The Effect of Deoxyfluorination on Intermolecular Interactions in the Crystal Structures of 1,6-Anhydro-2,3-epimino-hexopyranoses
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Synthesis
2.2. DFT Calculation Details
2.3. X-ray Crystallography
3. Results and Discussion
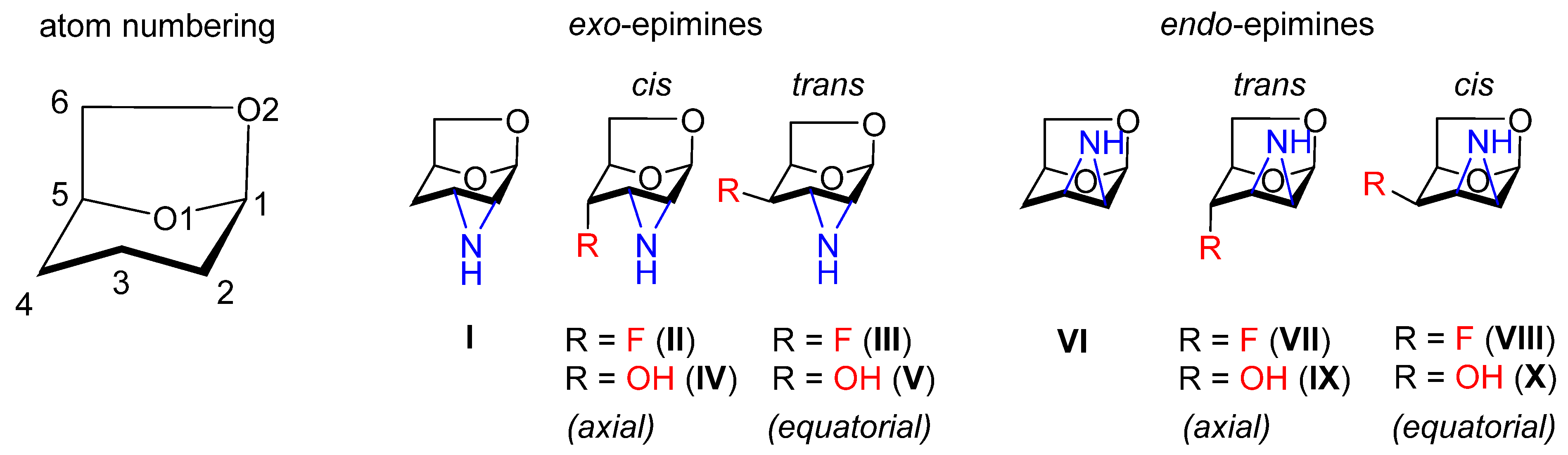
3.1. Nomenclature
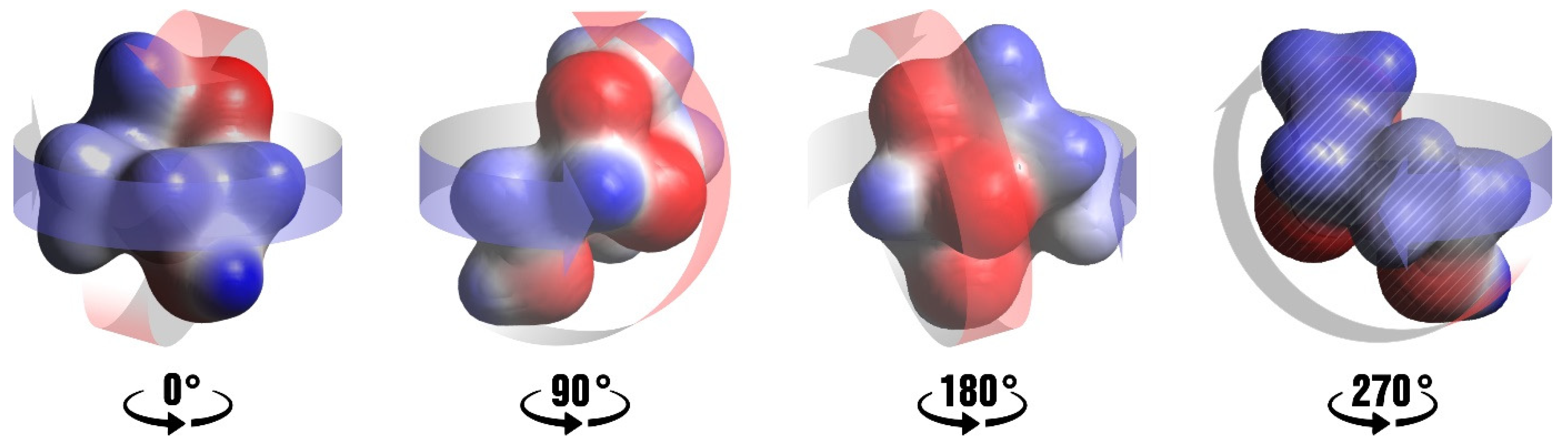
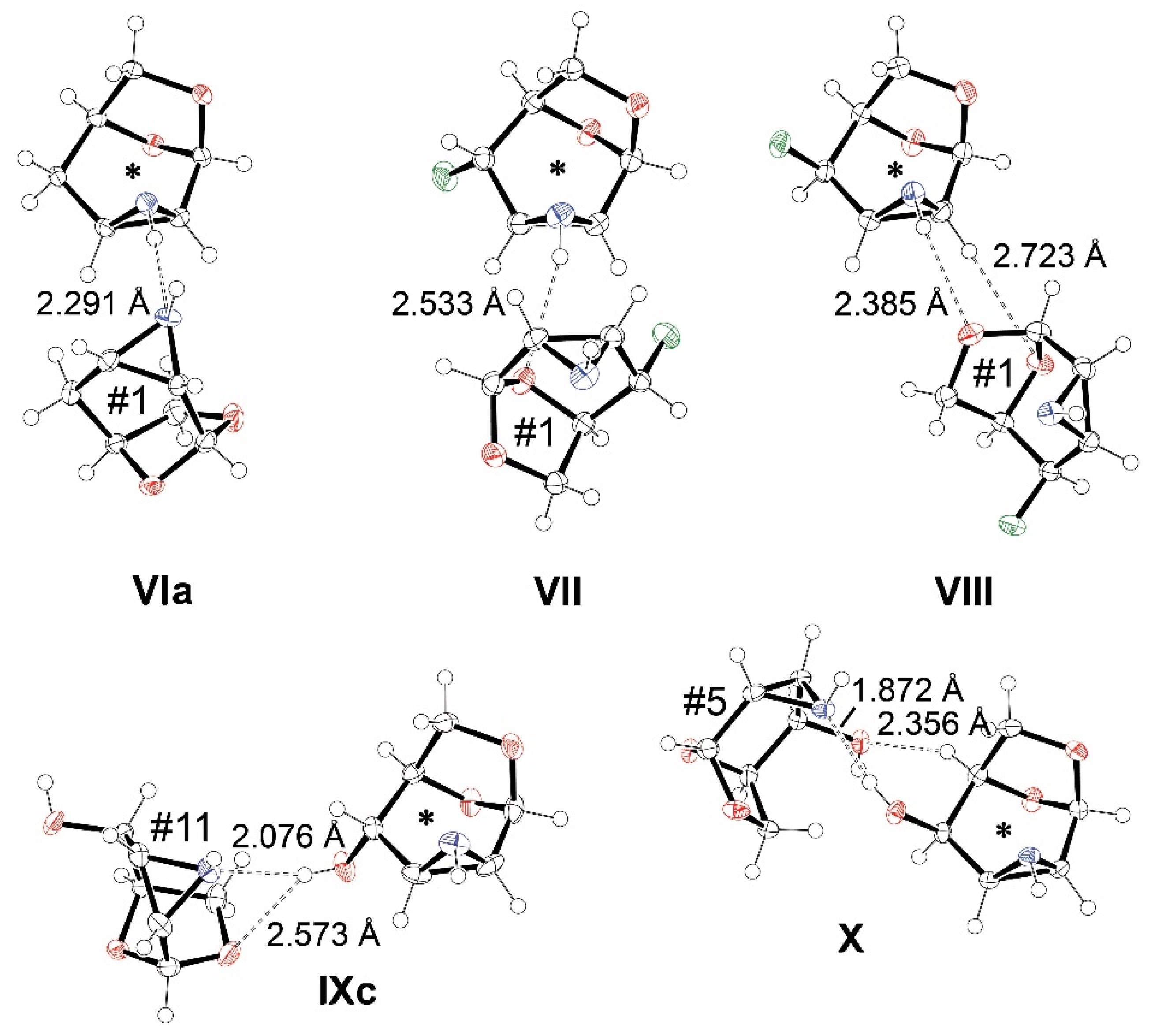
3.2. Options for the Formation of Intermolecular Interactions
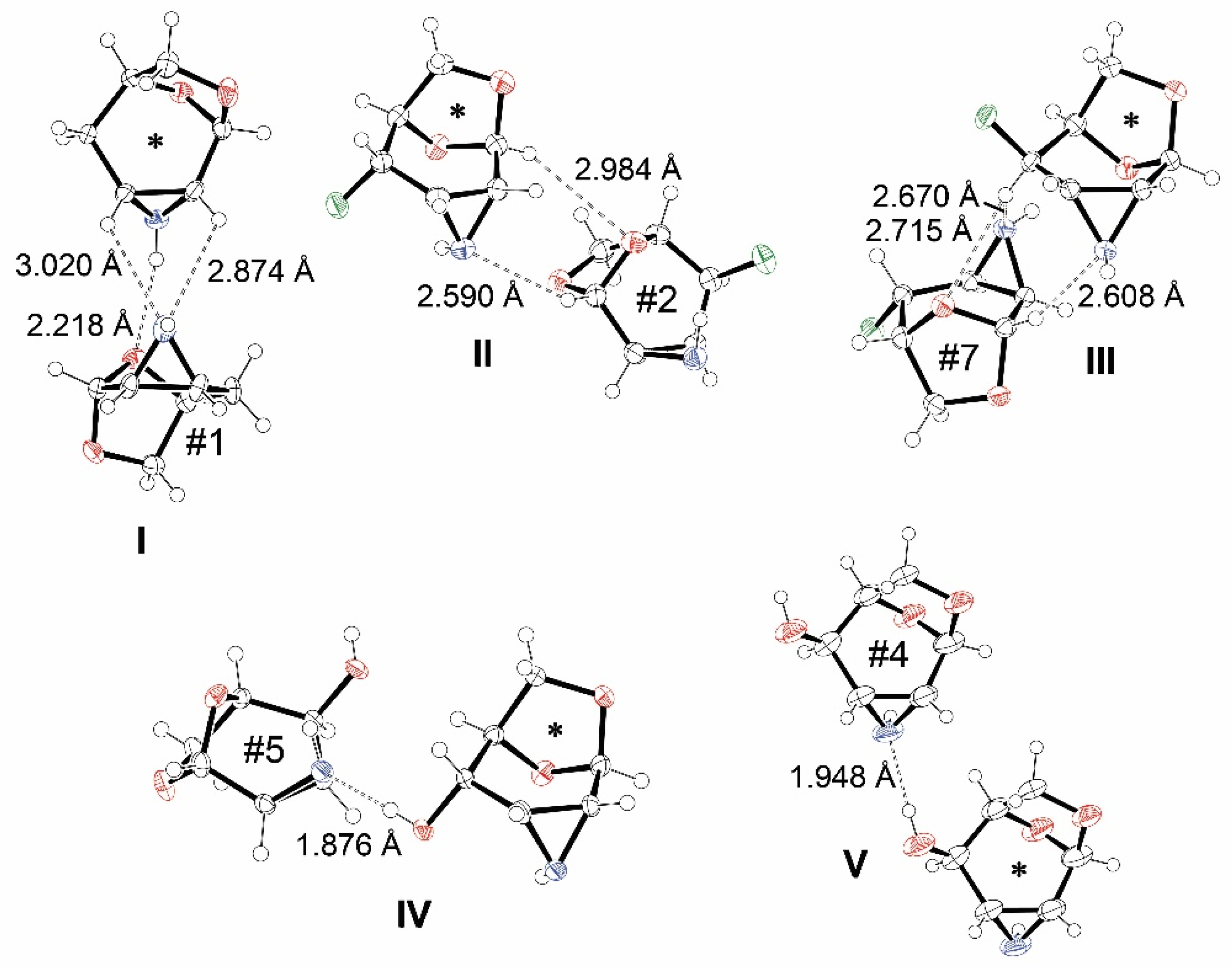
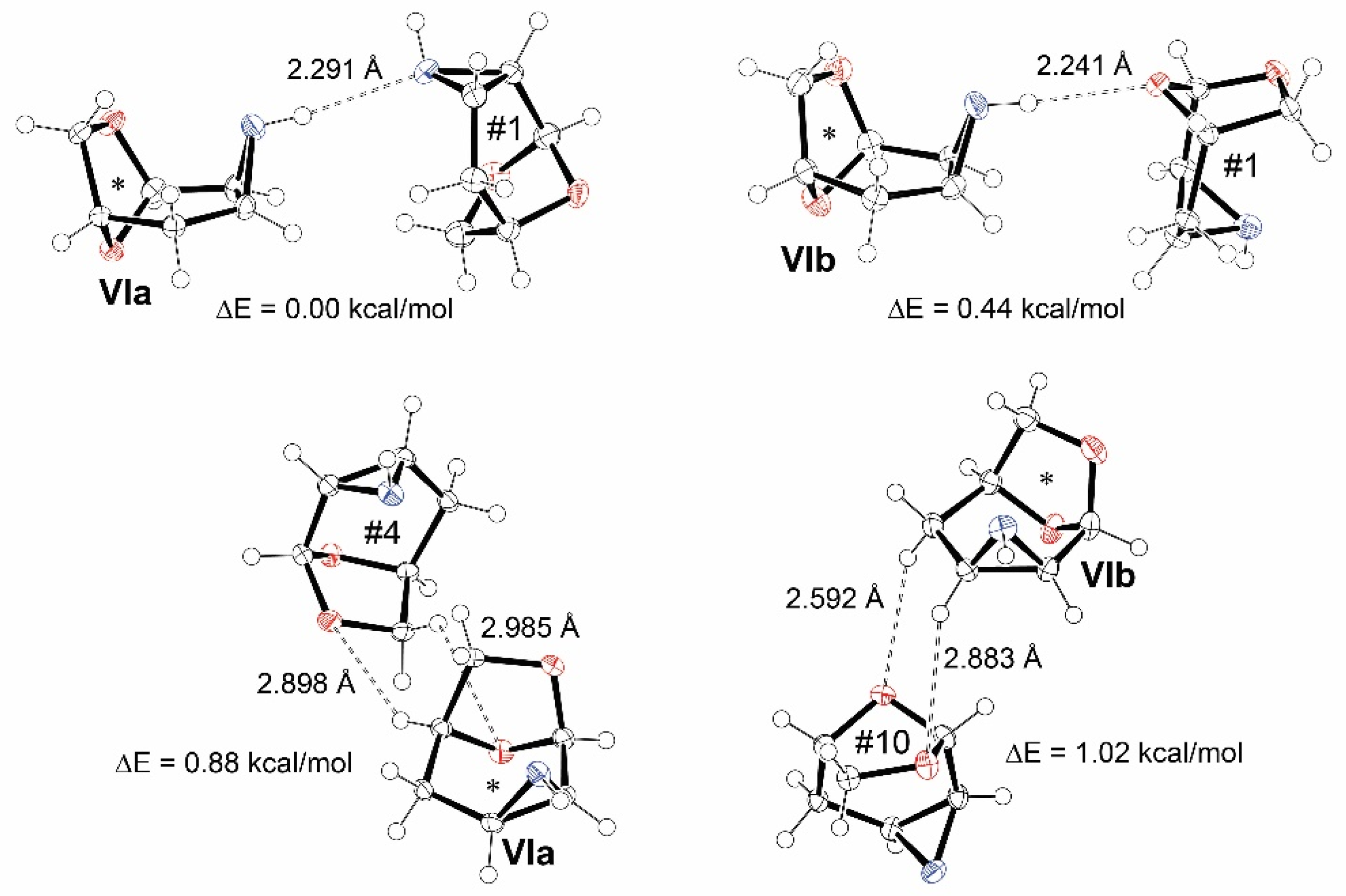
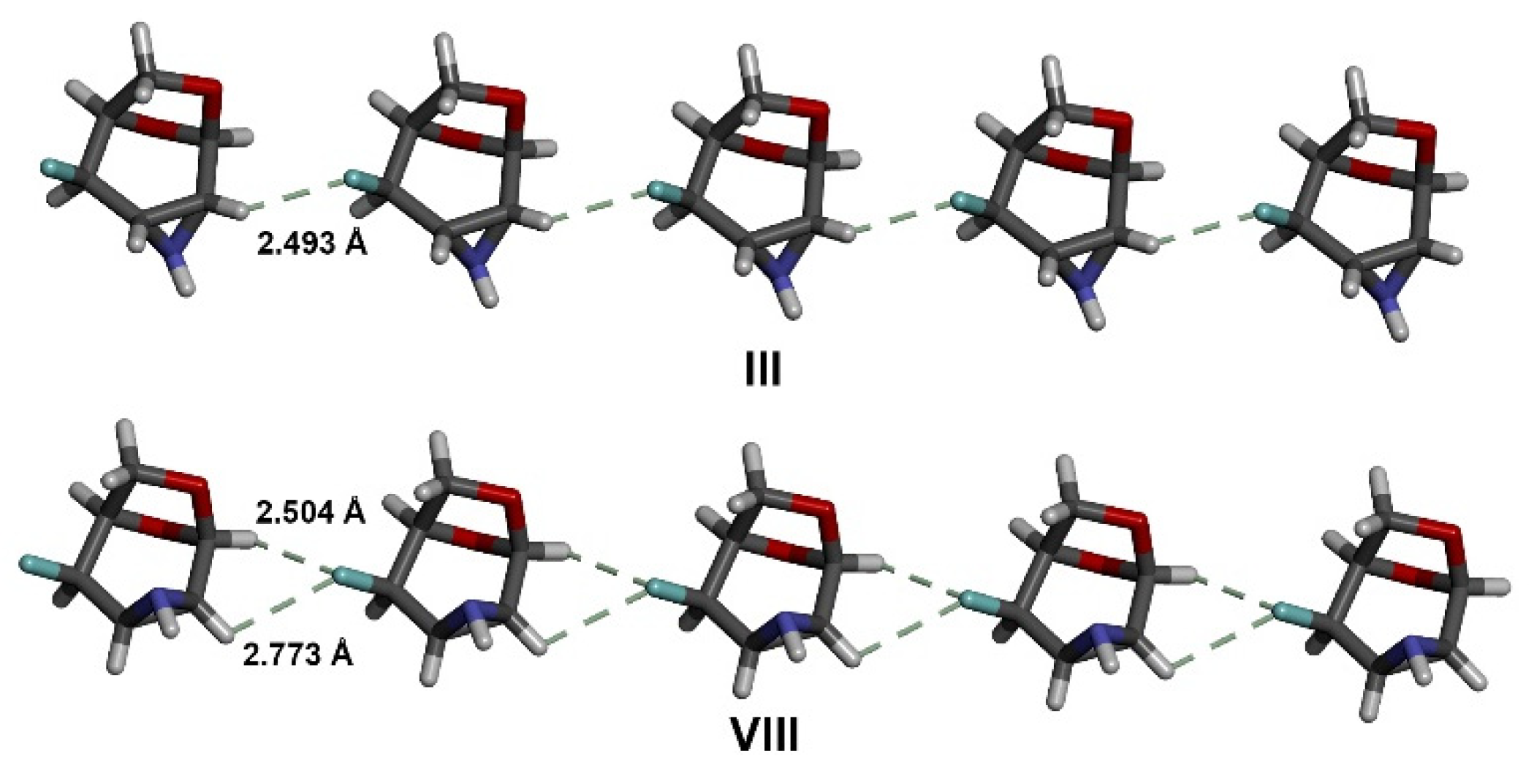
3.3. The Strongest Intermolecular Interactions Found in I–X
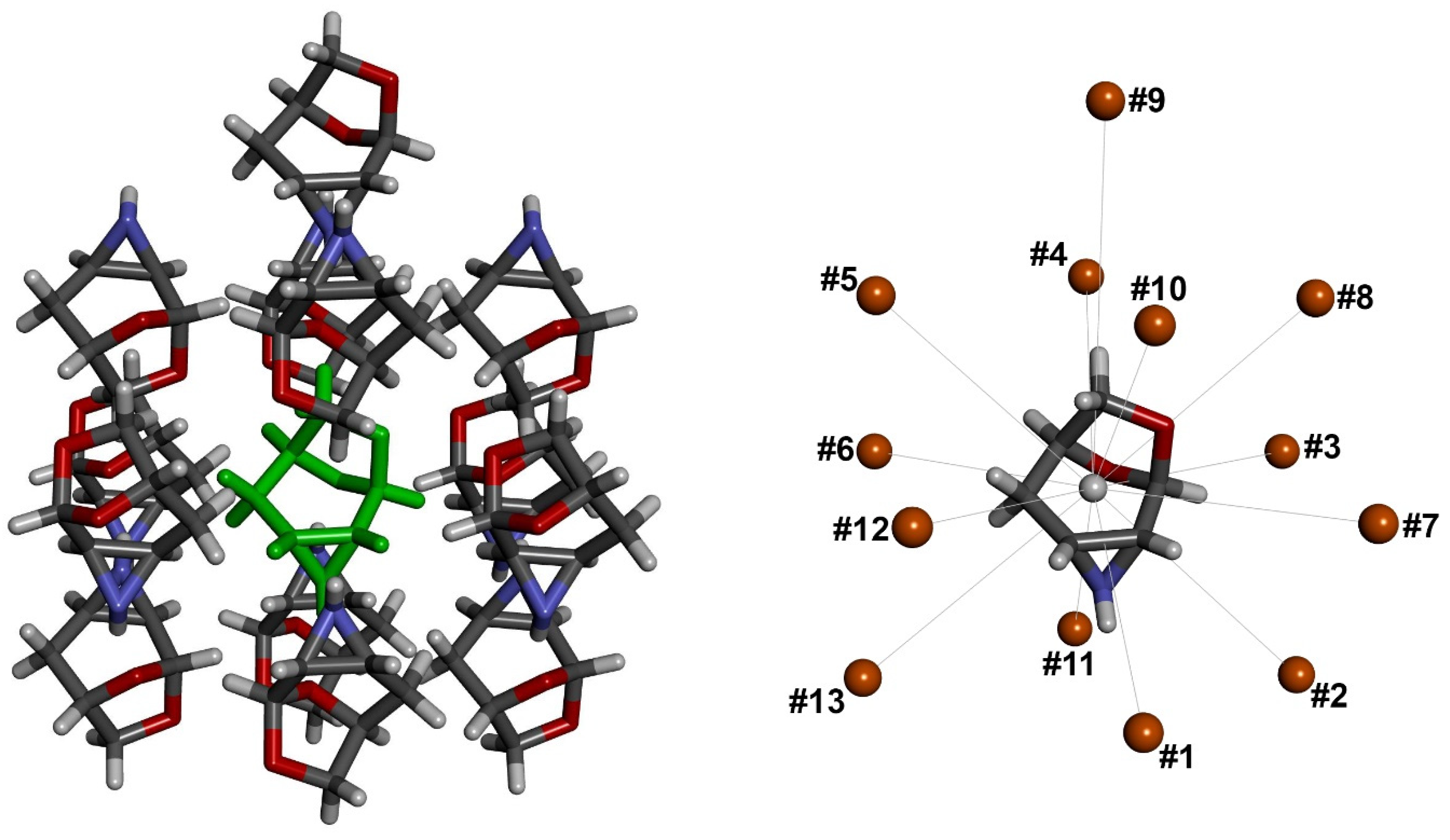
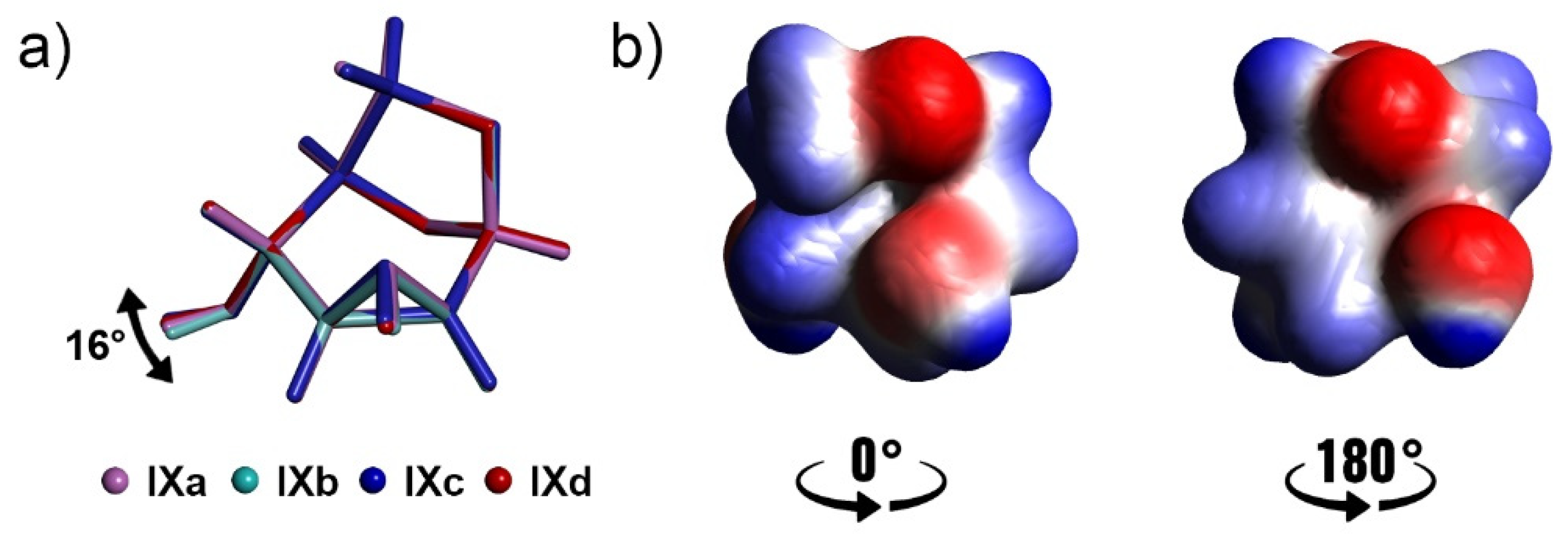
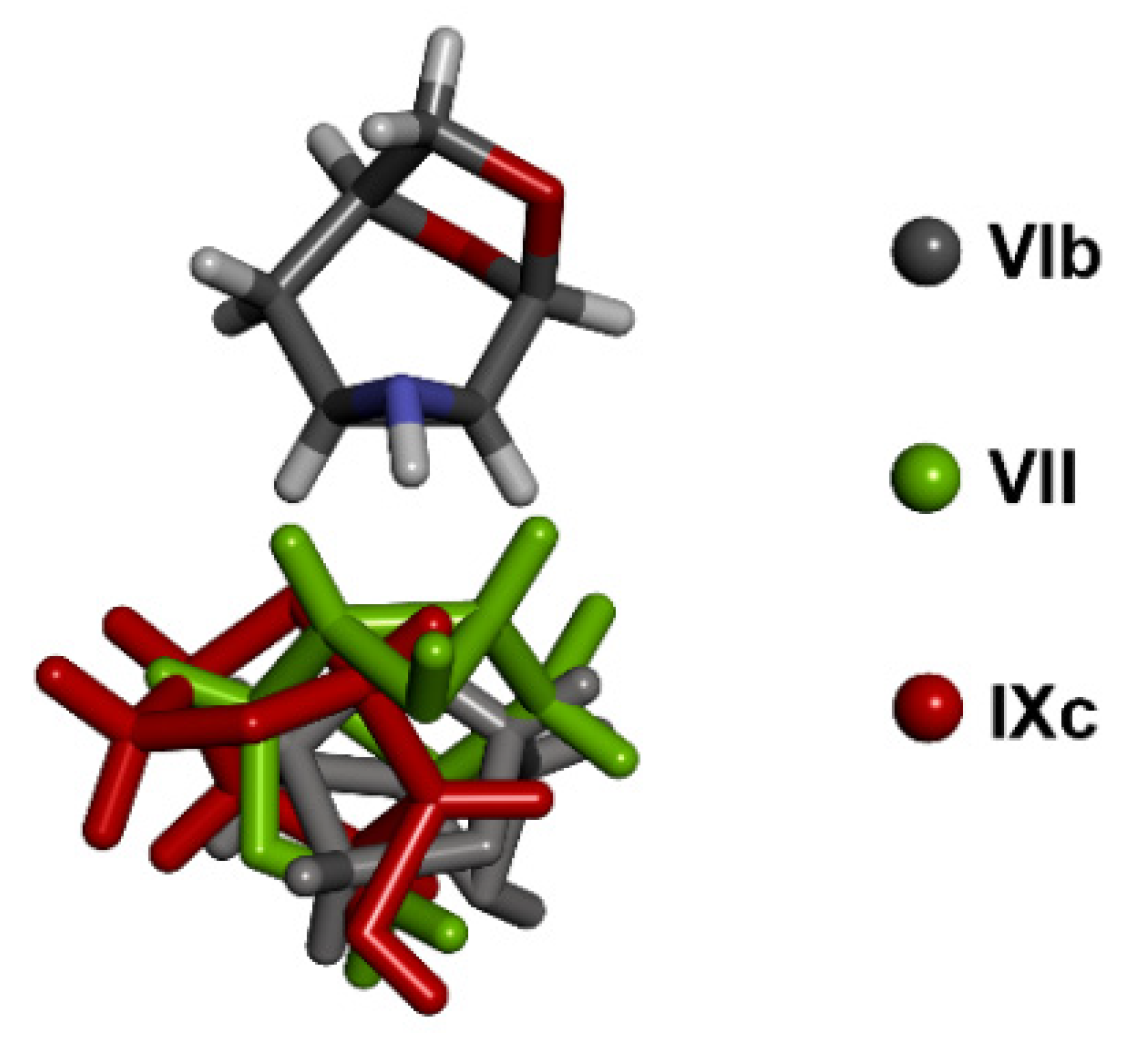
3.4. Comparison of Molecular Arrangements in Structures VI and IX Containing More Independent Molecules
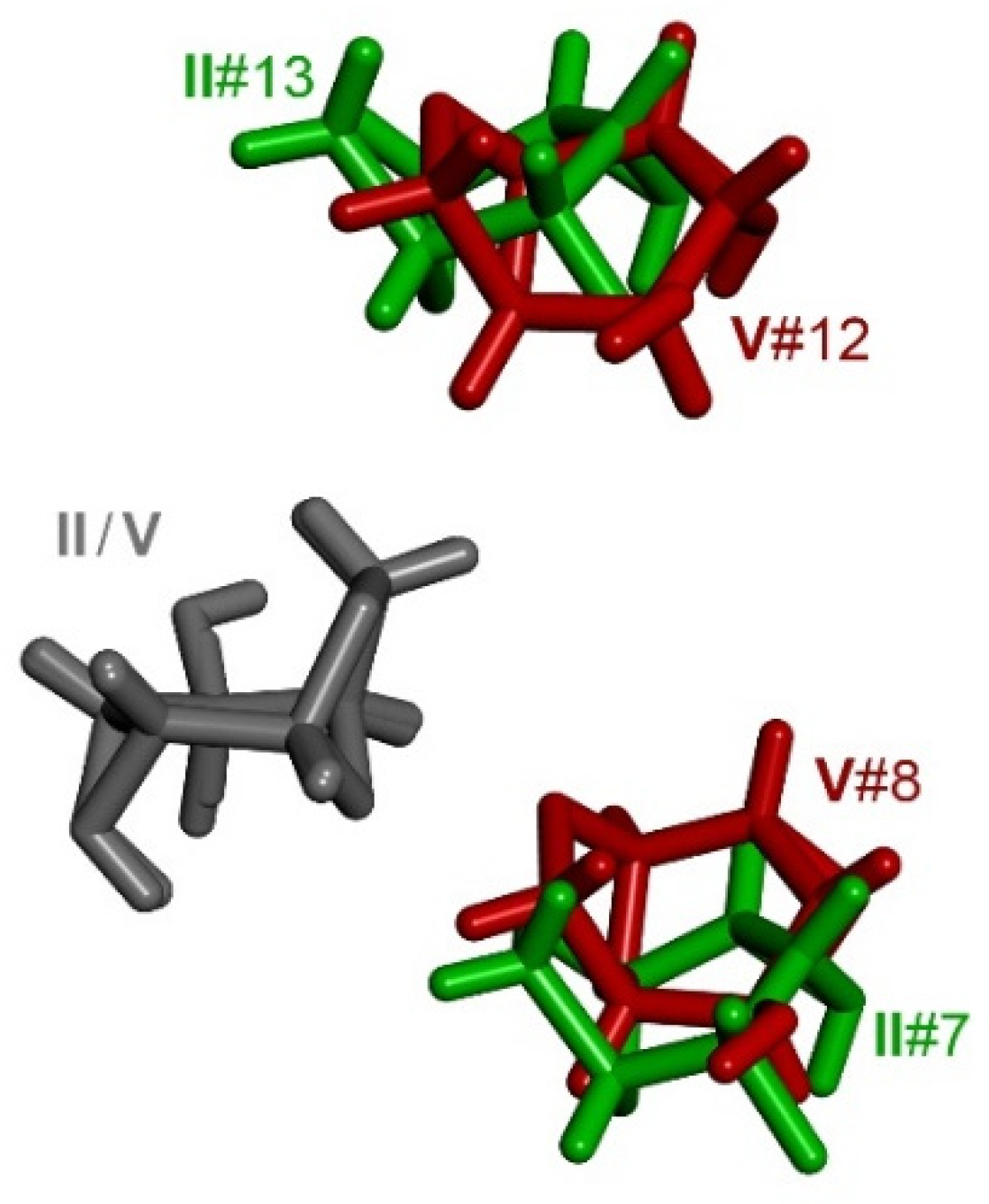
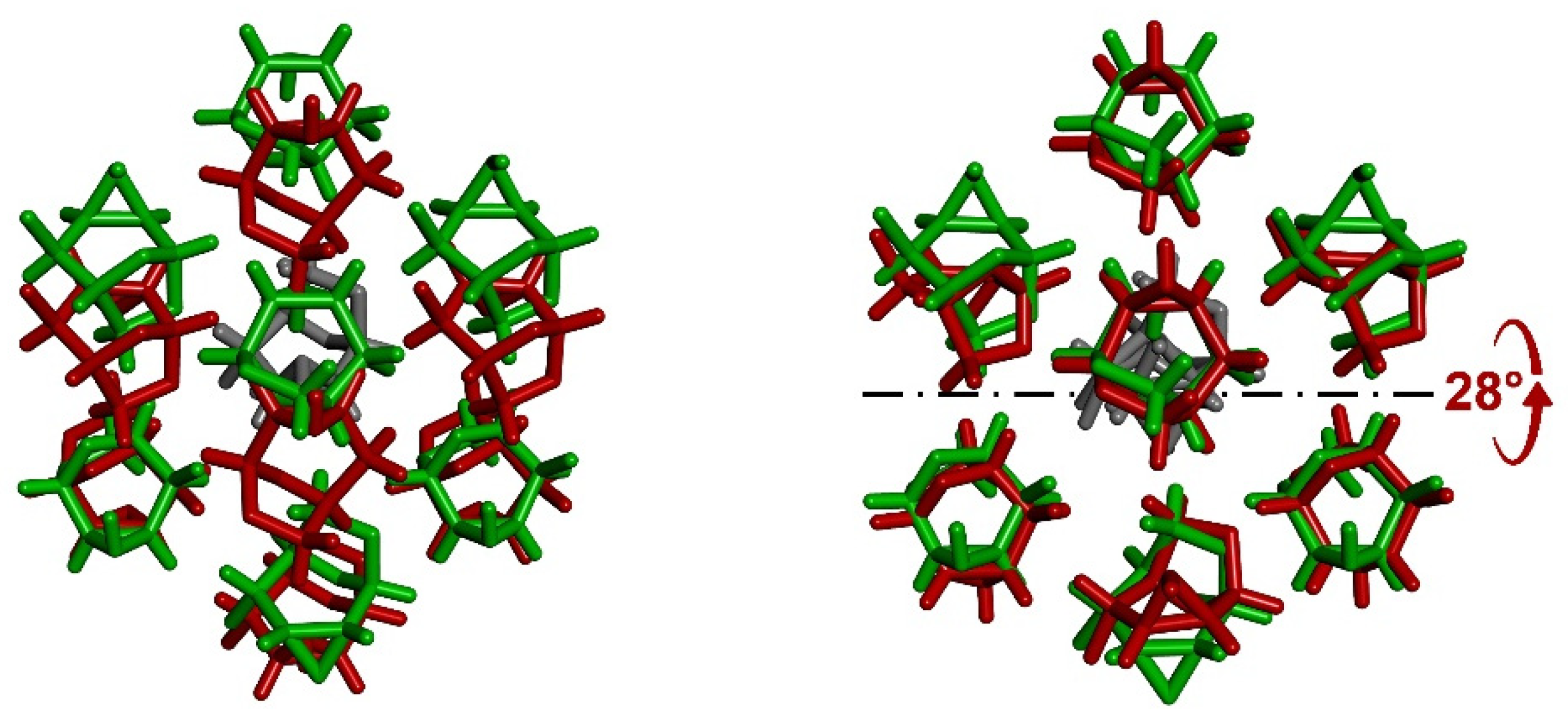
3.5. Comparison of Molecular Arrangements in Exo-Epimines I–V and in Endo-Epimines VI–X
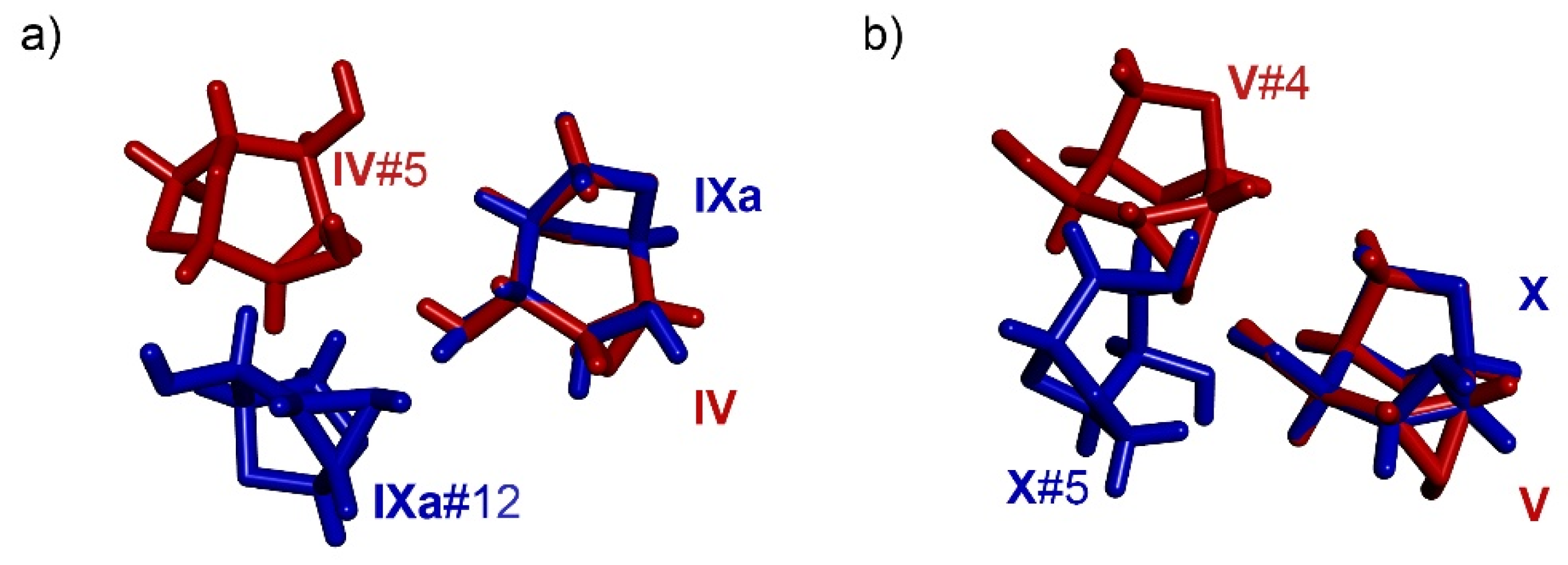
3.6. Comparison of Molecular Arrangements in the Ax- and Eq-Substituted Epimines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamburrini, A.; Colombo, C.; Bernardi, A. Design and synthesis of glycomimetics: Recent advances. Med. Res. Rev. 2020, 40, 495–531. [Google Scholar] [CrossRef]
