Mechanochemistry in Portugal—A Step towards Sustainable Chemical Synthesis
Abstract
:1. Introduction
1.1. Mechanochemical Methods
1.2. Mechanochemistry in Portugal
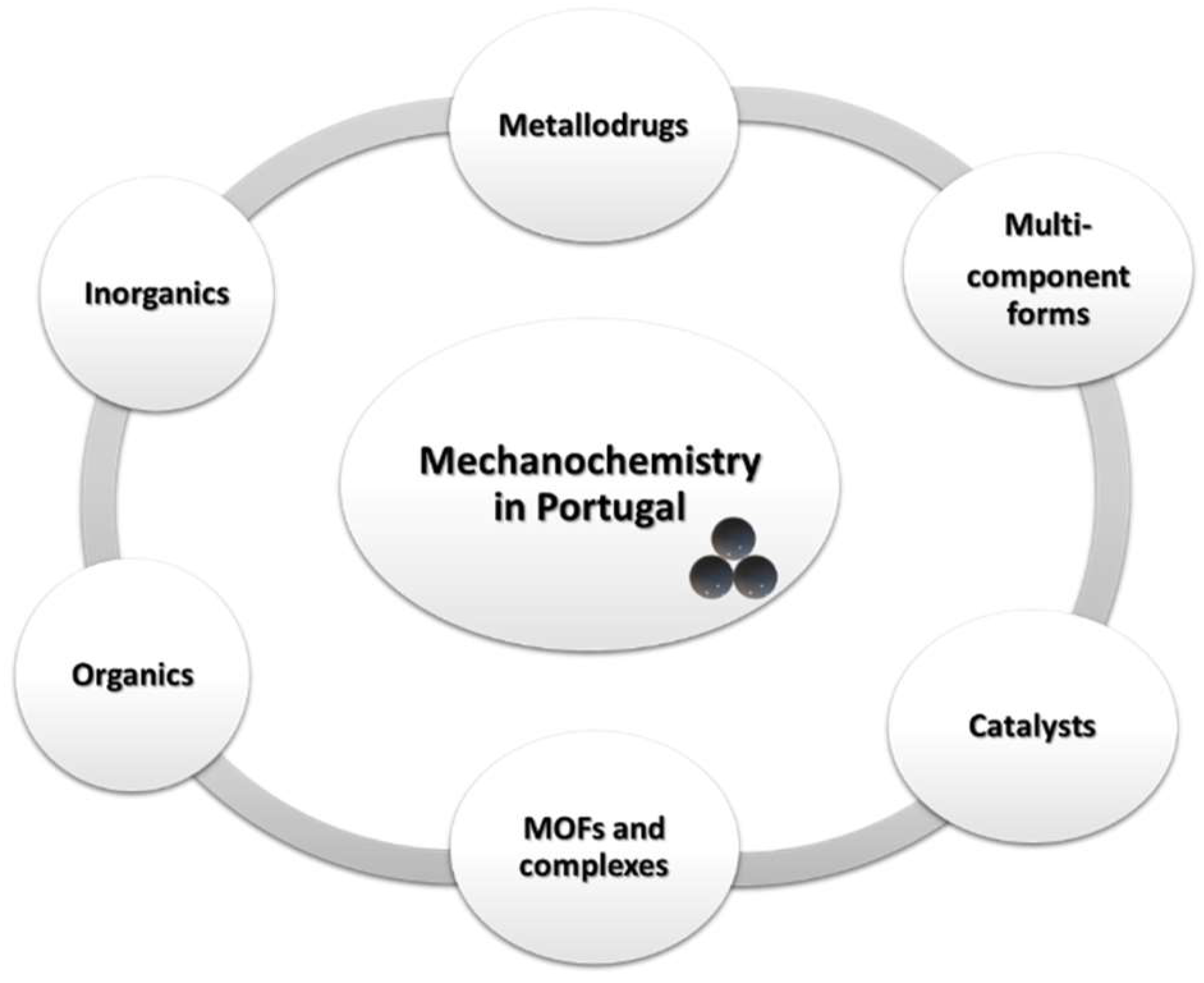
2. Developments of Mechanochemistry in Portugal
2.1. Mechanochemistry in the Synthesis of Polymorphs and Multicomponent Forms
2.1.1. Polymorphs
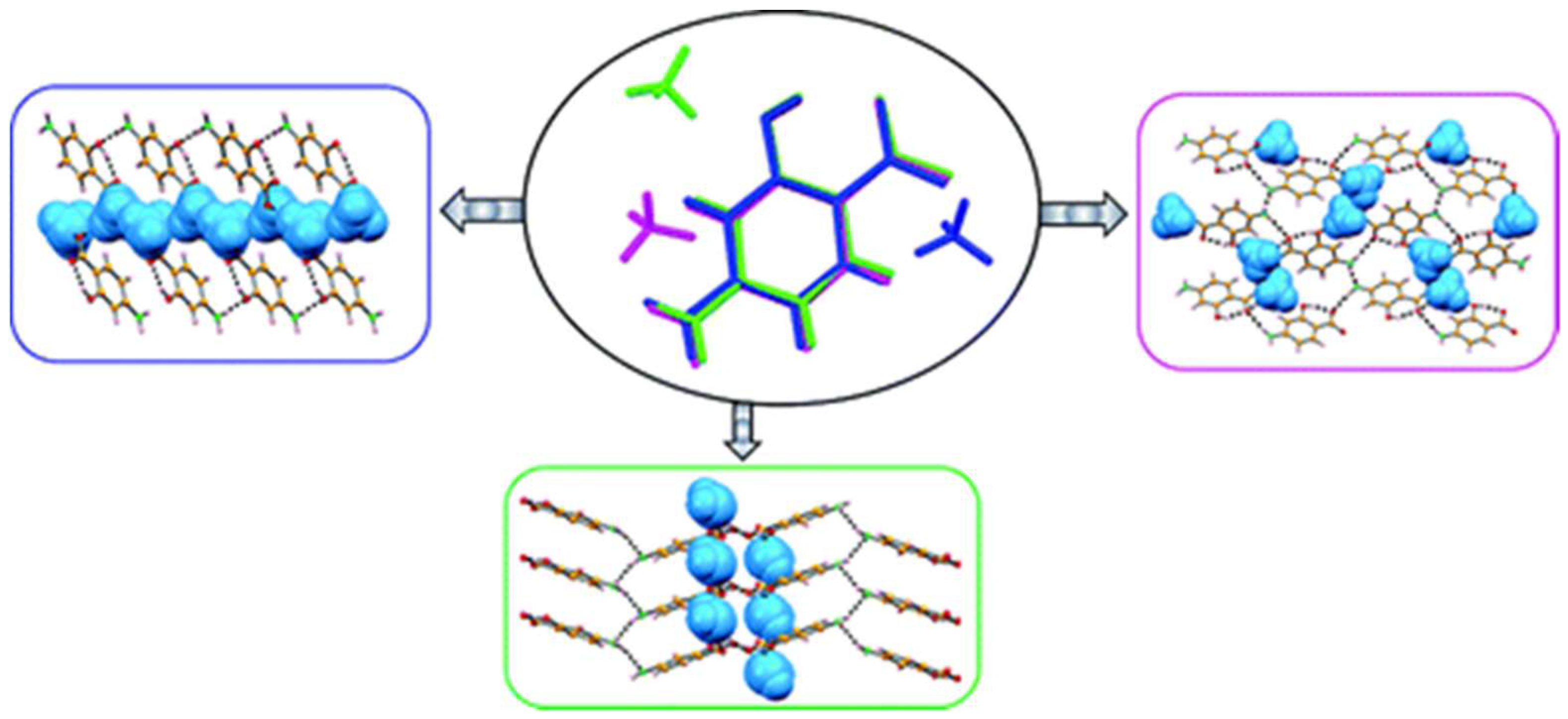
2.1.2. Screening and Synthesis of Multicomponent Crystal Forms
2.1.3. Improvement of Physicochemical Properties
2.1.4. Non-Crystalline Multicomponent Forms
2.2. Mechanochemistry in the Synthesis of BioMOFs and Coordination Polymers
2.3. Mechanochemistry in/for Catalysis
2.4. Mechanochemistry in the Synthesis of Organic and Inorganic Compounds
3. Final Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Azeleic Acid |
| AC | Activated Carbon |
| ACFs | Antibiotic Coordination Frameworks |
| ADA | Adamantylamine |
| AIDS | Acquired Immunodeficiency Syndrome |
| API | Active Pharmaceutical Ingredient |
| ASA | 4-Aminosalicylic Acid |
| ASD | Amorphous Solid Dispersion |
| BCS | Biopharmaceutical Classification System |
| BioMOFs | Bio-inspired Metal-organic Frameworks |
| BIP | 4,4′-Bipyridine |
| CAPRO | ξ-Caprolactam |
| CNTs | Carbon Nanotubes |
| COFs | Covalent-Organic Frameworks |
| CPs | Coordination Polymers |
| DABCO | Diazabicyclo[2.2.2]octane |
| DME | 1,2-Dimethoxyethane |
| FA | Fumaric Acid |
| Gly | Glycine |
| GO | Graphene Oxide |
| GRAS | Generally Regarded as Safe |
| HIV | Human Immunodeficiency Virus |
| HME | Hot-melt Extrusion |
| HPA | Heteropolyacids |
| ICC | Ionic Cocrystals |
| ILAG | Ion- and Liquid-assisted Grinding |
| ILs | Ionic Liquids |
| ISO | Isonicotinamide |
| IUPAC | International Union of Pure and Applied Chemistry |
| LAG | Liquid-Assisted Grinding |
| LDH | Layered Double Hydroxides |
| MA | Maleic Acid |
| MIR | Mid Infrared Spectroscopy |
| MLK | Montelukast |
| MOFs | Metal-Organic Frameworks |
| MORPH | Morpholine |
| NG | Neat Grinding |
| NMR | Nuclear Magnetic Ressonance |
| NSAID | Nonsteroidal Anti-Inflamatory Drug |
| PAT | Process Analytical Technology |
| PIP | Piperazine |
| PMMA | poly(methyl)methacrylate |
| POLAG | Polymer-Assited Grinding |
| SDM | Sulfadimethoxine |
| TBHP | Tert-butyl Hydroperoxide |
| Teflon | Polytetrafluoroethylene |
| TOF | Turnover Frequency |
| TON | Turnover Number |
| TRI | 4,4′-trimethylenedipiperidine |
| TSE | Twin Screw Extrusion |
| Υ-CD | Υ-cyclodextrin |
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- United Nations. 17 Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 21 December 2021).
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 28 July 2021).
- Colacino, E.; Porcheddu, A.; Charnay, C.; Delogu, F. From enabling technologies to medicinal mechanochemistry: An eco-friendly access to hydantoin-based active pharmaceutical ingredients. React. Chem. Eng. 2019, 4, 1179–1188. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef]
- Schiffmann, J.G.; Emmerling, F.; Martins, I.C.B.; Van Wüllen, L. In-situ reaction monitoring of a mechanochemical ball mill reaction with solid state NMR. Solid State Nucl. Magn. Reson. 2020, 109, 101687. [Google Scholar] [CrossRef]
