielded diethyl 2-acetamido-2-(4-nitrobenzyl)malonate 7. The reduction of the nitro group with iron yielded an amine derivative 8, and amide coupling of 8 with carboxylic acid derivative 3 yielded 9. Reducing the ethyl ester derivative 9 with lithium aluminum hydride (LAH) yielded compounds 10–12, and the hydrolysis of 12 with sodium hydroxide yielded an undesired product (Scheme 3). The reductive amination of 8 with aldehyde derivative 5 yielded 13.
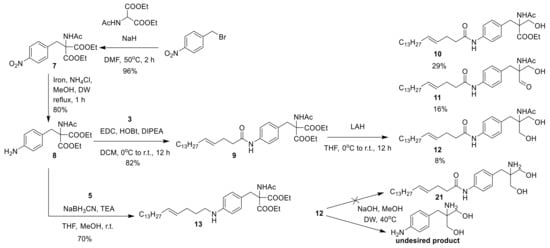
Scheme 3.
Synthesis of 10–13.
Reducing ethyl ester derivative 7 with calcium chloride yielded alcohol derivative 14. The deacetylation of 14 with 6 M HCl yielded amine salt derivative 15. The tert-butyloxycarbonyl protection of amine derivative 15 with di-tert-butyl dicarbonate yielded compound 16. The reduction of nitro derivative 16 with iron yielded amine derivative 17. The reductive amination of 17 with aldehyde derivative 5 yielded compound 18, and the Boc deprotection of 18 with 4 M HCl yielded 19. Amide coupling of 17 with carboxylic acid derivative 3 yielded 20, and its Boc deprotection with 4 M HCl yielded 21 (Scheme 4).
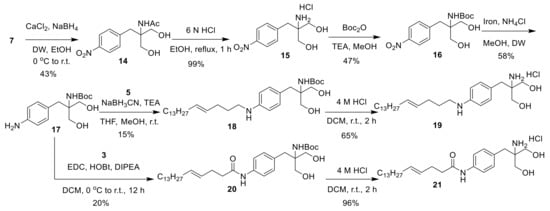
Scheme 4.
Synthesis of 19–21.
2.3. Evaluation of the Synthesized Serinolamide Derivatives as S1P1 Receptor Agonists
The ability of the compounds to internalize the S1P1 receptor from the cell surface was evaluated using a commercially available in vitro assay system to test the functionally antagonistic S1P1 receptor agonist activity of the synthesized serinolamide derivatives [13,14,15]. The efficacy of the synthetic compounds was expressed as a percentage of maximal efficacy at 1 μM of FTY720, a highly potent S1P1 agonist. In the S1P1 receptor internalization assay, compounds 12, 19, and 21 showed more than 80% efficacies at 30 μM. Notably, compound 19 showed good efficacy of 147%. In addition, compounds 4, 6, and 13 showed lower S1P1 receptor internalization efficacies than other derivatives (Table 1). The efficacy of compounds 19 and 21, in which a phenyl ring was introduced, was significantly improved compared to those of compounds 4 and 6, in which a phenyl ring was not introduced.

Table 1.
Effects of Serinolamide A derivatives on ligand binding to G protein-coupled receptor.
2.4. In Vivo Reduction of Peripheral Blood Lymphocyte Count by Treatment of Compounds 19 and 21 in Mice
S1P1 agonists, such as fingolimod, are known to induce peripheral lymphopenia by inhibiting S1P1-mediated lymphocyte outflow from lymphoid tissues. Lymphopenia caused by these drugs contributes to the therapeutic effect of autoimmune diseases such as multiple sclerosis [6,16]. Therefore, we investigated the effect of compounds to induce lymphopenia in blood by peripheral lymphocyte count (PLC) analysis (Figure 2). After intravenous administration of compounds 19, 21 (15 mg/kg, 30 mg/kg, maximum solubility concentration) and fingolimod (3 mg/kg) to mice, blood samples were collected by orbital bleed. At this time, there was no visual change compared to the vehicle treatment group containing the same amount of DMSO. As a result of measuring the number of lymphocytes in the blood, the number of lymphocytes started to decrease within 2.5 h after administration. In particular, the number of lymphocytes in the blood of mice administered 19 was significantly decreased during the first 5 h. In contrast to fingolimod, mice treated with 19 and 21 began to recover lymphocyte counts after 5 h. As a result of a single dose administration, peripheral lymphocyte counts in fingolimod-treated mice continued to decrease for 24 h post-dose. In contrast, lymphocyte counts in mice treated with compound 19 or 21 returned to near baseline levels, suggesting that the cardiac toxicity of fingolimod due to its long-term efficacy on lymphocyte reduction can be overcome (Figure 2) [17]. Collectively, these results suggest that administration of 19 and 21 can inhibit the egress of lymphocytes from lymphoid tissues to peripheral blood, and that lymphopenia can be reversed within 24 h.