- Uhrig, M.L.; Lantaño, B.; Postigo, A. Synthetic strategies for fluorination of carbohydrates. Org. Biomol. Chem. 2019, 17, 5173–5189. [Google Scholar] [CrossRef] [PubMed]
- Council, C.E.; Kilpin, K.J.; Gusthart, J.S.; Allman, S.A.; Linclau, B.; Lee, S.S. Enzymatic glycosylation involving fluorinated carbohydrates. Org. Biomol. Chem. 2020, 18, 3423–3451. [Google Scholar] [CrossRef] [PubMed]
- Bilska-Markowska, M.; Szwajca, A.; Marciniak, B. Design, properties and applications of fluorinated and fluoroalkylated N-containing monosaccharides and their analogues. J. Fluorine Chem. 2019, 227, 109364. [Google Scholar] [CrossRef]
- Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Linclau, B.; Arda, A.; Reichardt, N.C.; Sollogoub, M.; Unione, L.; Vincent, S.P.; Jimenez-Barbero, J. Fluorinated carbohydrates as chemical probes for molecular recognition studies. Current status and perspectives. Chem. Soc. Rev. 2020, 49, 3863–3888. [Google Scholar] [CrossRef] [PubMed]
- Diercks, T.; Ribeiro, J.P.; Cañada, F.J.; André, S.; Jiménez-Barbero, J.; Gabius, H.-J. Fluorinated Carbohydrates as Lectin Ligands: Versatile Sensors in 19F-Detected Saturation Transfer Difference NMR Spectroscopy. Chem. Eur. J. 2009, 15, 5666–5668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, J.P.; Diercks, T.; Jiménez-Barbero, J.; André, S.; Gabius, H.