- Quaresma, S.; André, V.; Fernandes, A.; Duarte, M.T. Mechanochemistry—A green synthetic methodology leading to metallodrugs, metallopharmaceuticals and bio-inspired metal-organic frameworks. Inorg. Chim. Acta 2017, 455, 309–318. [Google Scholar] [CrossRef]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The mechanical activation of covalent bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef]
- Achar, T.K.; Bose, A.; Mal, P. Mechanochemical synthesis of small organic molecules. Beilstein J. Org. Chem. 2017, 13, 1907–1931. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ying, P.; Wang, H.; Xiang, K.; Su, W. Mechanochemical Asymmetric Cross-Dehydrogenative Coupling Reaction: Liquid-Assisted Grinding Enables Reaction Acceleration and Enantioselectivity Control. Adv. Synth. Catal. 2020, 362, 893–902. [Google Scholar] [CrossRef]
- Dabral, S.; Wotruba, H.; Hernández, J.G.; Bolm, C. Mechanochemical Oxidation and Cleavage of Lignin β-O-4 Model Compounds and Lignin. ACS Sustain. Chem. Eng. 2018, 6, 3242–3254. [Google Scholar] [CrossRef]
- Fiss, B.G.; Richard, A.J.; Friščić, T.; Moores, A. Mechanochemistry for sustainable and efficient dehydrogenation/hydrogenation. Can. J. Chem. 2021, 99, 93–112. [Google Scholar] [CrossRef]
- Chauhan, P.; Chimni, S.S. Mechanochemistry assisted asymmetric organocatalysis: A sustainable approach. Beilstein J. Org. Chem. 2012, 8, 2132–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cave, G.W.V.; Raston, C.L.; Scott, J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 21, 2159–2169. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la, O. Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal–organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; Niu, H.; Cai, Y. Mechanochemical Construction 2D/2D Covalent Organic Nanosheets Heterojunctions Based on Substoichiometric Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 42035–42043. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, L.M.; Loots, L.; Barbour, L.J. Mechanochemical control of solvent content in a 1D coordination polymer. J. Coord. Chem. 2021, 74, 190–199. [Google Scholar] [CrossRef]
- Delori, A.; Friscic, T.; Jones, W. The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. CrystEngComm 2012, 14, 2350–2362. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptista, B.; Lopes, J.A.; Sarraguça, M.C. Pharmaceutical cocrystallization techniques. Advances and challenges. Int. J. Pharm. 2018, 547, 404–420. [Google Scholar] [CrossRef]
- Fiss, B.G.; Vu, N.-N.; Douglas, G.; Do, T.-O.; Friščić, T.; Moores, A. Solvent-Free Mechanochemical Synthesis of Ultrasmall Nickel Phosphide Nanoparticles and Their Application as a Catalyst for the Hydrogen Evolution Reaction (HER). ACS Sustain. Chem. Eng. 2020, 8, 12014–12024. [Google Scholar] [CrossRef]
- Gomollón-Bel, F. Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable. Chem. Int. 2019, 41, 12–17. [Google Scholar] [CrossRef]