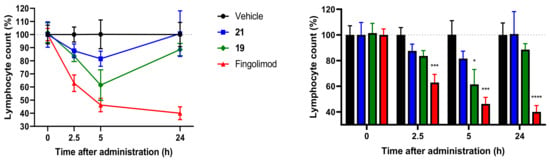
Figure 2.
Reduction of the blood lymphocyte count by the treatment of 19 and 21 in mice. Mice were intravenously administered with the vehicle (n = 8), 19 (15 mg/kg, n = 7), 21 (30 mg/kg, n = 10), or positive control fingolimod (3 mg/kg, n = 8). Blood lymphocyte counts were measured before (0 h) and after the administration (2.5 and 5, 24 h). Percentage of the lymphocyte count at the time before administration (0 h) was considered as a baseline (100%). * p < 0.05, *** p < 0.001, **** p < 0.0001 compared to vehicle-treated group (one-way ANOVA with Dunnett’s test). Data are presented as mean ± SEM.
3. Experimental Section
3.1. General Methods
All chemicals, reagents, and solvents were obtained from commercially available sources as reagent grades without further purification. The yields reported are for purified products and were not optimized. Synthesized compounds were checked by thin-layer chromatography (TLC) and 1H and 13C nuclear magnetic resonance (NMR), melting point (MP), high-resolution mass spectrometry (HRMS), and high-performance liquid chromatography (HPLC) analyses. Analytical thin-layer chromatography plates monitored reactions (Merck, Cat No. 1.05715, Darmstadt, Germany) and analyzed by ultraviolet light at 254 nm and 280 nm. The reactions were purified by MPLC (Biotage®, Isolera™ one, Uppsala, Sweden). The NMR spectra were recorded at 400 MHz (1H)/100 MHz (13C) using Bruker spectrometers (Billerica, USA.). Chemical shifts (δ) were reported in ppm downfield from tetramethylsilane (TMS). HPLC analysis was performed using a Waters E2695 system (Milford, USA.) equipped with a YMC-Triart C18 /S-5 μm /12 nm/ Lot No. 17452 (150 mm × 4.6 mm diameter). The HPLC data were recorded using the following parameters: DW (0.1% AcOH)/acetonitrile. Method A: 10/90 → 100/0 in 15 min, +5 min isocratic, flow rate of 0.5 mL/min to 1.0 mL/min, λ = 254 and 280 nm. HRMS was performed with electrospray ionization on a Q-Exactive (Thermo Fisher Scientific, Waltham, USA.) instrument. Specific rotation was measured with the autopol® III polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA).
3.2. General Procedure for Amide Coupling Reaction (Method A)
A mixture of carboxylic acid derivatives, EDC, HOBt, and DIPEA, was dissolved in dichloromethane ([C] ~ 0.1 M) and stirred for 20 min at room temperature. Amine derivatives were added into the reaction mixture and stirred overnight to afford serinolamide A. The reaction mixture was diluted with distilled water and extracted with ethyl acetate. The combined organic layer was dried with Na2SO4 and evaporated in vacuo. The obtained residue was purified by column chromatography on SiO2.
3.3. General Procedure for Boc Deprotection and Deacetylation Reaction (Method B)
To a mixture of NHBoc derivatives in dichloromethane or ethanol ([C] ~ 0.1 M), 4 M HCl was added, and the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was evaporated in vacuo.