-J.; Cañada, F.J. Fluorinated Carbohydrates as Lectin Ligands: 19F-Based Direct STD Monitoring for Detection of Anomeric Selectivity. Biomolecules 2015, 5, 3177–3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurfiřt, M.; Dračínský, M.; Červenková Šťastná, L.; Cuřínová, P.; Hamala, V.; Hovorková, M.; Bojarová, P.; Karban, J. Selectively Deoxyfluorinated N-Acetyllactosamine Analogues as 19F NMR Probes to Study Carbohydrate-Galectin Interactions. Chem. Eur. J. 2021, 27, 13040–13051. [Google Scholar] [CrossRef]
- O’Hagan, D.; Young, R.J. Accurate Lipophilicity (log P) Measurements Inform on Subtle Stereoelectronic Effects in Fluorine Chemistry. Angew. Chem. Int. Ed. 2016, 55, 3858–3860. [Google Scholar] [CrossRef]
- Denavit, V.; Lainé, D.; Bouzriba, C.; Shanina, E.; Gillon, É.; Fortin, S.; Rademacher, C.; Imberty, A.; Giguère, D. Stereoselective Synthesis of Fluorinated Galactopyranosides as Potential Molecular Probes for Galactophilic Proteins: Assessment of Monofluorogalactoside–LecA Interactions. Chem. Eur. J. 2019, 25, 4478–4490. [Google Scholar] [CrossRef]
- St-Gelais, J.; Cote, E.; Laine, D.; Johnson, P.A.; Giguere, D. Addressing the Structural Complexity of Fluorinated Glucose Analogues: Insight into Lipophilicities and Solvation Effects. Chem. Eur. J. 2020, 26, 13499–13506. [Google Scholar] [CrossRef]
- Baumann, A.; Marchner, S.; Daum, M.; Hoffmann-Röder, A. Synthesis of Fluorinated Leishmania Cap Trisaccharides for Diagnostic Tool and Vaccine Development. Eur. J. Org. Chem. 2018, 2018, 3803–3815. [Google Scholar] [CrossRef]