- Gečiauskaitė, A.A.; García, F. Main group mechanochemistry. Beilstein J. Org. Chem. 2017, 13, 2068–2077. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.G.; Halasz, I.; Crawford, D.E.; Krupicka, M.; Balaz, M.; Andre, V.; Vella-Zarb, L.; Niidu, A.; Garcia, F.; Maini, L.; et al. European Research in Focus: Mechanochemistry for Sustainable Industry (COST Action MechSustInd). Eur. J. Org. Chem. 2020, 2020, 8–9. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 9640–9643. [Google Scholar] [CrossRef]
- Friscić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and liquid-assisted grinding: Improved mechanochemical synthesis of metal-organic frameworks reveals salt inclusion and anion templating. Angew. Chem. Int. Ed. Engl. 2010, 49, 712–715. [Google Scholar] [CrossRef]
- Hasa, D.; Rauber, G.S.; Voinovich, D.; Jones, W. Cocrystal Formation through Mechanochemistry: From Neat and Liquid-Assisted Grinding to Polymer-Assisted Grinding. Angew. Chem.-Int. Ed. 2015, 54, 7371–7375. [Google Scholar] [CrossRef]
- Hasa, D.; Carlino, E.; Jones, W. Polymer-Assisted Grinding, a Versatile Method for Polymorph Control of Cocrystallization. Cryst. Growth Des. 2016, 16, 1772–1779. [Google Scholar] [CrossRef]
- Crawford, D.; Casaban, J.; Haydon, R.; Giri, N.; McNally, T.; James, S.L. Synthesis by extrusion: Continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 2015, 6, 1645–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, D.; Grepioni, F.; Andre, V.; Duarte, M.T. Drug-containing coordination and hydrogen bonding networks obtained mechanochemically. CrystEngComm 2009, 11, 2618–2621. [Google Scholar] [CrossRef]
- Andre, V.; Hardeman, A.; Halasz, I.; Stein, R.S.; Jackson, G.J.; Reid, D.G.; Duer, M.J.; Curfs, C.; Duarte, M.T.; Friscic, T. Mechanosynthesis of the Metallodrug Bismuth Subsalicylate from Bi2O3 and Structure of Bismuth Salicylate without Auxiliary Organic Ligands. Angew. Chem.-Int. Ed. 2011, 50, 7858–7861. [Google Scholar] [CrossRef] [PubMed]
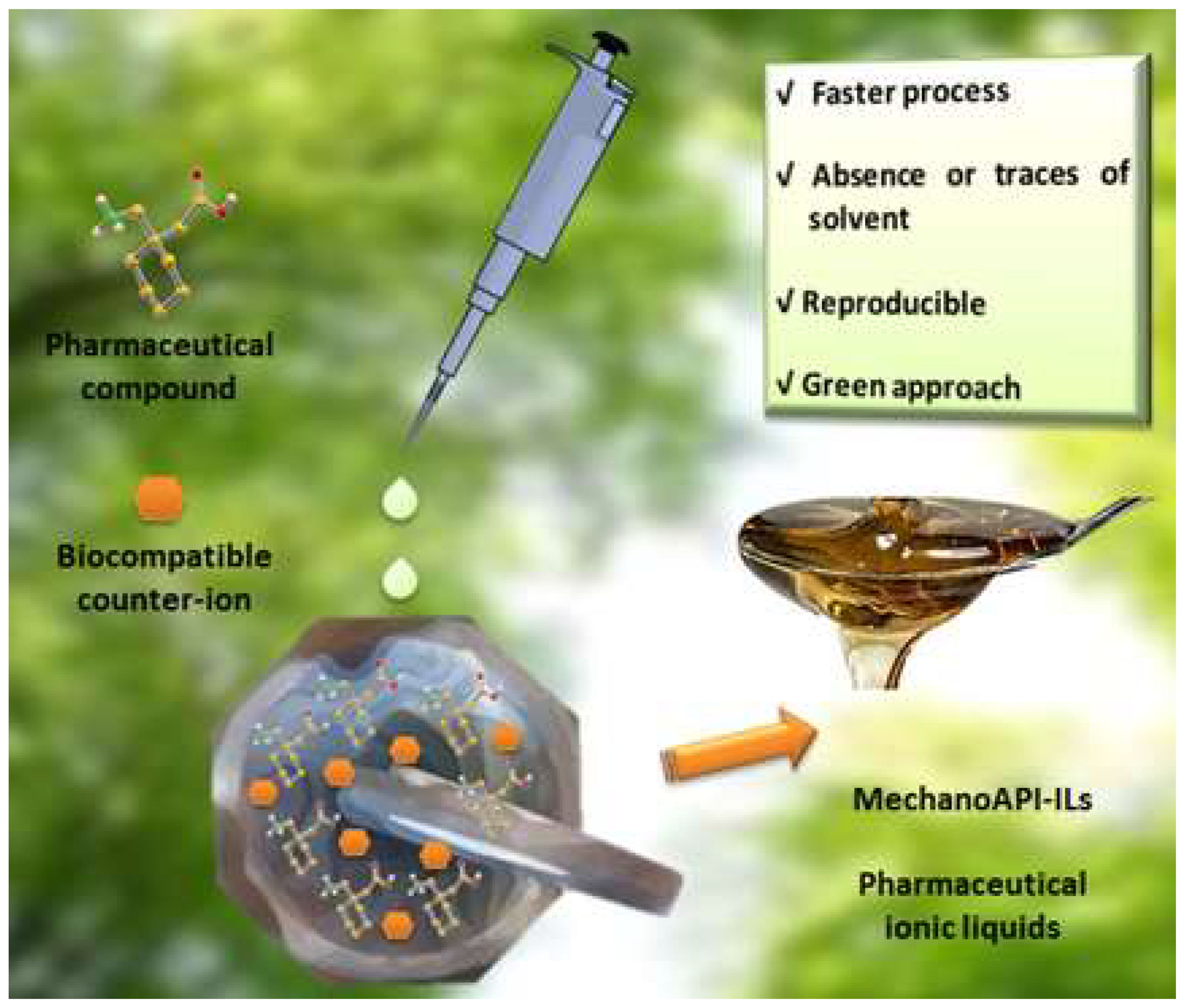
- Martins, I.C.B.; Oliveira, M.C.; Diogo, H.P.; Branco, L.C.; Duarte, M.T. MechanoAPI-ILs: Pharmaceutical Ionic Liquids Obtained through Mechanochemical Synthesis. ChemSusChem 2017, 10, 1360–1363. [Google Scholar] [CrossRef]
- André, V.; da Silva, A.R.F.; Fernandes, A.; Frade, R.; Garcia, C.; Rijo, P.; Antunes, A.M.M.; Rocha, J.; Duarte, M.T. Mg- and Mn-MOFs Boost the Antibiotic Activity of Nalidixic Acid. ACS Appl. Bio Mater. 2019, 2, 2347–2354. [Google Scholar] [CrossRef]
- Gomes, C.S.B.; Gomes, P.T.; Duarte, M.T. α-Diimine transition-metal complexes: Mechanochemistry-A new synthetic approach. J. Organomet. Chem. 2014, 760, 101–107. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: The Design of Organic Solids; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Andre, V.; Cunha-Silva, L.; Duarte, M.T.; Santos, P.P. First Crystal Structures of the Antihypertensive Drug Perindopril Erbumine: A Novel Hydrated Form and Polymorphs alpha and beta. Cryst. Growth Des. 2011, 11, 3703–3706. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Rubini, K.; Polito, M.; Brescello, R.; Cotarca, L.; Duarte, M.T.; Andre, V.; Piedade, M.F.M. Polymorphic gabapentin: Thermal behaviour, reactivity and interconversion of forms in solution and solid-state. New J. Chem. 2008, 32, 1788–1795. [Google Scholar] [CrossRef]