3.4. General Procedure for the Reductive Amination with Aldehyde (Method C)
A mixture of aldehyde derivatives in methanol and tetrahydrofuran in a ratio of 1:1 ([C] ~ 0.1 M) was added a mixture of amine derivatives with triethylamine. The resulting suspension was stirred at room temperature (0.5 h). Then, sodium cyanoborohydride was added and stirred at room temperature. The reaction mixture was evaporated in vacuo. The product residue was washed with ethyl acetate and distilled water. The combined organic layer was dried with anhydrous Na2SO4 and evaporated in vacuo. The obtained residue was purified by column chromatography on SiO2.
3.4.1. Synthesis of Serinolamide A (1)
Using Method A, 3 (50 mg, 0.17 mmol), EDC (68 mg, 0.44 mmol), HOBt (85 mg, 0.63 mmol) and DIPEA (0.23 mL, 1.33 mmol), 26 (44 mg, 0.28 mmol) gave 40 mg (61%) of serinolamide A as clear oil; Rf = 0.34 (n-hexane/EtOAc 1/2); {[α]D25 = +2.78 (c = 0.18, CHCl3)}[Lit.10 [α]D25 = +1.97 (c = 0.18, CHCl3)]; IR (KBr): ν 3361, 2955, 2917, 2848, 1732, 1616, 1469 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.43–5.47 (m, trans–2H), 4.37–4.40 (m, 1H), 3.54–3.78 (m, 5H), 3.47 (s, 3H), 3.01–2.83 (m, 3H), 2.30–2.43 (m, 4H), 1.95–1.98 (m, 2H), 1.24–1.32 (m, 22H), 0.88 (t, J = 7.0 Hz, CH3); 13C NMR (CDCl3,100 MHz) δ 174.3, 131.6, 128.4, 70.9, 62.0, 58.9, 57.4, 34.2, 33.5, 32.5, 31.9, 29.6, 29.6, 29.5, 29.3, 29.2, 28.0, 22.6, 14.1; HRMS (M + H)+ (ESI+) 384.3478 [M + H]+ (calcd for C23H45NO3H+ 384.3477).
3.4.2. Synthesis of Serinolamide B (2)
Using Method A, 3 (120 mg, 0.42 mmol), EDC (169 mg, 1.09 mmol), HOBt (212 mg, 1.15 mmol) and DIPEA (0.6 mL, 3.29 mmol), 27 (96 mg, 0.68 mmol) gave 45 mg (49%) of serinolamide B as clear oil; Rf = 0.13 (n-hexane/EtOAc 1/1); mp: 84–86 °C; [α]D25 = −17.22 (c = 0.18, CHCl3); IR (KBr): ν 3291, 2917, 2849, 1639, 1543, 1466 cm−1; 1H NMR (CDCl3, 400 MHz) δ 6.19–6.21 (m, NH), 5.37–5.48 (m, trans–2H), 4.02–4.07 (m, 1H), 3.75–3.78 (m, 1H), 3.61–3.65 (m, 1H), 3.54–3.58 (m, 1H), 3.47–3.51 (m, 1H), 3.34 (s, OCH3), 3.16–3.19 (m, 1H), 2.24–2.31 (m, 4H), 1.92–1.97 (m, 2H), 1.23–1.30 (m, 22H), 0.86 (t, J = 7.0 Hz, CH3); 13C NMR (CDCl3, 100 MHz) δ 173.1, 132.1, 127.9, 72.9, 63.8, 59.2, 50.5, 36.6, 32.5, 31.9, 29.6, 29.6, 29.5, 29.4, 29.3, 29.2, 28.6, 22.6, 14.1; HRMS (M + H)+ (ESI+) 370.3321 [M + H]+ (calcd for C22H43NO3H+ 370.3321).