- Johannes, M.; Reindl, M.; Gerlitzki, B.; Schmitt, E.; Hoffmann-Röder, A. Synthesis and biological evaluation of a novel MUC1 glycopeptide conjugate vaccine candidate comprising a 4’-deoxy-4’-fluoro-Thomsen–Friedenreich epitope. Beilstein J. Org. Chem. 2015, 11, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selnick, H.G.; Hess, J.F.; Tang, C.; Liu, K.; Schachter, J.B.; Ballard, J.E.; Marcus, J.; Klein, D.J.; Wang, X.; Pearson, M.; et al. Discovery of MK-8719, a Potent O-GlcNAcase Inhibitor as a Potential Treatment for Tauopathies. J. Med. Chem. 2019, 62, 10062–10097. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-Y.; Chen, C.-Y.; Tsai, T.-I.; Li, S.-T.; Lin, K.-H.; Cheng, Y.-Y.; Ren, C.-T.; Cheng, T.-J.R.; Wu, C.-Y.; Wong, C.-H. Immunogenicity Study of Globo H Analogues with Modification at the Reducing or Nonreducing End of the Tumor Antigen. J. Am. Chem. Soc. 2014, 136, 16844–16853. [Google Scholar] [CrossRef]
- Oberbillig, T.; Mersch, C.; Wagner, S.; Hoffmann-Röder, A. Antibody recognition of fluorinated MUC1 glycopeptide antigens. Chem. Commun. 2012, 48, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.A.; Liu, Y.; Leon, E.; Allman, S.A.; Friedrich, N.; Saouros, S.; Curry, S.; Soldati-Favre, D.; Davis, B.G.; Feizi, T.; et al. Detailed insights from microarray and crystallographic studies into carbohydrate recognition by microneme protein 1 (MIC1) of Toxoplasma gondii. Protein Sci. 2009, 18, 1935–1947. [Google Scholar] [CrossRef]