- Friscic, T.; Trask, A.V.; Motherwell, W.D.S.; Jones, W. Guest-directed assembly of caffeine and succinic acid into topologically different heteromolecular host networks upon grinding. Cryst. Growth Des. 2008, 8, 1605–1609. [Google Scholar] [CrossRef]
- da Silva, J.L.F.; da Piedade, M.F.M.; Andre, V.; Domingos, S.; Martins, I.C.B.; Duarte, M.T. The Lisbon Supramolecular Green Story: Mechanochemistry towards New Forms of Pharmaceuticals. Molecules 2020, 25, 2705. [Google Scholar] [CrossRef]
- André, V.; Fernandes, A.; Santos, P.P.; Duarte, M.T. On the Track of New Multicomponent Gabapentin Crystal Forms: Synthon Competition and pH Stability. Cryst. Growth Des. 2011, 11, 2325–2334. [Google Scholar] [CrossRef]
- Andre, V.; Braga, D.; Grepioni, F.; Duarte, M.T. Crystal Forms of the Antibiotic 4-Aminosalicylic Acid: Solvates and Molecular Salts with Dioxane, Morpholine, and Piperazine. Cryst. Growth Des. 2009, 9, 5108–5116. [Google Scholar] [CrossRef]
- André, V.; Shemchuk, O.; Grepioni, F.; Braga, D.; Duarte, M.T. Expanding the Pool of Multicomponent Crystal Forms of the Antibiotic 4-Aminosalicylic Acid: The Influence of Crystallization Conditions. Cryst. Growth Des. 2017, 17, 6417–6425. [Google Scholar] [CrossRef]
- André, V.; Duarte, M.T.; Braga, D.; Grepioni, F. Polymorphic Ammonium Salts of the Antibiotic 4-Aminosalicylic Acid. Cryst. Growth Des. 2012, 12, 3082–3090. [Google Scholar] [CrossRef]
- Castro, R.A.E.; Ribeiro, J.D.B.; Maria, T.M.R.; Silva, M.R.; Yuste-Vivas, C.; Canotilho, J.; Eusebio, M.E.S. Naproxen Cocrystals with Pyridinecarboxamide Isomers. Cryst. Growth Des. 2011, 11, 5396–5404. [Google Scholar] [CrossRef]
- Perpetuo, G.L.; Chierice, G.O.; Ferreira, L.T.; Fraga-Silva, T.F.C.; Venturini, J.; Arruda, M.S.P.; Bannach, G.; Castro, R.A.E. A combined approach using differential scanning calorimetry with polarized light thermomicroscopy in the investigation of ketoprofen and nicotinamide cocrystal. Thermochim. Acta 2017, 651, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Évora, A.O.L.; Castro, R.A.E.; Maria, T.M.R.; Ramos Silva, M.; Canotilho, J.; Eusébio, M.E.S. Lamotrigine: Design and synthesis of new multicomponent solid forms. Eur. J. Pharm. Sci. 2019, 129, 148–162. [Google Scholar] [CrossRef] [PubMed]
- di Martino, P.; Guyot-Hermann, A.M.; Conflant, P.; Drache, M.; Guyot, J.C. A new pure paracetamol for direct compression: The orthorhombic form. Int. J. Pharm. 1996, 128, 1–8. [Google Scholar] [CrossRef]
- André, V.; M. da Piedade, M.F.; Duarte, M.T. Revisiting paracetamol in a quest for new co-crystals. CrystEngComm 2012, 14, 5005–5014. [Google Scholar] [CrossRef]