3.4.3. Synthesis of (E)-N-(1,3-dihydroxypropan-2-yl)octadec-4-enamide (4)
Using Method A, 3 (180 mg, 0.63 mmol), EDC (254 mg, 1.64 mmol), HOBt (122 mg, 0.90 mmol) and DIPEA (0.86 mL, 4.93 mmol), commercially available serinol (158 mg, 1.01 mmol) gave 80 mg (33%) of 4 as a white solid; Rf = 0.1 (n-hexane/EtOAc 1/1); mp: 119–121 °C; IR (KBr): ν 3285, 2955, 2917, 2849, 1637, 1545, 1465 cm−1; 1H NMR (CDCl3, 400 MHz) δ 6.19 (br, 1H), 5.36–5.53 (m, trans–2H), 3.88–3.97 (m, 1H), 3.83–3.86 (m, 2H), 3.76–3.81 (m, 2H), 2.40–2.42 (m, 2H), 2.27–2.34 (m, 4H), 1.94–1.99 (m, 2H), 1.23–1.33 (m, 22H), 0.87 (t, J = 7.0 Hz, CH3); 13C NMR (DMSO-d6, 100 MHz) δ 171.9, 130.7, 129.4, 60.6, 53.2, 35.8, 32.4, 31.7, 29.5, 29.4, 29.4, 29.3, 29.1, 29.0, 28.7, 22.5, 14.4; HRMS (M + H)+ (ESI+) 356.3165 [M + H]+ (calcd for C21H41NO3H+ 356.3164).
3.4.4. Synthesis of (E)-2-(octadec-4-en-1-ylamino)propane-1,3-diol (6)
Using Method C, 5 (100 mg, 0.37 mmol), commercially available serinol (38 mg, 0.41 mmol), triethylamine (0.15 mL, 1.11 mmol) and sodium cyanoborohydride (46.5 mg, 0.74 mmol) gave 18 mg (14%) of 6 as yellow oil; Rf = 0.1 (n-hexane/EtOAc 1/1); IR (KBr): ν 3291, 2918, 2850, 1636, 1389, 1358 cm−1; 1H NMR (CD3OD, 400 MHz) δ 5.41–5.59 (m, trans–2H), 3.74–3.86 (m, 4H), 3.25–3.29 (m, 1H), 3.09–3.13 (m, 2H), 2.12–2.17 (m, 2H), 1.98–2.06 (m, 2H), 1.78–1.84 (m, 2H), 1.24–1.32 (m, 22H), 0.88 (t, J = 7.0 Hz, CH3); 13C NMR (CD3OD, 100 MHz) δ 132.1, 127.7, 60.3, 57.5, 44.6, 32.1, 31.6, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 28.8, 25.6; HRMS (M + H)+ (ESI+) 342.3372 [M + H]+ (calcd for C21H43NO2H+ 342.3372).
3.4.5. Synthesis of diethyl (E)-2-acetamido-2-(4-(octadec-4-enamido)benzyl)malonate (9)
Using Method A, 3 (105 mg, 0.37 mmol), EDC (77 mg, 0.49 mmol), HOBt (38 mg, 0.27 mmol) and DIPEA (0.26 mL, 1.5 mmol), 8 (100 mg, 0.3 mmol) gave 150 mg (82%) of 9 as clear oil; Rf = 0.53 (n-hexane/EtOAc 1/1); IR (KBr): ν 3235, 2848, 1742, 1707, 1512 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.40 (d, J = 8.2 Hz, 2 ArH), 7.22 (s, CONH), 6.94 (d, J = 8.3 Hz, 2 ArH), 6.52 (s, NH), 5.42–5.57 (m, trans–2H), 4.22–4.29 (m, 1H), 3.60 (s, CH2), 2.40 (br, 4H), 2.02 (s, COCH3), 1.96–1.99 (m, 2H), 1.25–1.30 (m, 22H), 0.87 (t, J = 7.0 Hz, CH3); 13C NMR (CDCl3, 100 MHz) δ 169.0, 167.4, 137.0, 132.5, 130.9, 130.4, 128.0, 119.5, 67.2, 62.6, 37.6, 37.2, 32.5, 31.9, 29.6, 29.6, 29.5, 29.4, 29.3, 29.1, 28.4, 23.0, 22.6, 14.1, 14.0; HPLC purity: 15.2 min, 100%; HRMS (M + H)+ (ESI+) 587.4060 [M + H]+ (calcd for C34H54N2O6H+ 587.4060).