- Mehta, G.; Sen, S. Probing Fluorine Interactions in a Polyhydroxylated Environment: Conservation of a C–F···H–C Recognition Motif in Presence of O–H···O Hydrogen Bonds. Eur. J. Org. Chem. 2010, 2010, 3387–3394. [Google Scholar] [CrossRef]
- Müller, K. Simple Vector Considerations to Assess the Polarity of Partially Fluorinated Alkyl and Alkoxy Groups. CHIMIA Int. J. Chem. 2014, 68, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudin, A.K. Aziridines and Epoxides in Organic Synthesis; John Wiley and Sons: Weinheim, Germany, 2006; pp. 1–492. [Google Scholar]
- Sweeney, J.B. Aziridines: Epoxides’ ugly cousins? Chem. Soc. Rev. 2002, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Stanković, S.; D’Hooghe, M.; Catak, S.; Eum, H.; Waroquier, M.; Van Speybroeck, V.; De Kimpe, N.; Ha, H.-J. Regioselectivity in the ring opening of non-activated aziridines. Chem. Soc. Rev. 2012, 41, 643–665. [Google Scholar] [CrossRef]
- Degennaro, L.; Trinchera, P.; Luisi, R. Recent Advances in the Stereoselective Synthesis of Aziridines. Chem. Rev. 2014, 114, 7881–7929. [Google Scholar] [CrossRef] [PubMed]
- Botuha, C.; Chemla, F.; Ferreira, F.; Pérez-Luna, A. Aziridines in Natural Product Synthesis. In Heterocycles in Natural Product Synthesis; John Wiley and Sons: Weinheim, Germany, 2011; pp. 1–39. [Google Scholar]
- Karban, J.; Kroutil, J. Chemistry of carbohydrate aziridines. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2006; Volume 60, pp. 27–101. [Google Scholar]