- Duberg, M.; Nyström, C. Studies on direct compression of tablets XVII. Porosity—Pressure curves for the characterization of volume reduction mechanisms in powder compression. Powder Technol. 1986, 46, 67–75. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Fábián, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving Mechanical Properties of Crystalline Solids by Cocrystal Formation: New Compressible Forms of Paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Castro, R.A.E.; Leitão, J.M.M.; Esteves da Silva, J.C.G. At-line green synthesis monitoring of new pharmaceutical co-crystals lamivudine:theophylline polymorph I and II, quantification of polymorph I among its APIs using FT-IR spectroscopy and MCR-ALS. J. Pharm. Biomed. Anal. 2019, 169, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Évora, A.O.L.; Bernardes, C.E.S.; Piedade, M.F.M.; Conceição, A.C.L.; Minas da Piedade, M.E. Energetics of Glycine Cocrystal or Salt Formation with Two Regioisomers: Fumaric Acid and Maleic Acid. Cryst. Growth Des. 2019, 19, 5054–5064. [Google Scholar] [CrossRef]
- Zabransky, M.; Alves, P.C.; Bravo, C.; Duarte, M.T.; Andre, V. From pipemidic acid molecular salts to metal complexes and BioMOFs using mechanochemistry. CrystEngComm 2021, 23, 1099–1109. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Andre, V.; Tan, D.; Duarte, M.T.; Lancaster, R.W.; Karamertzanis, P.G.; Friscic, T. Molecular Recognition of Steroid Hormones in the Solid State: Stark Differences in Cocrystallization of beta-Estradiol and Estrone. Cryst. Growth Des. 2015, 15, 1492–1501. [Google Scholar] [CrossRef]
- Friscic, T.; Lancaster, R.W.; Fabian, L.; Karamertzanis, P.G. Tunable recognition of the steroid alpha-face by adjacent pi-electron density. Proc. Natl. Acad. Sci. USA 2010, 107, 13216–13221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Évora, A.O.L.; Castro, R.A.E.; Maria, T.M.R.; Rosado, M.T.S.; Ramos Silva, M.; Matos Beja, A.; Canotilho, J.; Eusébio, M.E.S. Pyrazinamide-Diflunisal: A New Dual-Drug Co-Crystal. Cryst. Growth Des. 2011, 11, 4780–4788. [Google Scholar] [CrossRef]
- Shemchuk, O.; Tsenkova, B.K.; Braga, D.; Duarte, M.T.; Andre, V.; Grepioni, F. Ionic Co-Crystal Formation as a Path Towards Chiral Resolution in the Solid State. Chem.-Eur. J. 2018, 24, 12564–12573. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Martins, M.; Fernandes, A.; André, V.; Duarte, M.T. An insight into dapsone co-crystals: Sulfones as participants in supramolecular interactions. CrystEngComm 2013, 15, 8173–8179. [Google Scholar] [CrossRef] [Green Version]
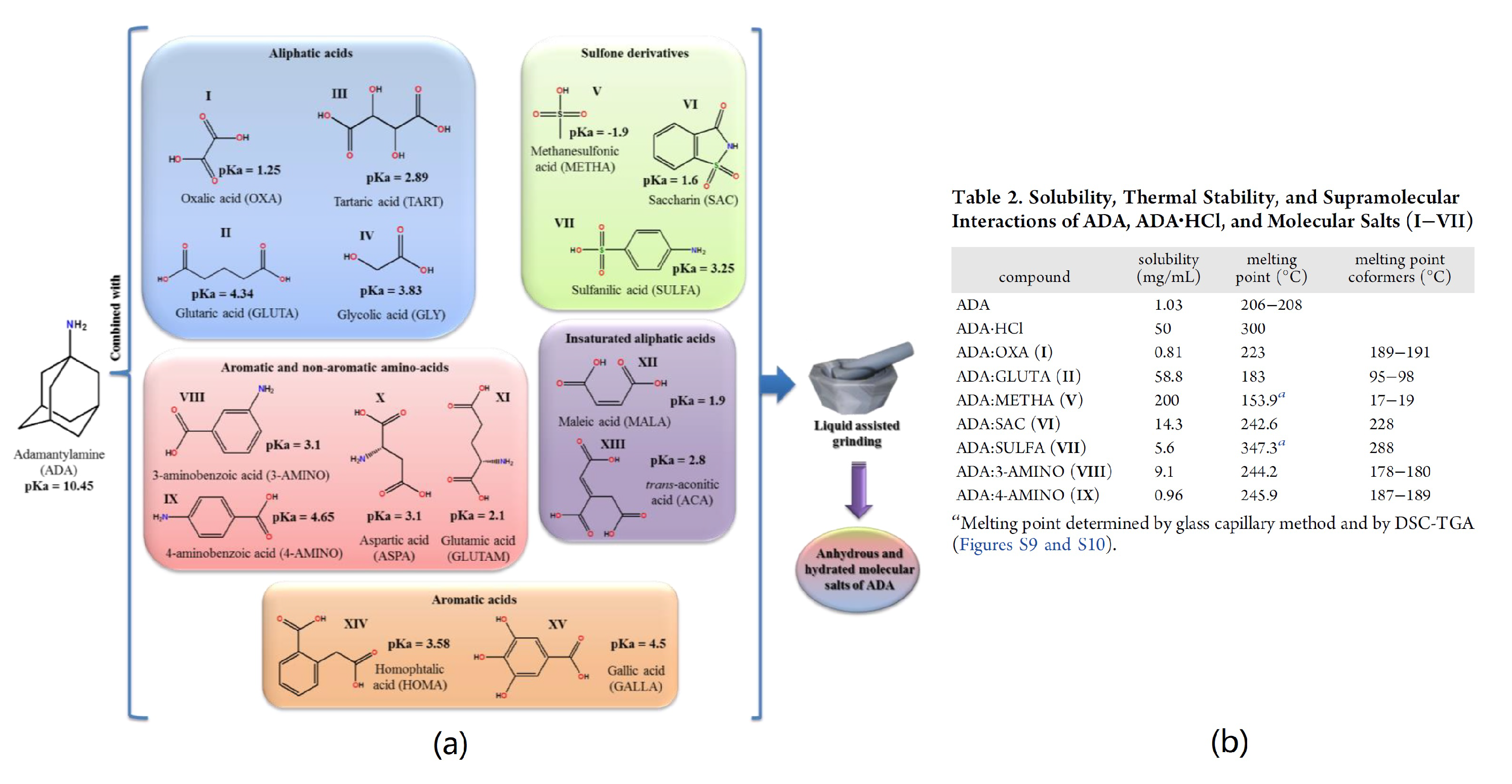
- Martins, I.C.B.; Sardo, M.; Alig, E.; Fink, L.; Schmidt, M.U.; Mafra, L.; Duarte, M.T. Enhancing Adamantylamine Solubility through Salt Formation: Novel Products Studied by X-ray Diffraction and Solid-State NMR. Cryst. Growth Des. 2019, 19, 1860–1873. [Google Scholar] [CrossRef]
- Domingos, S.; Fernandes, A.; Duarte, M.T.; Piedade, M.F.M. New Multicomponent Sulfadimethoxine Crystal Forms: Sulfonamides as Participants in Supramolecular Interactions. Cryst. Growth Des. 2016, 16, 1879–1892. [Google Scholar] [CrossRef]
- Martins, I.C.B.; Sardo, M.; Santos, S.M.; Femandes, A.; Antunes, A.; Andre, V.; Mafra, L.; Duarte, M.T. Packing Interactions and Physicochemical Properties of Novel Multicomponent Crystal Forms of the Anti-Inflammatory Azelaic Acid Studied by X-ray and Solid-State NMR. Cryst. Growth Des. 2016, 16, 154–166. [Google Scholar] [CrossRef]
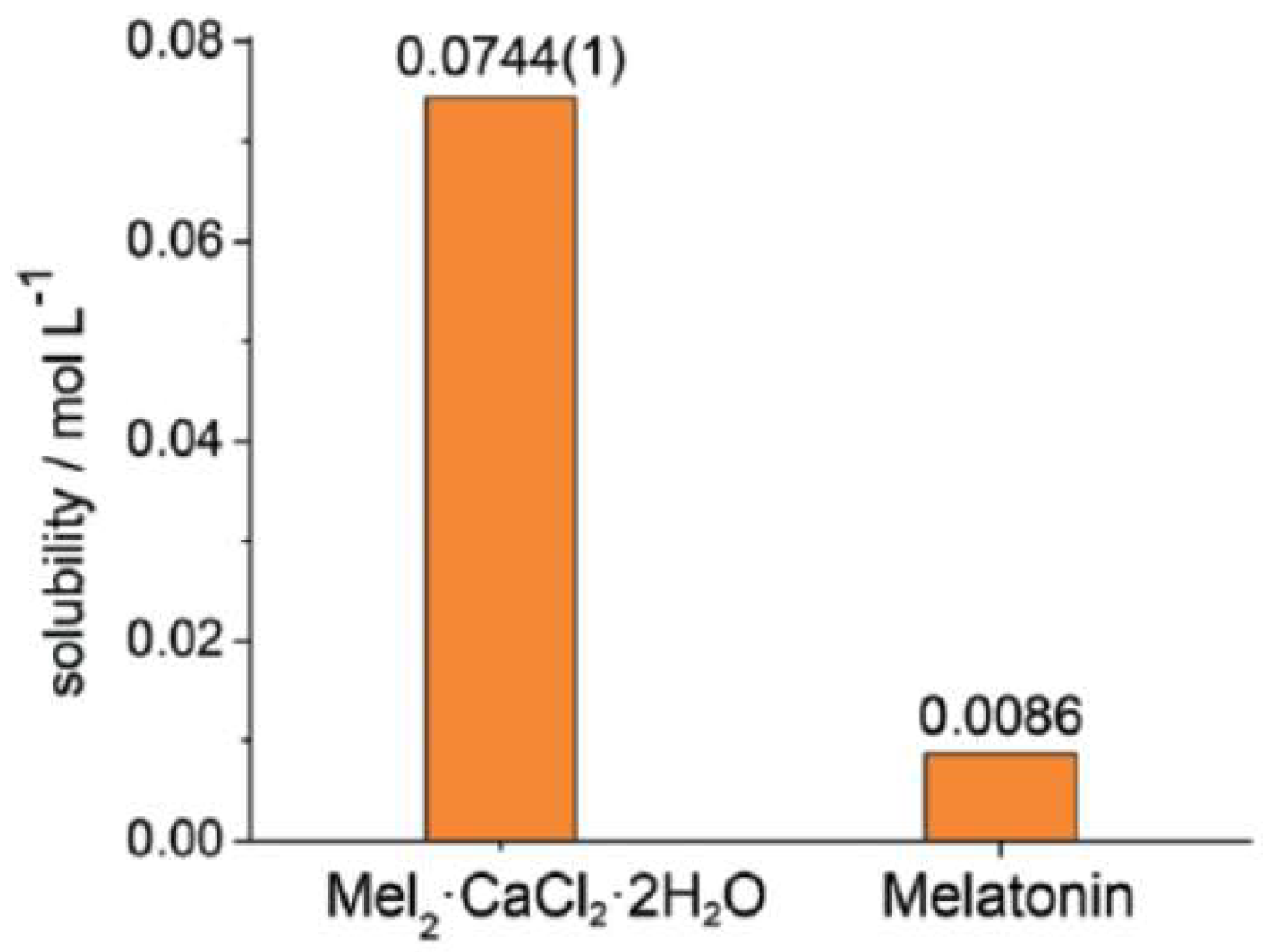