3.4.6. Synthesis of 10, 11, 12
A mixture of 9 (110 mg, 0.19 mmol) in tetrahydrofuran (5 mL) was added lithium aluminum hydride (36 mg, 0.94 mmol) at 0 oC for 5 min. The resulting suspension was stirred at room temperature for 12 h. The mixture was filtered through a pad of celite and the solvent was evaporated. The residue was purified by column chromatography to give 10 (30 mg, 29%, white solid, Rf = 0.5); mp: 69–71 °C, 11 (15 mg, 16%, ivory solid, Rf = 0.33); mp: 84–86 °C, 12 (8 mg, 8%, ivory solid, Rf = 0.16) (n-hexane/EtOAc 1/5); mp: 130–132 °C; 10 IR (KBr): ν 3158, 2845, 2765, 1731, 1698, 1478 cm−1; 1H NMR (CDCl3, 400 MHz) δ 7.42 (d, J = 8.4 Hz, 2 ArH), 7.13 (s, CONH), 7.05 (d, J = 8.4 Hz, 2 ArH), 5.86–5.88 (m, NH), 5.43–5.58 (m, trans–2H), 4.81–4.86 (m, 1H), 4.14–4.20 (m, CH2), 3.05–3.14 (m, 2H), 2.41 (m, 4H), 1.98–1.99 (m, 5H), 1.24–1.31 (m, 28H), 0.87 (t, J = 7.0 Hz, CH3); 13C NMR (CDCl3, 100 MHz) δ 171.5, 170.7, 169.5, 136.9, 132.5, 131.6, 129.8, 128.0, 119.8, 61.5, 53.1, 37.6, 37.3, 32.5, 31.9, 29.6, 29.6, 29.6, 29.5, 29.4, 29.3, 29.1, 28.4, 23.2, 22.6, 14.1; HPLC purity: 15.2 min, 94.5%; HRMS (M + H)+ (ESI+) 545.3954 [M + H]+ (calcd for C32H52N2O5H+ 545.3954). 11 IR (KBr): ν 3234, 2954, 2914, 2848, 1742, 1707, 1511, 1470 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.62 (s, CHO), 7.43 (d, J = 8.2 Hz, 2 ArH), 7.17 (s, CONH), 7.09 (d, J = 8.3 Hz, 2 ArH), 5.94–5.96 (m, NH), 5.43–5.56 (m, trans–2H), 4.67–4.72 (m, 1H), 3.11–3.15 (m, 2H), 2.40–2.41 (m, 4H), 1.96–2.01 (m, 5H), 1.25–1.31 (m, 25H), 0.87 (t, J = 7.0 Hz, CH3); 13C NMR (CDCl3, 100 MHz) δ 198.7, 170.8, 170.0, 136.9, 132.6, 131.2, 129.8, 127.9, 120.1, 59.8, 37.6, 34.4, 32.5, 31.9, 29.6, 29.6, 29.5, 29.4, 29.3, 29.1, 28.4, 23.0, 22.6, 14.1; HPLC purity: 7.7 min, 99.0%; HRMS (M + H)+ (ESI+) 501.3692 [M + H]+ (calcd for C30H48N2O4H+ 501.3692). 12 IR (KBr): ν 3342, 2953, 2914, 2849, 1686, 1515, 1470 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.45–7.47 (m, 2 ArH), 7.17–7.19 (m, 2 ArH), 5.44–5.55 (m, trans–2H), 3.58–3.69 (m, 4H), 3.02 (s, CH2), 2.37–2.41 (m, 4H), 1.99–2.00 (m, 2H), 1.96 (s, COCH3), 1.26–1.36 (m, 22H), 0.91 (t, J = 7.0 Hz, CH3); 13C NMR (CD3OD, 100 MHz) δ 172.7, 172.5, 136.8, 132.5, 131.5, 130.5, 128.0, 119.8, 119.6, 61.8, 61.7, 36.6, 36.0, 34.2, 32.1, 31.6, 29.3, 29.3, 29.2, 29.0, 28.7, 28.4, 22.3, 13.0; HPLC purity: 14.0 min, 98.6%; HRMS (M + H)+ (ESI+) 503.3849 [M + H]+ (calcd for C30H50N2O4H+ 503.3848).