- Karban, J.; Kroutil, J.; Budesinsky, M.; Sykora, J.; Cisarova, I. Ring-Opening Reactions of Aziridines Fused to a Conformationally Locked Tetrahydropyran Ring. Eur. J. Org. Chem. 2009, 36, 6399–6406. [Google Scholar] [CrossRef]
- Karban, J.; Sýkora, J.; Kroutil, J.; Císařová, I.; Padělková, Z.; Buděšínský, M. Synthesis of All Configurational Isomers of 1,6-Anhydro-2,3,4-trideoxy-2,3-epimino-4-fluoro-β-d-hexopyranoses. J. Org. Chem. 2010, 75, 3443–3446. [Google Scholar] [CrossRef]
- Karban, J.; Budesinsky, M.; Cerny, M.; Trnka, T. Synthesis and NMR spectra of 1,6-anhydro-2,3-dideoxy-2,3-epimino- and 1,6-anhydro-3,4-dideoxy-3,4-epimino-β-d-hexopyranoses. Collect. Czech. Chem. Commun. 2001, 66, 799–819. [Google Scholar] [CrossRef]
- Karban, J.; Budesinsky, M.; Kroutil, J. Synthesis of 1,6-anhydro-2,3,4-trideoxy-2,3-epimino- and 1,6-anhydro-2,3,4-trideoxy-3,4-epimino-β-d-hexopyranoses and their NMR and infrared spectra. Collect. Czech. Chem. Commun. 2004, 69, 1939–1954. [Google Scholar] [CrossRef]
- Kroutil, J.; Trnka, T.; Buděšínský, M.; Černý, M. Preparation of 2,3-dideoxy-2,3-epimino and 3,4-dideoxy-3,4-epimino derivatives of 1,6-anhydro-β-d-hexopyranoses by Mitsunobu reaction. Collect. Czech. Chem. Commun. 1998, 63, 813–825. [Google Scholar] [CrossRef]
- Cerny, M.; Elbert, T.; Pacak, J. Syntheses with anhydro sugars. 21. Preparation of 1,6-anhydro-2,3-dideoxy-2,3-epimino-β-d-mannopyranose and its conversion to 2-amino-1,6-anhydro-2-deoxy-beta-mannopyranose. Collect. Czech. Chem. Commun. 1974, 39, 1752–1767. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02.; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool. Version 1.2.0. 2016. Available online: http://avogadro.cc/ (accessed on 30 August 2021).