- Shemchuk, O.; André, V.; Duarte, M.T.; Braga, D.; Grepioni, F. Mechanochemical preparation of molecular and ionic co-crystals of the hormone melatonin. CrystEngComm 2019, 21, 2949–2954. [Google Scholar] [CrossRef]
- Shemchuk, O.; André, V.; Duarte, M.T.; Taddei, P.; Rubini, K.; Braga, D.; Grepioni, F. Molecular Salts of l-Carnosine: Combining a Natural Antioxidant and Geroprotector with “Generally Regarded as Safe” (GRAS) Organic Acids. Cryst. Growth Des. 2017, 17, 3379–3386. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Nolasco, M.M.; Ribeiro-Claro, P.; Almeida Paz, F.A.; Braga, S.S. Preformulation Studies of the γ-Cyclodextrin and Montelukast Inclusion Compound Prepared by Comilling. J. Pharm. Sci. 2019, 108, 1837–1847. [Google Scholar] [CrossRef]
- Fernandes, J.A.; Sardo, M.; Mafra, L.; Choquesillo-Lazarte, D.; Masciocchi, N. X-ray and NMR Crystallography Studies of Novel Theophylline Cocrystals Prepared by Liquid Assisted Grinding. Cryst. Growth Des. 2015, 15, 3674–3683. [Google Scholar] [CrossRef]
- Simões, M.F.; Pereira, A.; Cardoso, S.; Cadonau, S.; Werner, K.; Pinto, R.M.A.; Simões, S. Five-Stage Approach for a Systematic Screening and Development of Etravirine Amorphous Solid Dispersions by Hot-Melt Extrusion. Mol. Pharm. 2020, 17, 554–568. [Google Scholar] [CrossRef]
- Simões, M.F.; Nogueira, B.A.; Tabanez, A.M.; Fausto, R.; Pinto, R.M.A.; Simões, S. Enhanced solid-state stability of amorphous ibrutinib formulations prepared by hot-melt extrusion. Int. J. Pharm. 2020, 579, 119156. [Google Scholar] [CrossRef] [PubMed]
- Mullers, K.C.; Wahl, M.A.; Pinto, J.F. Multilayer laminar co-extrudate as a novel controlled release dosage form. Eur. J. Pharm. Sci. 2013, 49, 491–498. [Google Scholar] [CrossRef]
- Mullers, K.C.; Wahl, M.A.; Pinto, J.F. Production of dosage forms for oral drug delivery by laminar extrusion of wet masses. Eur. J. Pharm. Biopharm. 2013, 84, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Wahl, M.A.; Pinto, J.F. Delivery of Drugs from Laminar Co-Extrudates Manufactured by a Solvent-Free Process at Room Temperature. J. Pharm. Sci. 2014, 103, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.D.; Chávez, F.V.; Maria, T.M.R.; Eusébio, M.E.S.; Sebastião, P.J.; Martín-Ramos, P.; Figueirinhas, J.L.; Silva, M.R. Synthesis of liquid crystals based on hydrogen-bonding of 4-(Octyloxy) benzoic acid with 4-alkylbenzoic acids. Mol. Cryst. Liq. Cryst. 2016, 630, 87–101. [Google Scholar] [CrossRef]
- Miranda, M.D.; Chavez, F.V.; Maria, T.M.R.; Eusebio, M.E.S.; Sebastiao, P.J.; Silva, M.R. Self-assembled liquid crystals by hydrogen bonding between bipyridyl and alkylbenzoic acids: Solvent-free synthesis by mechanochemistry. Liq. Cryst. 2014, 41, 1743–1751. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L. (Ed.) Mechanochemistry: A Tool in the Synthesis of Catalysts, Metallodrugs and Metallophamacuticals. In ICOMC Silver/Gold Jubilee Celebratory Book; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Andre, V.; Alves, P.C.; Duarte, M.T. Exploring antibiotics as ligands in metal-organic and hydrogen bonding frameworks: Our novel approach towards enhanced antimicrobial activity (mini-review). Inorg. Chim. Acta 2021, 525, 120474. [Google Scholar] [CrossRef]