3.4.7. Synthesis of diethyl (E)-2-acetamido-2-(4-(octadec-4-en-1-ylamino)benzyl)malonate (13)
Using Method C, 5 (165 mg, 0.62 mmol), 8 (200 mg, 0.62 mmol), triethylamine (0.26 mL, 1.86 mmol) and sodium cyanoborohydride (78 mg, 1.24 mmol) gave 150 mg (70%) of 13 as a white solid; Rf = 0.3 (n-hexane/EtOAc 1/2); mp: 67–69 °C; IR (KBr): ν 3205, 2946, 2814, 1673, 1480 cm−1; 1H NMR (CDCl3, 400 MHz) δ 6.79 (d, J = 8.3 Hz, 2 ArH), 6.52 (s, CONH), 6.47 (d, J = 8.4 Hz, 2 ArH), 5.41–5.43 (m, trans–2H), 4.22–4.28 (m, 4H), 3.56–3.63 (m, 1H), 3.51 (s, 2H), 3.05–3.08 (m, 2H), 1.96–2.09 (m, 7H), 1.63–1.67 (m, 2H), 1.25–1.30 (m, 28H), 0.87 (t, J = 7.0 Hz, CH3); 13C NMR (CDCl3, 100 MHz) δ 168.9, 167.7, 147.5, 131.4, 130.6, 129.1, 123.2, 122.5, 67.4, 62.4, 43.4, 37.0, 32.5, 31.9, 30.1, 29.6, 29.6, 29.5, 29.5, 29.3, 29.2, 23.0, 22.6, 14.1, 14.0; HPLC purity: 17.5 min, 95.5%; HRMS (M + H)+ (ESI+) 573.4268 [M + H]+ (calcd for C34H56N2O5H+ 573.4267).
3.4.8. Synthesis of (E)-2-amino-2-(4-(octadec-4-en-1-ylamino)benzyl)propane-1,3-diol (19)
Using Method B, 18 (70 mg, 0.12 mmol) and 4 M HCl in dioxane (0.16 mL, 0.64 mmol) gave 37 mg (65%) of 19 as a brown solid; mp: 135–137 °C (decomp.); [α]D25 = +1.11 (c = 0.18, EtOH); IR (KBr): ν 3301, 2919, 2850, 1664, 1513, 1412, 1163 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.57 (br, 4 ArH), 5.39–5.55 (m, trans–2H), 3.51–3.58 (m, 4H), 3.32–3.39 (m, 2H), 3.13 (s, CH2), 2.13–2.18 (m, 2H), 1.98–2.03 (m, 2H), 1.86 (br, 2H), 1.26–1.37 (m, 22H), 0.91 (t, J = 6.9 Hz, CH3); 13C NMR (CD3OD, 100 MHz) δ 136.4, 134.5, 132.3, 132.1, 127.6, 122.8, 61.0, 60.2, 51.7, 35.3, 32.2, 31.6, 29.4, 29.3, 29.2, 29.1, 28.9, 28.9, 25.5, 22.3, 13.1; HPLC purity: 7.8 min, 96.1%; HRMS (M + H)+ (ESI+) 447.3951 [M + H]+ (calcd for C28H50N2O2H+ 447.3950).