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Hobza, P.; Müller-Dethlefs, K. Non-Covalent Interactions: Theory and Experiment; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Stasyuk, O.A.; Sedlak, R.; Guerra, C.F.; Hobza, P. Comparison of the DFT-SAPT and Canonical EDA Schemes for the Energy Decomposition of Various Types of Noncovalent Interactions. J. Chem. Theory Comput. 2018, 14, 3440–3450. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2017/1, Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 2017. [Google Scholar]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J.J. CRYSTALS version 12: Software for guided crystal structure analysis. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Discovery Studio Visualizer, Version 17.2.0.16349; BIOVIA, Dassault Systèmes: San Diego, CA, USA, 2016.
- Moss, G.P. Basic terminology of stereochemistry (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]




| H | F-cis | F-trans | OH-cis | OH-trans | |
|---|---|---|---|---|---|
| exo ΔEout-in kcal/mol | 1.62 (I) | 3.31 (II) | 0.83 (III) | 3.88 (IV) | 0.86 (V) |
| endo ΔEout-in kcal/mol | 0.24 (VI) | 0.54 (VIII) | −0.54 (VII) | 0.69 (X) | 0.27 (IX) |
| OH···A (ΔE) | D···OH (ΔE) | NH···A (ΔE) | D···NH (ΔE) | |
|---|---|---|---|---|
| IXa | NHb (1.11) | not specific | NHc (3.18) | OHb (1.11) |