- Andre, V.; Quaresma, S.; da Silva, J.L.F.; Duarte, M.T. Exploring mechanochemistry to turn organic bio-relevant molecules into metal-organic frameworks: A short review. Beilstein J. Org. Chem. 2017, 13, 2416–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaresma, S.; Andre, V.; Antunes, A.M.M.; Cunha-Silva, L.; Duarte, M.T. Gabapentin Coordination Networks: Mechanochemical Synthesis and Behavior under Shelf Conditions. Cryst. Growth Des. 2013, 13, 5007–5017. [Google Scholar] [CrossRef]
- Andre, V.; Galego, F.; Martins, M. Mechanochemical Assembly of Nalidixic Acid Bioinspired Metal-Organic Compounds and Complexes toward Improved Solubility. Cryst. Growth Des. 2018, 18, 2067–2081. [Google Scholar] [CrossRef]
- Bravo, C.; Galego, F.; Andre, V. Hydrogen bonding networks of nalidixic acid-copper (II) complexes. CrystEngComm 2019, 21, 7199–7203. [Google Scholar] [CrossRef]
- Alves, P.C.; Rijo, P.; Bravo, C.; Antunes, A.M.M.; Andre, V. Bioactivity of Isostructural Hydrogen Bonding Frameworks Built from Pipemidic Acid Metal Complexes. Molecules 2020, 25, 2374. [Google Scholar] [CrossRef]
- Quaresma, S.; Andre, V.; Antunes, A.M.M.; Vilela, S.M.F.; Amariei, G.; Arenas-Vivo, A.; Rosal, R.; Horcajada, P.; Duarte, M.T. Novel Antibacterial Azelaic Acid BioMOFs. Cryst. Growth Des. 2020, 20, 370–382. [Google Scholar] [CrossRef]
- Quaresma, S.; Alves, P.C.; Rijo, P.; Duarte, M.T.; Andre, V. Antimicrobial Activity of Pyrazinamide Coordination Frameworks Synthesized by Mechanochemistry. Molecules 2021, 26, 1904. [Google Scholar] [CrossRef] [PubMed]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schuth, F. Mechanochemical Synthesis of Catalytic Materials. Chem.-Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Fontolan, E.; Alegria, E.; Kopylovich, M.N.; Bertani, R.; Pombeiro, A.J.L. The influence of multiwalled carbon nanotubes and graphene oxide additives on the catalytic activity of 3d metal catalysts towards 1-phenylethanol oxidation. J. Mol. Catal. A Chem. 2017, 426, 557–563. [Google Scholar] [CrossRef]
- Soliman, M.M.A.; Peixoto, A.F.; Ribeiro, A.P.C.; Kopylovich, M.N.; Alegria, E.; Pombeiro, A.J.L. Mechanochemical Preparation of Pd(II) and Pt(II) Composites with Carbonaceous Materials and Their Application in the Suzuki-Miyaura Reaction at Several Energy Inputs. Molecules 2020, 25, 2951. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L. New Trends in C-C Cross-Coupling Reactions: The Use of Unconventional Conditions. Molecules 2020, 25, 5506. [Google Scholar] [CrossRef]
- Matias, I.A.S.; Ribeiro, A.P.C.; Ferraria, A.M.; do Rego, A.M.B.; Martins, L. Catalytic Performance of a Magnetic Core-Shell Iron (II) C-Scorpionate under Unconventional Oxidation Conditions. Nanomaterials 2020, 10, 2111. [Google Scholar] [CrossRef]
- Gurbanov, A.V.; Martins, L.; Kopylovich, M.N.; Sutradhar, M.; Zubkov, F.I.; Mahmudov, K.T.; Pombeiro, A.J.L. Mechanochemical and Conventional Synthesis of Copper (II) Coordination Polymers Bearing Arylhydrazone of Acetoacetanilide and Their Catalytic Activity in Conversion of Acetone to Acetic Acid. ChemistrySelect 2020, 5, 7923–7927. [Google Scholar] [CrossRef]