3.4.9. Synthesis of (E)-N-(4-(2-amino-3-hydroxy-2-(hydroxymethyl)propyl)phenyl)octadec-4-enamide (21)
Using Method B, 20 (12 mg, 0.02 mmol) and 4 M HCl in dioxane (0.02 mL, 0.08 mmol) gave 10 mg (96%) of 21 as an ivory solid; mp: 156–158 °C (decomp.); [α]D25 = +7.22 (c = 0.18, EtOH); IR (KBr): ν 3288, 2916, 2849, 1655, 1598, 1511, 1414, 1119, 1050 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.54 (d, J = 8.3 Hz, 2 ArH), 7.27 (d, J = 8.3 Hz, 2 ArH), 5.43–5.56 (m, trans–2H), 3.55 (s, 4H), 2.99 (s, CH2), 2.38–2.43 (m, 4H), 1.98–2.03 (m, 2H), 1.27–1.34 (m, 22H), 0.92 (t, J = 7.0 Hz, CH3); 13C NMR (CD3OD, 100 MHz) δ 131.5, 130.5, 129.6, 128.0, 120.1, 61.0, 60.4, 36.6, 35.4, 32.1, 31.6, 29.3, 29.3, 29.1, 29.0, 28.7, 28.4, 22.3, 13.0; HPLC purity: 8.8 min, 99.1%; HRMS (M + H)+ (ESI+) 461.3743 [M + H]+ (calcd for C28H48N2O3H+ 461.3743).
3.5. Cell Culture
For S1P1 receptor internalization assay, PathHunter® EDG1 HEK 293 cells (93-0784C1; DiscoverX, Fremont, CA, USA.) were cultured in DMEM containing 10% (v/v) fetal bovine serum (Biowest), 100 U/mL penicillin-streptomycin (Gibco), 0.25 μg/mL puromycin (Invivogen), and 200 μg/mL hygromycin B (Invitrogen). Cells were incubated at 5% CO2 in a 37 °C humidified atmosphere.
3.6. S1P1 Receptor Internalization Assay
The S1P1 receptor internalization activity of synthesized compounds was evaluated using PathHunter® EDG1 total GPCR internalization HEK293 cell line (93-0784C1; DiscoverX). The cell lines are engineered to co-express two fragments of β-galactosidase at S1P1 receptor and endosome, respectively. The endocytosis of receptor leads β-galactosidase to complemented form, and the internalization activity was measured by chemiluminescent signal. The HEK293 EDG1 cells (1 × 104 cells/well) in cell plating 28 reagent (DiscoverX) were seeded in 96-well white plates and incubated overnight at 37 °C. The test compounds were prepared in cell plating 28 Reagent (DiscoverX) and treated for 3 h at 37 °C. Then, 50 μL of detection reagent (PathHunter® Detection Kit, 93-0001L; DiscoverX) was added to the wells and incubated for 1 h at room temperature in the dark. The chemiluminescent signals were measured at all wavelengths using a microplate reader (SpectraMax®i3; Molecular Devices).
3.7. Measurement of Peripheral Lymphocyte Count
Compound 19 and fingolimod were dissolved in 5% DMSO and distilled water, and 21 was dissolved in 50% DMSO and distilled water (final volume was 20 μL). All test compounds were intravenously administered to B6C3H mice (10 wks, 20 g). Blood samples were obtained from retro-orbital sinus of the mice under anesthesia (4% isoflurane) at different time points and were collected into a K2-EDTA-coated tube. Blood lymphocyte counts were measured using an automatic blood cell counter (Horiba).
4. Conclusions
In this study, we optimized previous synthetic methods for serinolamides A and B. Structural similarities with FTY720 indicated that serinolamides may act as S1P1 receptor agonists. We synthesized a series of derivatives and evaluated their efficacy in S1P1 receptor internalization. Compounds 19 and 21 were rationally designed by hybridization of serinolamide A with the FTY720 scaffold and exhibited favorable efficacies in S1P1 receptor internalization. Finally, we confirmed that compound 19 in vivo activity by effectively reducing the number of blood lymphocytes in mice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27092818/s1, Scheme S1: Synthesis of 26 and 27.
Author Contributions
S.J.P. and J.K. (Jushin Kim) carried out the experimental work and wrote the paper; J.K. (Jushin Kim), H.J.K. and R.K. participated in the discussion of biological activities; J.W.C., S.J.P. and Y.K. constructed the target compound structure; S.J.P., E.H.L. and B.K. synthesized the compounds; J.K. (Jaehwan Kim), S.K. and J.K. (Jushin Kim) conducted PLC experiment and discussed the biological activities; J.-H.P. and K.D.P. directed and supervised the whole experimentation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea [NRF-2018M3A9C8016849] and the Korea Institute of Science and Technology (KIST Institutional Program (2E31522).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or the Supplementary Materials file.