| 2.034 Å | 2.439 Å | 2.034 Å | ||
| IXb | NHa (1.67) | not specific | OHd (2.32) | OHa (1.67) |
| 2.193 Å | 2.394 Å | 2.193 Å | ||
| IXc | NHd (0.00) | OHd (1.59) | O1a (3.03) | NHa (3.18) |
| 2.076 Å | 2.216 Å | 2.123 Å | 2.439 Å | |
| IXd | OHc (1.59) | NHb (2.32) | O1b (2.17) | OHc (0.00) |
| 2.216 Å | 2.394 Å | 2.240 Å | 2.076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubec, M.; Císařová, I.; Karban, J.; Sýkora, J. The Effect of Deoxyfluorination on Intermolecular Interactions in the Crystal Structures of 1,6-Anhydro-2,3-epimino-hexopyranoses. Molecules 2022, 27, 278. https://doi.org/10.3390/molecules27010278
Jakubec M, Císařová I, Karban J, Sýkora J. The Effect of Deoxyfluorination on Intermolecular Interactions in the Crystal Structures of 1,6-Anhydro-2,3-epimino-hexopyranoses. Molecules. 2022; 27(1):278. https://doi.org/10.3390/molecules27010278
Chicago/Turabian StyleJakubec, Martin, Ivana Císařová, Jindřich Karban, and Jan Sýkora. 2022. "The Effect of Deoxyfluorination on Intermolecular Interactions in the Crystal Structures of 1,6-Anhydro-2,3-epimino-hexopyranoses" Molecules 27, no. 1: 278. https://doi.org/10.3390/molecules27010278
APA StyleJakubec, M., Císařová, I., Karban, J., & Sýkora, J. (2022). The Effect of Deoxyfluorination on Intermolecular Interactions in the Crystal Structures of 1,6-Anhydro-2,3-epimino-hexopyranoses. Molecules, 27(1), 278. https://doi.org/10.3390/molecules27010278