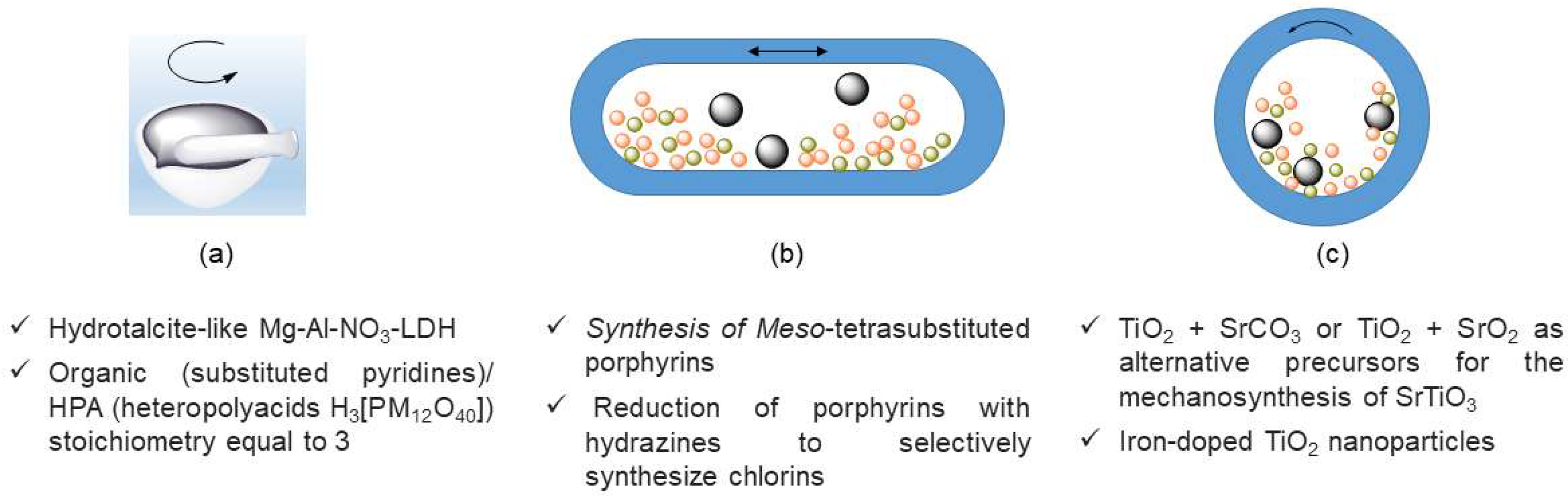
- Ay, A.N.; Zumreoglu-Karan, B.; Mafra, L. A Simple Mechanochemical Route to Layered Double Hydroxides: Synthesis of Hydrotalcite-Like Mg-Al-NO3-LDH by Manual Grinding in a Mortar. Z. Anorg. Allg. Chem. 2009, 635, 1470–1475. [Google Scholar] [CrossRef]
- Monteiro, J.F.; Ferreira, A.A.L.; Antunes, I.; Fagg, D.P.; Frade, J.R. Thermodynamic restrictions on mechanosynthesis of strontium titanate. J. Solid State Chem. 2012, 185, 143–149. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Azevedo, S.; Fernandes, F.; Freitas, E.; Pereira, M.; Tavares, C.J.; Lanceros-Mendez, S.; Teixeira, V. Synthesis of iron-doped TiO2 nanoparticles by ball-milling process: The influence of process parameters on the structural, optical, magnetic, and photocatalytic properties. J. Mater. Sci. 2014, 49, 7476–7488. [Google Scholar] [CrossRef]
- Santos, F.M.; Nogueira, H.I.S.; Cavaleiro, A.M.V.; Gomes, E.D.; Belsley, M.S. A new synthetic route for compounds prepared from Keggin heteropolyacids and pyridine derivatives. Inorg. Chim. Acta 2017, 455, 600–606. [Google Scholar] [CrossRef]
- Pineiro, M.; Gomes, C.; Peixoto, M. Mechanochemical in situ generated gas reactant for the solvent-free hydrogenation of porphyrins. Green Chem. Lett. Rev. 2021, 14, 338–343. [Google Scholar] [CrossRef]
- Gomes, C.; Peixoto, M.; Pineiro, M. Porphyrin synthesis using mechanochemistry: Sustainability assessment. J. Porphyr. Phthalocyanines 2019, 23, 889–897. [Google Scholar] [CrossRef]
- Stroh, J.; Feiler, T.; Ali, N.Z.; da Piedade, M.E.M.; Emmerling, F. Mechanistic Insights into a Sustainable Mechanochemical Synthesis of Ettringite. ChemistryOpen 2019, 8, 1012–1019. [Google Scholar] [CrossRef]
- Stroh, J.; Ali, N.Z.; Maierhofer, C.; Emmerling, F. Ettringite via Mechanochemistry: A Green and Rapid Approach for Industrial Application. ACS Omega 2019, 4, 7734–7737. [Google Scholar] [CrossRef]
- Gomes, C.; Vinagreiro, C.S.; Damas, L.; Aquino, G.; Quaresma, J.; Chaves, C.; Pimenta, J.; Campos, J.; Pereira, M.; Pineiro, M. Advanced Mechanochemistry Device for Sustainable Synthetic Processes. ACS Omega 2020, 5, 10868–10877. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, V.; Duarte, M.T.; Gomes, C.S.B.; Sarraguça, M.C. Mechanochemistry in Portugal—A Step towards Sustainable Chemical Synthesis. Molecules 2022, 27, 241. https://doi.org/10.3390/molecules27010241
André V, Duarte MT, Gomes CSB, Sarraguça MC. Mechanochemistry in Portugal—A Step towards Sustainable Chemical Synthesis. Molecules. 2022; 27(1):241. https://doi.org/10.3390/molecules27010241
Chicago/Turabian StyleAndré, Vânia, M. Teresa Duarte, Clara S. B. Gomes, and Mafalda C. Sarraguça. 2022. "Mechanochemistry in Portugal—A Step towards Sustainable Chemical Synthesis" Molecules 27, no. 1: 241. https://doi.org/10.3390/molecules27010241
APA StyleAndré, V., Duarte, M. T., Gomes, C. S. B., & Sarraguça, M. C. (2022). Mechanochemistry in Portugal—A Step towards Sustainable Chemical Synthesis. Molecules, 27(1), 241. https://doi.org/10.3390/molecules27010241