Acknowledgments
The author would like to thank the National Research Foundation of Korea [NRF-2018M3A9C8016849] and the Korea Institute of Science and Technology (KIST Institutional Program (2E31522).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors upon the reasonable request.
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- O’Gorman, C.; Lucas, R.; Taylor, B. Environmental Risk Factors for Multiple Sclerosis: A Review with a Focus on Molecular Mechanisms. Int. J. Mol. Sci. 2012, 13, 11718–11752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [PubMed]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H. T cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.; Riddy, D.M.; Stamp, C.; Bradley, M.E.; McGuiness, N.; Sattikar, A.; Guerini, D.; Rodrigues, I.; Glaenzel, A.; Dowling, M.R.; et al. Investigating the Molecular Mechanisms through which FTY720-P Causes Persistent S1P(1) Receptor Internalization. Br. J. Pharmacol. 2014, 171, 4797–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of Lymphocyte Egress into Blood and Lymph by Distinct Sources of Sphingosine-1-Phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, G.T.; Maceyka, M.; Milstien, S.; Spiegel, S. Targeting the Sphingosine-1-Phosphate Axis in Cancer, Inflammation and Beyond. Nat. Rev. Drug Discov. 2013, 12, 688–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, M.; Pereira, A.R.; Debonsi, H.M.; Ligresti, A.; Di Marzo, A.; Gerwick, W.H. A Cannabinomimetic Lipid from a Marine Cyanobacterium. J. Nat. Prod. 2011, 74, 2313–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahalawat, S.; Pandey, S.K. Enantioselective total synthesis of (+)-serinolamide A. RSC Adv. 2015, 5, 41013. [Google Scholar] [CrossRef]
- Gao, Y.R.; Guo, S.H.; Zhang, Z.X.; Mao, S.; Zhang, Y.L.; Wang, Y.Q. Concise synthesis of (+)-serinolamide A. Tetrahedron Lett. 2013, 54, 6511–6513. [Google Scholar] [CrossRef]
- Ghotekar, G.S.; Mujahid, M.; Muthukrishnan, M. Total Synthesis of Marine Natural Products Serinolamide A and Columbamide D. ACS Omega. 2019, 4, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Lescop, C.; Müller, C.; Mathys, B.; Birker, M.; de Kanter, R.; Kohl, C.; Hess, P.; Nayler, O.; Rey, M.; Sieber, P.; et al. Novel S1P1 receptor agonists–Part 4: Alkylaminomethyl substituted aryl head groups. Eur. J. Med. Chem. 2016, 116, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Bolli, M.H.; Lescop, C.; Birker, M.; de Kanter, R.; Hess, P.; Kohl, C.; Nayler, O.; Rey, M.; Sieber, P.; Velker, J.; et al. Novel S1P1 receptor agonists–Part 5: From amino-to alkoxy-pyridines. Eur. J. Med. Chem. 2016, 115, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Yeon, S.K.; Kim, Y.; Kim, H.J.; Kim, S.; Kim, J.; Choi, J.W.; Kim, B.; Lee, E.H.; Seo, S.H.; et al. Discovery of Novel Sphingosice-1-Phosphate-1 Receptor Agonists for the Treatment of Multiple Sclerosis. J. Med. Chem. 2022, 65, 3539–3562. [Google Scholar] [CrossRef] [PubMed]
- Marsolais, D.; Rosen, H. Chemical Modulators of Sphingosine-1-Phosphate Receptors as Barrier-Oriented Therapeutic Molecules. Nat. Rev. Drug Discov. 2009, 8, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, F.L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H.G.; Timony, G.A.; Martinborough, E.; Rosen, H.; et al. Ozanimod (RPC1063) is a Potent Sphingosine-1-Phosphate Receptor-1 (S1P(1)) and Receptor-5 (S1P(5)) Agonist with Autoimmune Disease-Modifying Activity. Br. J. Pharmacol. 2016, 173, 1778–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).